The role, formation and characterization of LiC6 in composite lithium anodes
XUE Zhou-qing, WANG Zi-you, ZHANG Jun-dong, LU Yang, HUANG Wen-ze,CHEN Ai-bing, ZHAO Chen-zi,*, Kuzmina Elena, Karaseva Elena,Kolosnitsyn Vladimir, FAN Li-zhen, ZHANG Qiang,*
(1. School of Materials Science and Engineering, Beijing Institute of Technology, Beijing 100081, China;2. Advanced Research Institute of Multidisciplinary Science, Beijing Institute of Technology, Beijing 100081, China;3. Beijing Key Laboratory of Green Chemical Reaction Engineering and Technology, Department of Chemical Engineering,Tsinghua University, Beijing 100084, China;4. Tanwei College, Tsinghua University, Beijing 100084, China;5. College of Chemical and Pharmaceutical Engineering, Hebei University of Science and Technology, Shijiazhuang 050018, China;6. Ufa Institute of Chemistry UFRC RAS, Ufa 450054, Russia;7. Beijing Advanced Innovation Center for Materials Genome Engineering, Institute of Advanced Materials and Technology,University of Science and Technology Beijing, Beijing 100083, China)
Abstract: To solve the problems of large volume changes and the formation of lithium dendrites in lithium metal batteries, the incorporation of carbon into the lithium metal anodes has gained considerable attention due to its excellent chemical and electrochemical stability, as well as its exceptional mechanical strength that allows repeated cycling. This review provides a comprehensive overview of the formation of lithiated graphite (LiC6) and its role in both bulk and electrode-electrolyte interface regions during lithium plating and stripping. In bulk form, LiC6 has excellent lithiophilic properties, reducing the overpotential for lithium nucleation and promoting uniform lithium deposition. Additionally, when LiC6 is introduced at the electrode-electrolyte interfaces, it improves contact between the electrode and electrolyte by acting as a buffering layer, thereby reducing interfacial impedance. Finally, prospects and challenges for the development of Li/C composite anodes are discussed.
Key words: Lithium-carbon composite anodes;Lithiated graphite (LiC6);Lithiophilic chemistry;Lithium dendrite growth;Lithium plating/stripping
1 Introduction
With carbon neutrality and sustainable development goals in prospect, energy storage systems become an essential approach to employing the intermittent and dispersed renewable energies for future power grids[1-3]. Among the various types of energy storage devices, rechargeable lithium batteries are widely applied due to their efficiency, affordability,and portability[4-14]. Nevertheless, current commercial lithium-ion batteries seem unable to meet the evergrowing energy storage demands[15-20]. Lithium metal batteries (LMBs), on the other hand, are regarded as one of the most promising next-generation energy storage systems, as lithium metal anode has an extremely high theoretical specific capacity (3 860 mAh g-1) and low electrochemical potential (-3.04 Vvs.SHE)[21-25].
However, the formation of lithium dendrites, extreme volumetric fluctuation, and rapid fracture of lithium metal are significant challenges for the practical application of LMBs[26-31]. Many emerging innovations have been proposed to enhance the performances of working Li metal anode, including electrolyte design[32-35], interfacial modification[36-38], introducing solid-state electrolytes[39-40], and constructing composite anodes[41-42]. Among them, composite anodes that integrate lithium metal into 3-dimensional hosts (3D hosts) stand out as a promising approach[43]. The origins of the advantages brought by 3D hosts are as follows: (1) The 3D host material provides a porous matrix to encapsulate metallic lithium, effectively reducing volumetric changes, promoting uniform lithium plating, and enhancing the mechanical strength of the anode[44-47]. (2) The large specific area and high conductivity of certain 3D hosts effectively reduce the local current density on the composite anode, therefore further suppressing lithium dendrite growth and metallic lithium pulverization[45,48-49]. (3) Numerous interconnecting pores and lithiophilic surface areas offered by the 3D framework enable the efficient transport of lithium ions,which magnifies the benefits mentioned above[42,45,50].
The emerging 3D hosts can be classified into 2 categories: insulate hosts (such as polymers and glass fibers) and conductive hosts (such as metallic or carbon-based materials). The carbon-based hosts exhibit not only excellent chemical and electrochemical stability[51-56], but also extraordinary mechanical strength during repeated cycling, which promotes the constant sizing and thus higher safety levels for batteries[42-43,57-60]. Consequently, lithium-carbon (Li/C)composite anodes are given great attention and being widely studied.
The superior properties of Li/C composite anodes are largely attributed to the lithiophilic LiC6,which isin-situformed during the composing process of lithium and carbon[70]. The lithiophilicity of the LiC6surface enables the uniform nucleation and growth of lithium metal[71-73]. And the 3D hosts provide sufficient space to accommodate volume expansion during lithium plating[46,50,73-76]. Aiming at a deeper insight into the interfacial evolution of Li/C composite anodes and further improvement on composite anode-based battery design, it is crucial to understand the way that LiC6functions[48,58,71,77].
The development of LiC6traces back to the practical applications of Li/C composite anodes. Fig. 1 illustrates the most important events in carbon materials for lithium metal batteries.

Fig. 1 The timeline of typical events in the development stages of LiC6-contained composite anodes in lithium metal batteries[48-49, 61-69]
In this contribution, the recent advances in Li/C composite anodes are summarized, along with an insightful discussion on the role of LiC6in rechargeable lithium batteries (Fig. 2). The pathways for generating composite anodes are presented, followed by a clarification of the evolution mechanisms related to LiC6in both the bulk phase and at the interface The design principles of Li/C composite anodes are outlined, highlighting major opportunities and providing a blueprint for their future advancements.
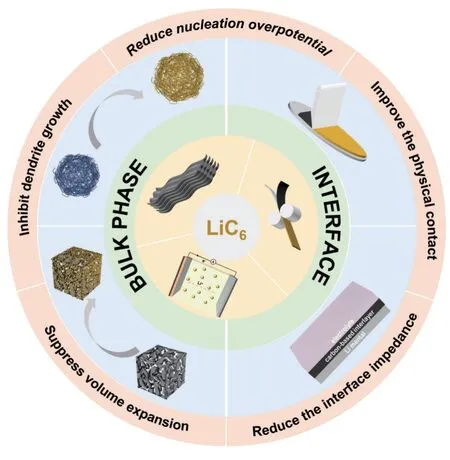
Fig. 2 Summary of the roles of LiC6 in lithium composite anodes
2 LiC6 in the battery
LiC6compounds are formed throughin situintercalation reactions between lithium metal and carbonbased materials[78]. Lithiophilic LiC6can enhance the intercalation detachment process during bulk phase interaction and act as a buffer layer for electrolytes and interfaces during interfacial interaction.
2.1 LiC6 generation in composite anodes
2.1.1 Lithiated carbon anode
The widely accepted method for LiC6fabrication is by generating a Li/C composite anode. This can be achieved by adding molten lithium to carbon or by bringing lithium into contact with carbon during the construction of a lithium metal battery. The chemical reaction between graphite and Li metal are illustrated in Fig. 3a: Li++ 6C→LiC6, ΔG= -10.59 kJ mol-1. The process of inserting Li atoms into carbon is thermodynamically spontaneous, as indicated by Gibbs free energy variation[79]. After Li intercalation, the bonds between carbon framework and Li+in the electrolyte are strengthened, leading to the generation of lithiophilic of LiC6.

Fig. 3 (a) Schematic of the mechanism of graphite reacted with Li metal to generate LiC6. (b) Different lithiation stages of a carbon-based anode during charging. Reproduced with permission[81]. Copyright 2018 American Chemical Society
The lithium kinetic processes in Li/C composites involve several stages[80]:
(1) Lithium ions are transported from the electrolyte to the carbon surface.
(2) During the charge transfer process, lithium ions react with carbon matrices and are transported into the carbon anode through the interface, forming the Li/C composites. The reaction continues and the lithium-ion contents in the carbon matrices gradually increase until all the carbon matrices are fully lithiated into LiC6.
(3) Following the formation of LiC6, the nucleation and growth of lithium metal occur as a result of the lower potential.
The evolution of LiC6during charge and discharge processes is distinct. The charging process involve a sequential lithiation process of Li+insertion into graphite (Fig. 3b). This progressive process comprises several stages: initial solid solution state (original graphite), stage III (from LiC24to LiC18), stage II(from LiC18to LiC12), and the final stage I (from LiC12to LiC6)[72]. The anode materials in different lithiation stages are distinguished by different colors, transitioning from black (LiC18) to silvery-black (LiC12), and finally to golden yellow (LiC6) at the end of the charge[81].
During the discharge process, as metallic lithium is fully consumed from the composite anode, the concentration of LiC6gradually decreases[82]. The carbon skeleton still participates in the discharge process,leading to structural changes that give rise to the formation of LiC12. Subsequently, the phase with lower lithium content gradually becomes dominant.Considering practical battery design, the anodes will retain an amount of excessive lithium, typically maintaining a negative/positive (N/P) ratio between 1.05 and 1.20[83]. This approach not only preserves the lithiophilicity and structural stability of the framework but also compensates for capacity loss during the cycling process.
Li metal has a low melting point of 180.5 °C and good fluidity at temperatures above 200 °C[84-85].Hence, injecting molten Li through direct thermal injection is a routine strategy for pre-storing lithium in the 3D hosts[86-94]. For instances, Cui’s group described an effective layered Li-reduced graphene oxide (Li-rGO) anode made by injecting molten Li into the surface of rGO (Fig. 4a)[65]. The rGO offers the excellent framework for accommodating the infusion of Li metal, which contributes to a stable plating stripping process and significantly mitigates the volume change. The emergence of lithiophilic LiC6facilitates the even distribution of lithium ions into the gaps within the layer of rGO. This anode was found to maintain a capacity of up to 3 390 mAh g-1with good rate capability and stable cycling, as well as a low hysteresis in a full-cell matched with the LiCoO2cathode. The molten Li can not only be added into layered carbon matrices, but also can be combined with 3D carbon nanotube networks. Chen’s group demonstrated impressive results by soaking CNT particles in molten Li metal under stirring (Fig. 4b)[95]. Molten lithium is spontaneously absorbed into CNT particles to form Li-CNT composite particles. Compared with bare lithium, the carbon nanotube frame structure of Li-CNT composite anodes can inhibit dendrite growth and form stable SEI. In addition, the larger specific surface area of Li-CNT particles provides internal space to accommodate the volume expansion during lithium plating. The full cell composed of Li-CNT composite anode and LiFePO4(LFP) cathode exhibits a CE of 90.1% in a prolonged 300 cycles at 1 C. Thus,the construction of various lithium carbon structures is deemed suitable through the process of making composite anodes via molten lithium.
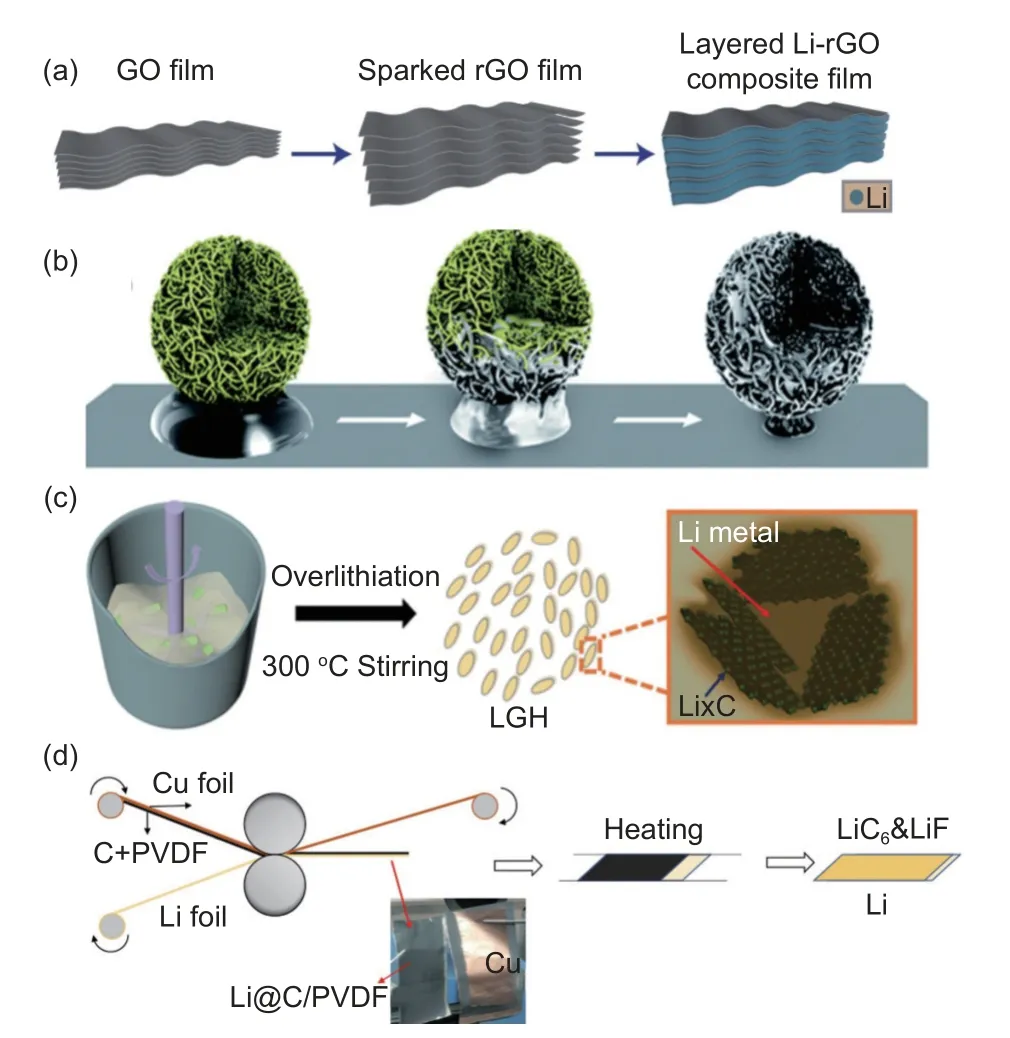
Fig. 4 (a) Schematic illustration for the fabrication of the Li-rGO composite film by lithium metal melting. Reproduced with permission[65]. Copyright 2016, Springer Nature. (b) The construction of the Li-CNT composite anode. Reproduced with permission[95]. Copyright 2013, Royal Society of Chemistry. (c) The fabrication process of Li-graphite composite anode by the stirring and melting method. Reproduced with permission[99]. Copyright 2018, Elsevier. (d) Formation process of Li@LiC6&LiF-x composite anode materials. Reproduced with permission[104]. Copyright 2021 American Chemical Society
Achieving a better N/P ratio and avoiding excess lithium while improving energy density can be lucratively achieved through the melting method by precisely controlling the Li content. However, pairing excess lithium (averaging 300% in current studies) with the positive electrode inevitably reduces the battery’s energy density[96-98]. Therefore, prioritizing cost savings and minimizing the waste of lithium is strongly considered for large-scale production through the melting method. Tu, Xia and co-workers demonstrated a successful large-scale fabrication of lithiumgraphite composite anodes (LGH)viaa simple onestep stirring-melting process (Fig. 4c)[99]. Notably, this process allows for the precise control of the mass load of Li metal, thereby avoiding an excessive amount of Li in the complete battery. Stirring method accelerates the contact reaction between molten lithium and layered graphite sheets, resulting in a high-density surface featuring LiC6nucleation sites. This surface enables to reduce local current density during the stripping/plating process, allowing for more uniform plating of lithium. These advantages realize stable operation of LGH symmetrical cells for up to 100 cycles at 5 mA cm2with a capacity of 1.5 mAh cm-2, and lithium utilization rate per cycle is 25%.
The use of the melting method in preparing LiC6enables the uniform reaction between lithium and carbon, resulting in a homogeneous phase distribution in the composite anode. At high temperatures, lithium metal exhibits good fluidity, facilitating uniform contact between lithium and carbon-based materials and favorable lithiophilic properties throughout the material. However, the melting method necessitates a strict inert atmosphere and specialized equipment, which can increase production costs. At the same time, when the composite anode is prepared on a large scale by molten lithium injection, the hidden risk of high temperature will possibly damage the lithiophilic surface[100-101]. To address this issue, a simple and safe method of roller pressing was explored to take advantage of lithium metal’s good ductility at room temperature. Gao’s group designed the rolling with heat treatment strategy to fabricate the Li@LiC6&LiF composite Li anode. (Fig. 4d). In this composite anode,LiC6is derived from the direct contact reaction of lithium and graphite. LiC6composite anode is successfully fabricated by direct contact between Li and carbon, which is triggered by the lower Li ion diffusion barrier of LiC6(0.05 eV) than that of Li (0.13 eV).The homogenization of nucleation and growth get profits from the excellent lithiophilic properties. Notably, LiC6material has a high lithium-ion diffusion coefficient (2×10-7cm2s-1)[102-103], which effectively promotes the interfacial diffusion kinetics of lithiumion. The rapid ionic diffusion eliminates the excessiv1 Li accumulation induced dendrite. The Li@LiC6&LiF coupled with Ni-rich NCM cathode realized a capacity retention rate of 94% after 100 cycles at 0.1 C.
2.1.2 Anode-free carbon anode
The electrochemical reaction of LiC6generation during the battery cycle process is enabled by using lithium-free carbon materials as the anode. This approach reduces the pre-treatment process of materials,minimizes the weight of the negative electrode, and increases the overall energy density of the battery by producing LiC6in situ. Due to the absent of pre-lithiation, the lithiophobic anode free carbon anode may suffer from inhomogeneous Li deposition leading to subsequent Li dendrite. Essential structural design for carbon material should be adopted to solve these problems.
Hence, increasing specific area of carbon matrices is the basic strategy in a lithium free anode.Kang et al. reported the synthesis of three-dimensional (3D) carbon materials (CMs) to modify electrodes using a high-temperature carbonization method(Fig. 5a)[105]. During lithiation, CMs were converted in situ to LiC6. The lithiophilic LiC6dispersed lithiumion flow, resulting in uniform lithium nucleation. In addition, the 3D structure provides a large specific surface area, combined with lithiophilic LiC6, results in a synergistic effect that facilitates uniform and controlled lithium growth avoiding dendrite formation.Therefore, the CMs-modified electrode achieved a high Coulombic efficiency of 97% even after 300 cycles of long life. During the electroplating process of lithium in a battery, the unavoidable volume expansion can make the internal stress distribution within the battery uneven, which can adversely affect the cycle life of the battery. Although the large surface area of the electrode material can potentially delay the growth of dendrites, the volume expansion will still destroy the anodic structure, deteriorating the anodic lifespan. Therefore, He’s group reports a 3D graphene frame (3D-GF)[106], an interconnected hollow sphere with large internal spaces (Fig. 5b). The tough graphene skeleton can stabilize the structure of the lithium metal anode. The macropores within the sphere offer a larger volume to alleviate the significant volume changes during the lithium deposition.This selective lithium deposition effectively prevents the formation of lithium dendrites at a deposition capacity of up to 10 mAh cm-2. The anodic structure combining high specific area with special three-dimensional design can overcome the dendrite formation and volume expansion simultaneously. Wan’s group proposed to attach spherical carbon granules to the three-dimensional conducting skeleton as the anode to facilitate the long cycle of the battery(Fig. 5c)[107]. This novel approach benefits from the three-dimensional skeleton’s ability to accommodate volume expansion. Besides, the spherical carbon granules increase the surface area to suppress the Li dendrite filaments[108-110]. The lithiophilic LiC6formed in initial charging improves the wettability of lithium to spherical C, promoting stable lithium deposition and replenish the Li loss in subsequent cycles. The prepared anode exhibits a lithium utilization rate above 95% and maintains stable plating/peeling performance over 500 cycles. At the same time, the full cell matched with LiFePO4strike a long lifespan of 1 000 cycles at a surplus Li of merely 5%.

Fig. 5 (a) Lithium process on a three-dimensional carbon modified electrode. Reproduced with permission[105]. Copyright 2019, Elsevier;(b) Schematic diagrams showing the Li nucleation and growth process on the 3D-GF. Reproduced with permission[106]. Copyright 2019, American Chemical Society. (c) Schematic diagrams of lithium plating process on the CMN. Reproduced with permission[107]. Copyright 2017,American Chemical Society
2.2 The roles of LiC6 in a working battery
The lithiophilic properties of LiC6in the Li/C composite anode can reduce the nucleation overpotential of lithium and promote uniform growth during lithium deposition. The Li behaviors for Li-carbon composites include multiple steps as the formation of LiC6, the ion transport and the subsequent Li nucleation and growth[111-115]. Lithium deposition is triggered by a certain overpotential. When lithium metal directly contacts with the electrolyte, SEI forms on the surface of the anode. Solvated lithium ions traverse the SEI, undergo desolvation, and subsequently reach the anode[116-117]. The resulting LiC6, featuring a lithiophilic functional site, can decrease the nucleation overpotential of lithium metal deposition. Compared to lithium deposited directly on copper foil, the higher surface energy deposited on LiC6realizes a smaller wetting angle during lithium deposition.which results in the smaller deposited nucleation overpotential of <0.1 V[118].
2.2.1 Bulk phases
LiC6in bulk phase plays important roles in reducing overpotential of lithium-ion insertion, promoting uniformity of lithium deposition. The intrinsic lithiophilicity of LiC6skeleton can protect the surface of the negative electrode, which improves the performance, stability, and lifespan of the battery.
However,the primary carbon is lithiophobic,which cannot deliver satisfy performances. A pre-lithiation on forming LiC6for carbon matrices is essential. In order to explore the lithiophilicity of the carbon materials, Li’s group compared the wettability of Li in pure carbon materials and lithiated carbon materials (Fig. 6a)[68]. They conducted apparent contact angle (ACA) measurements of molten lithium liquid(Liliq) droplets on porous graphite paper (PCP) and drops on prelithiated PCP. As shown in the Fig. 6a,Liliq’s ACA on PCP is 142°, while Liliq’s ACA on prelithiated PCP becomes less than 90°. At the same time, Li is permeated into the pores and diffused into the whole porous substrate rapidly even in 2 s. The above phenomenon proves that prelithiated PCP exhibited a transition from lithiophobicity (C6) to lithiophilicit1 (LiC6). Comparing with bare carbon matrices, Li began to grow along the lithiated LiC6fiber, forming a core-shell structure with LiC6fiber as the core and excess Li as the shell. Li-PCP composite materials exhibit better performance than bare Li foils,displaying over 200 stable cycles.

Fig. 6 (a) Li wettability of PCP, lithiated PCP. ACA measurements of(I, II) PCP and (IV, V) lithiated PCP. Digital photos of a Li droplet on (III)PCP and (VI) lithiated PCP after cooling down to room temperature[68].(b) Schematic diagrams of electrochemical plating/stripping process of lithium on the CNT sponges. Reproduced with permission[119]. Copyright 2019,American Chemical Society; (c) Schematic diagrams of coralloid layered composite electrode. Reproduced with permission[120]. Copyright 2018, Elsevier. (d) Schematic of the Li-EG composite anode fabrication process. Reproduced with permission[121]. Copyright 2019, Royal Society of Chemistry
The carbon framework conducts electricity and decreases local current density, while a stable composite structure helps handle volume changes during repeated electrochemical plating and stripping.
Based on the above, a prelithiation procedure forming lithiophilic LiC6is important. Many researchers have adopted pre-lithiation processes for various carbonaceous hosts to change lithiophobicity to lithiophilicity for guiding uniform lithium plating. Wang and colleagues reported a commercial carbon nanotube (CNT) sponge as a 3D current collector to improve the electrochemical performance of the battery(Fig. 6b)[119]. During battery discharge, lithium is intercalated into CNT interlayers forming LiC6, then Li metal is plated on the surface nanopores when the voltage reaches 0 V. Since the “pre-lithiation” behavior generates LiC6on the surface and the affinity of the carbon bulk phase, inducing the Li nucleation with low over-potential. In addition, the high specific surface area of carbon nanotubes (113.8 m2g-1) increases the density of lithium nucleation sites and reduces the local current density, which ensures the uniformity of lithium deposition on carbon nanotube matrices. Therefore, dendrite-free lithium plating with high capacity of 10.0 mAh cm-2was achieved on the CNT sponge.
Adding more lithiophilic components into LiC6matrices can substantially improve the Li plating.Zhang’s group uses a silver coating to guide the molten Li permeation on fabricating the structured CF electrode (Fig. 6c)[120]. Taking advantage of the siphonic effect of a silver coating, molten lithium is automatically infused into the CF/Ag framework and rapidly diffuses throughout the entire electrode. The CF/Ag-Li anode exhibits structural stability on suppressing the volume changes during repetitive lithium plating/stripping processes. The LiC6formed in the CF graphite crystal and Ag nanoparticles both promote the uniform deposition of lithium in a carbon fiber frame. Therefore, the full cell constructed with S demonstrates a high initial discharge capacity of the 785 mAh g-1and a high-capacity retention rate of 64.3% after 400 cycles at 0.5 C.
LiC6can act on the bulk phase not only by molten lithium injection, but also by mixing heating and pressurization. He and colleagues heated and pressurized Expanded Graphite (EG) and Li powder to form a lithium-expanded graphite composite anode (Li-EG)(Fig. 6d)[121]. According to BET tests, the specific surface area and pore volume of EG were 21.398 m2g-1and 0.170 3 cm3g-1, respectively. The abundant porosity contributes to providing sufficient space for lithium metal deposition. More importantly, EGin situreacts with lithium metal to form LiC6. LiC6has good affinity for lithium ions and electronic conductivity, providing sufficient sites for lithium nucleation and effectively reducing the overpotential during charging and discharging. In addition, the formation of a stable SEI helps to reduce interfacial side reactions.Therefore, the Li-EG composite negative electrode exhibits excellent electrochemical performance. The prepared electrode exhibits excellent cycle stability of more than 1 500 cycles even at ultra-high current densities of 10 mA cm-2.
During the process of lithium removal, dead lithium production from the lithium metal anode can reduce the utilization efficiency and cycle life of working batteries. In regular circumstances, the formation of dead lithium in Li metal through only conversion mechanism is inevitable. The de-intercalating process in Li carbon materials can realize much higher utilization efficiency. The lithium potential of the Li/C composite anode is 0.1 V, higher than the 0 V potential of metal lithium removal. Therefore, realizing the de-intercalating mechanism of the Li/C composite anode can reduce the generation of dead lithium and improve the cycle stability of the electrode. Zhang’s group puts forward a successive conversion-deintercalation (CTD) delithiation mechanism (Fig. 7) to regulate the deposition and shedding behavior of lithium by manipulating the overpotential of the lithium anode in the cycle[48]. This CTD mechanism contributes to improving lithium metal utilization efficiency reducing dead lithium generation, and enhancing cycle stability. During the initial cycles, the lithium metal is plated and stripped at potential less than that of LiC6(0.1 Vvs. Li/Li+), indicating that the capacity is completely contributed by the conversion mechanism. In subsequent cycles, the accumulation of dead lithium increases the anodic interfacial overpotential. When the overpotential reaches LiC6intercalating potential,the delithiation will be triggered in the LiC6and Li metal simultaneously. At this time, the delithiation mechanism converts from a single conversion mechanism to the CTD delithiation mechanism. The intercalation reaction between lithium metal and graphite enables the rapid replenishment of graphite by lithium ions. The speed of lithium supplementation of graphite is greater than the speed of Li metal stripping, allowing for continuous de-intercalation and conversion of Li+. This reduces the accumulation of dead lithium on the surface of lithium metal in subsequent cycles. The electrochemical testing shows that the full cell with the CTD delithiation mechanism can maintain up to 210 cycles, which is higher than that of a bare Li anode that can only last for 110 cycles.
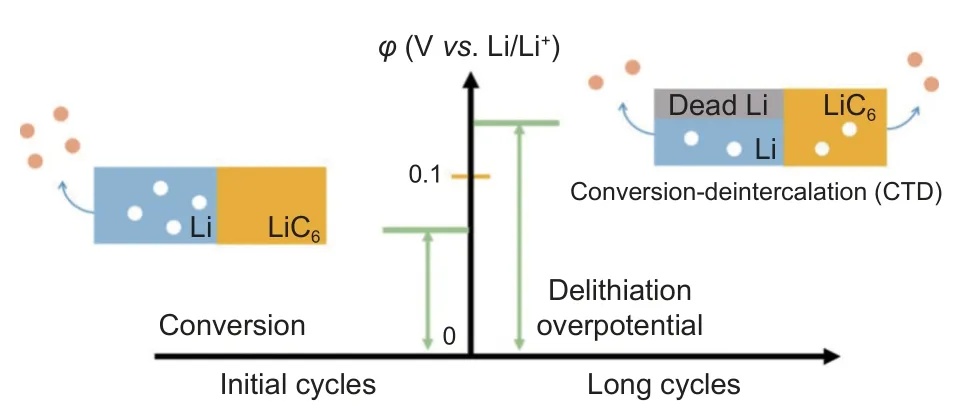
Fig. 7 Diagram of the overpotential and delithium mechanism in composite anode during initial and long cycles. Reproduced with permission[48].Copyright 2022, American Chemical Society
During the battery formation process, the electrochemical method involves the gradualin situformation of LiC6from lithium ions and carbon skeleton matrices, followed by the subsequent deposition of lithium onto the LiC6skeleton. Consequently, the electrochemical methods offer simplicity, cost-effectiveness, and higher efficiency. However, this method suffers from uneven lithium metal deposition on the anode surface, which will shorten the battery cycle life. Therefore, the preparation of a lithiophilic composite anode using the electrochemical method requires specific considerations for the uniform structure and surface design of the carbon materials.
2.2.2 Interface
The LiC6bulk can not only improve the anodic performance in liquid electrolyte batteries, its intrinsic lithiophilic nature can also work as sole interface to adjust Li interfacial properties, guiding the homogenously Li plating and stripping in both liquid electrolyte and solid-state batteries.
LiC6can work as powerful interlayer to improve the solid-state batteries. The solid-state batteries suffer from the poor initial interfacial contact and unstable interfacial Li kinetics at the Li/solid-state electrolyte (SSW) interfaces. The LiC6can act as a buffer layer to improve the initial anodic contact and reduce the interface impedance. Li-garnet solid-state batteries suffer from the poor interfacial contact. Shao’s group reported a method to draw a graphite-based soft interface on the surface of the electrolyte with a pencil to enhance the interface connection (Fig. 8a)[122].The reaction between the graphite-based interfacial layer and metallic lithium forms LiC6, obtaining a lithiated connection interface with good lithiophilic and electronic conductivity. This strategy significantly improves the wettability and interface contact between SSE and lithium metal, effectively reducing the interface impedance. Lithium ions can be evenly distributed at the interface, so that the battery has excellent cycle stability of more than 1 000 cycles. In polymer based solid-state electrolytes, LiC6is also a recommended interfacial aider. Shen’s group[123]used the scraping method instead of manual pencil drawing for large-scale preparation (Fig. 8b). The graphite-modified solid electrolyte (GSE) has a high electrochemical potential window of exceeding 4.6 V and a high ionic conductivity of 3.08×10-4S cm-1at room temperature. The graphite modification layer and lithium undergo a spontaneous reaction to produce a lithiophilic LiC6layer, which significantly improves interface contact and stability, enhances current distribution uniformity, and reduces the impedance of NCM622/SE/Li batteries. Moreover, the assembled NCM622/G-SE/Li cell exhibits an initial capacity of 160 mAh g-1at 0.5 C and the capacity remains above 100 mAh g-1after 200 cycles.
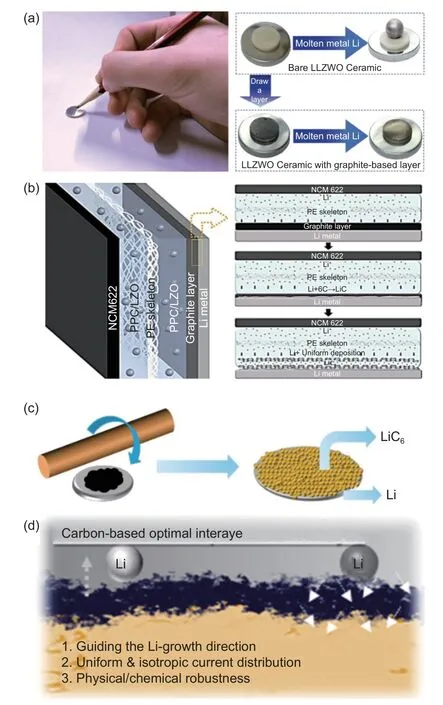
Fig. 8 (a) A pencil sketch of the preparation of a graphite-based interfacial layer and a wetting behavior comparison between molten lithium metal on LALZWO ceramics with and without the interfacial layer. Reproduced with permission[122]. Copyright 2018, American Chemical Society.(b) Schematic diagram of the graphite-modified composite solid electrolyte membrane. Reproduced with permission[123]. Copyright 2020, American Chemical Society; (c) Schematic diagram of the preparation process of the heterogeneous interface layer[75]. (d) Schematic diagram of the mechanism of action of carbon-based optimal interlayer[124]
Multiple methods can help to import LiC6interfaces in the solid-state batteries. Zhou’s group reported that graphitized carbon nanospheres as a modified material modified a layer of LiC6heterogeneous interface layer on the surface of the metal lithium anode by rolling on lithium foil (Fig. 8c)[75]. The increase in surface area by this LiC6layer can homogenize the current density and Li ion flux on the surface of the lithium foil. The heterogeneous interface layer can significantly improve the reversibility and uniformity of Li electrochemical plating and stripping, inhibiting dendrite growth and maintaining the interfacial stability.Rolling method possesses universality which is promising for industrial production.
In addition to drawing graphite layers to the electrolyte surface, Kang’s group designed a slurry casted method for fabricating carbon-based interlayer to induce directed growth of lithium metal (Fig. 8d)[124].After introducing a layer of lithium metal seed layer,the lithiated LiC6can induce the rapid preferential deposition of lithium metal in the anode and interlayer,thereby effectively reducing the nucleation barrier.Furthermore, the utilization of amorphous carbon as an intermediate layer preserves the stability of the interface after cycling. The full cell assembled with the interlayer shows excellent electrochemical performance with a capacity retention rate of up to 99.6%after 250 cycles at 2.5 mA cm-2and a cumulative lithium metal capacity exceeding 1 000 mAh cm-2.
2.3 Advanced characterization methods
In recent years, researchers have realized that real-time monitoring and tracking the evolution of lithium metal electrodes at the nano- and micro-scale during cycling is important to better understand the electrochemical performance of the electrode. With the development of advanced characterization techniques, there are more methods to track the morphological evolution, chemical components transition,structure and dynamics of lithium-carbon.
In situ optical microscopy (OM) is an optical instrument to extract information such as volume and morphology change in working battery, which is a non-destructive, non-contact and non-vacuum method[125-126]. A typical example of in situ light microscopy is the real-time observation on the dynamic morphological evolution of the Li/CP@LiC6electrodes[127]. Cross-sectional images of Li@CP/LiC6electrodes measured at 0.5 and 3 mAh cm-2. The Li plating/stripping evolutions are shown in Fig. 9a. The original Li@CP/LiC6electrode is covered with a uniform lithiophilic LiC6and has a thin Li metal bottom layer. No Li dendrite formation/growth was observed during the plating process. LiC6with strong lithiophilic properties makes most of the Li metal stored in the pores of the electrode, so that the volume changes are obviously suppressed. During the stripping process,no dead lithium accumulation was observed at the electrode/electrolyte interface thanks to the three-dimensional space of the electrode. In order to visually observe the deposition behavior of lithium ions and study the influence of lithiophilic sites on the deposition process, Wang et al.[128]conducted in situ OM observation of lithium dendrite growth and electrode volume expansion of Cu, Ag/HC and other electrodes in electrochemical processes at a current density of 4 mA cm-2. It was found that with the continuous increase of lithium deposition capacity, Ag/HC electrode showed the best performance to inhibit volume expansion and abnormal growth of lithium dendrites throughout the excess deposition of lithium.
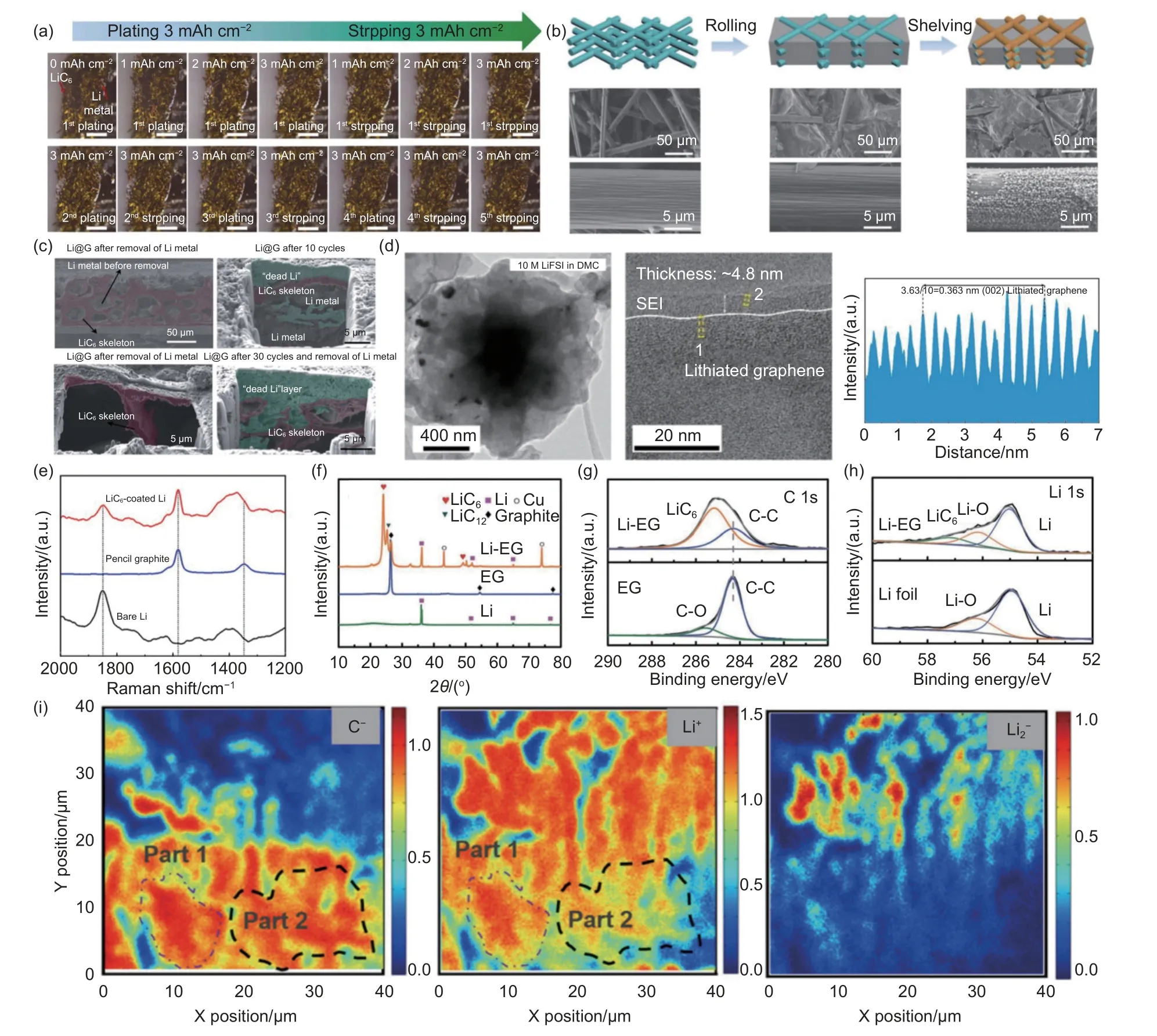
Fig. 9 (a) Operado optical microscopic image of the Li@CP/LiC6 electrode-electrolyte interface. Reproduced with permission[127]. Copyright 2022, Elsevier.(b) Schematic illustration and high-resolution SEM images for pristine CF, initial Li/CF composite anode, and Li/CF composite anode shelving for 72 h with the formation of LiC6 layers. Reproduced with permission[49]. Copyright 2019, John Wiley and Sons; (c) Removal of cross-sectional SEM and FIB-SEM images of LiC6 and dead lithium backbones in the anterior and posterior Li@G anodes of lithium metal. Reproduced with permission[130]. Copyright 2021, Royal Society of Chemistry. (d) High-resolution cryo-EM image of lithium graphene shell. Reproduced with permission[77]. Copyright 2019, American Chemical Society.(e) Raman spectroscopy of bare lithium, pencil graphite and lithium coated with LiC6. (f) XRD spectra of Li, EG, Li-EG. (g) C 1s XPS spectra for EG and Li-EG. (h) Li and Li-EG for Li 1s XPS spectrum[121]. (i) the C-, Li+, and Li2- maps of the graphite electrode after 15 cycles by TOF-SIMS after depth sputtering[48]
Scanning electron microscopy (SEM) mainly uses various physical signals excited by secondary electrons and backscattered electrons when scanning the sample surface to modulate imaging. Utilizing SEM can enable the observation of processes such as LiC6particle formation, growth of lithium dendrites on the negative electrode surface, and volume changes of composite anodes before and after battery cycling through cross-sectional analysis. Coupling with inert focused ion beam (FIB), the samples can be cut and the inside structure of samples can be captured by SEM. The FIB-SEM is a more powerful tool to analyze the surface and bulk simultaneously[129].
Zhang et al. used SEM to characterize the Li/CF composite anode obtained by the roll pressing method.Under rolling pressure, metallic lithium was pressed into the gaps between carbon fibers as shown in Fig. 9b[49]. After 72 h of standing, further magnification revealed small particles of 100-200 nm in size that were uniformly and densely distributed on the CF surface, and could be identified as LiC6compound.The corn granular LiC6layer constructs a three-dimensional interconnected lithiophilic framework in the composite anode.
In order to determine the spatial distribution of LiC6and metal Li in the Li@G anode, Liu et al. characterized the internal structure using a focused ion beam (FIB)-SEM[130]. In Fig. 9c, the two components of metallic lithium and LiC6can be clearly distinguished: the metallic lithium is tightly surrounded by the LiC6framework (marked in light red). Cross-sectional SEM images show many irregular cavities within the electrode, which are thought to be removed lithium. The remaining skeleton is considered to be the structural skeleton of the Li@G anode, which is mainly composed of LiC6. It forms a continuous network supporting the composite anode. The FIB-SEM image in Fig. 9c reveals that most of the Li metal is attached to the walls of the LiC6framework, and the abundant LiC6framework provides a continuous electronic pathway for the recycling of “dead Li”.
In situ electron microscopy (in situ TEM) can provide real-time high-resolution information such as microstructure changes, phase transitions and evolutions at specific observation sites[131-133].The spatial resolution can reach atomic scale and high temporal resolution can realize sub-millisecond level. To characterize the Li metal deposition in wrinkled graphene cage (WGC) in real batteries, cryo-electron microscopy, which is a recently developed method is used to characterize the active and sensitive battery materials under electrochemical conditions without damaging the sample[77]. In high-resolution images(Fig. 9d), the lattice spacing of lithiated graphene shells was measured to be 0.363 nm, significantly mitigating the volume expansion.
Raman spectroscopy is an effective technique for detecting vibrational frequencies and is widely used in materials research. As a sensitive technique for determining the structural information of graphite intercalation compounds[134], Fig. 9e shows the Raman spectra collected from bare lithium electrode, graphite, and LiC6. Graphite exhibits prominent peaks at 1 346 and 1 581 cm-1, corresponding to theDandGbands of crystalline graphite carbon. The spectrum of LiC6shows aGband peak at 1 581 cm-1, indicating the presence of LiC6. While theDband peak overlaps with the broad peak of bare Li, demonstrating the presence of graphite and lithium metal.
X-ray characterizations provides fast, precise,and non-destructive analysis of crystal structures,making it useful for studying the phase and crystal structure of matter in lithium metal batteries. In situ X-ray diffraction (XRD) allows for the detection of phase transitions and structural evolution during plating and stripping[135-136]. X-ray photoelectron spectroscopy (XPS) technology is an effective way to analyze elemental composition and content, chemical valence state, and chemical bonds of the material surface. As a typical example, He et al. observed the XRD spectrum of Li-EG and found the characteristic peak of LiC6due to EG embedding into the lithium metal[121], indicating the lithiation process of the negative electrode (Fig. 9f-h). Additionally, XPS spectra further confirmed the formation of LiC6. The C 1s XPS peak at 285.2 eV in the Li-EG spectrum belongs to LiC6, corresponding to the Li 1s peak at 57.1 eV.The lithiophilic LiC6is conducive to reducing the nucleation overpotential and inducing uniform lithium deposition.
Time-of-flight secondary ion mass spectrometry(TOF-SIMS) is one of the most cutting-edge and practical surface analysis techniques that accurately determines the composition of surface elements by bombarding the sample surface with an ion beam to generate secondary ions. Zhang et al. analyzed the distribution of lithium ions in the composite negative electrode after delithiation using TOF-SIMS (Fig. 9i)[48].The images show the lithium distributions in the electrode with different delithiation stages. The different intensities of lithium demonstrate the different lithiation level, indicating the removal of Li ion in weak Li intensity area. Additionally, based on the mapping of Li2-, partially deposited Li metal was found to remain on the surface of the graphite. The TOF-SIMS methods provide solid evidence on investigating the transformation mechanism shifts from a single intercalation mechanism to a delithiation-insertion conversion mechanism.
3 Conclusions and perspectives
Composite lithium metal anodes with carbonbased materials contributes to a highly reversible lithium plating and improved electrochemical performances. Among them, LiC6plays vital roles in the Li/C composite anode. This review provides an overview of LiC6formation and the mechanism of LiC6in the bulk and interface phases during plating and stripping processes. When LiC6functions in the bulk phase, its exceptional lithiophilic properties can reduce the nucleation overpotential of lithium, promoting uniform nucleation and growth of lithium deposits. When it acts at the interface between the electrode and the electrolyte, LiC6as a buffering layer can improve its contact and reduce interfacial impedance. Advanced characterization techniques, includingin-situmethods,can monitor the morphological evolution of LiC6in the electrode, allowing for a deeper understanding of a working Li/C composite anode.
At present, the construction lithium composite anode by introducing a 3D carbon skeleton still poses challenges. The bulk energy density of the battery decreases as the 3D skeleton increases the weight of the anodes. Furthermore, a larger specific surface area creates significant obstacles in achieving a stable electrolyte-electrode interfacial layer, as it results in larger electrochemical interfaces and consequently leads to more side reactions. Moreover, the feasibility of Li/C composite anodes and the lithium kinetic mechanism remain to be investigated, impeding the practical application of 3D skeletons.
To address the aforementioned challenges, the combination of lithiophilic materials with lightweight skeletons can significantly enhance the reversibility and reduce the impact on energy density. Considering that 3D skeletons possess a large contact area, it results in increased consumption of active materials.Therefore, an important direction for future lithium batteries is the design of advanced compatible electrolytes realizing high interfacial stability. Additionally,conducting in-depth research on the mechanism of lithium deposition kinetics on 3D carbon skeleton structures through the integration of multiple theoretical and experimental methods, such as finite element simulation and first-principles calculations, can provide valuable insights.
Although Li/C composite anodes have achieved remarkable accomplishments, further research is still needed, including:
(1) Exploring advanced materials characterization and simulation techniques. Advanced characterization methods such as NMR and theoretical simulation techniques can help to investigate the distribution and evolutions of LiC6in composite anodes, understand the working principles of composite electrodes,and afford theoretical support for improving battery performance.
(2) Optimize the electrode/electrolyte interface.The design of a highly stable electrode/electrolyte interface is critical for future applications of composite lithium anodes. This is because the 3D skeleton with high specific surface area in the composite lithium anode helps to reduce the local current density and inhibit the growth of lithium dendrites, which is desirable. However, this also leads to the challenge of increased battery side reactions, which can significantly reduce the coulomb efficiency. Therefore, it is necessary to balance the inhibition of lithium dendrite growth with the prevention of side reactions to achieve the best performance of composite lithium anodes in practical applications.
(3) Developing new manufacturing methods or optimization of existing composite processes. Electrodeposition and melt injection pre-lithiation methods are difficult to achieve uniform distribution of Li in the carbon-based matrix. The rolling method necessitates the skeleton material to possess good mechanical strength and flexibility. An environmentally friendly, low-cost and easy-to-operate synthetic processes are also crucial factors for the implementation of carbon-based hosts.
(4) Designing Li/C composite anodes with advanced carbon materials. The ideal carbon material is supposed to possess a combination of excellent electrical conductivity, high Li diffusion coefficient, good mechanical properties and stable solid-electrolyte interphase (SEI) generation. It is worth noting that these attributes may be found in one or more carbon materials. In the future, by integrating multiple disciplines, it will be possible to design composite lithium electrodes that exhibit high stability, high energy density,and excellent electrochemical performances. This interdisciplinary approach will facilitate advancements in the field of lithium batteries, leading to the development of more efficient and reliable energy storage systems.
The development of Li/C composite anodes has greatly improved the plating/stripping process. In the future, in-depth research on LiC6is beneficial for developing lithium metal batteries, improving the energy density, cycle life, and safety of batteries to promote their practical application.
Acknowledgements
This work was supported by the Beijing Municipal Natural Science Foundation (Z200011), National Natural Science Foundation of China (22108151,22109084, 22209092, 22061132002), National Key Research and Development Program(2021YFB2500300), S&T Program o1 Hebei(22344402D), Tsinghua-Jiangyin Innovation Special Fund (TJISF) and the Institute of Strategic Research,Huawei Technologies Co., Ltd.
- 新型炭材料的其它文章
- Carbon-based materials for advanced lithium metal batteries based on carbon units of different dimensions
- The role of carbon materials in suppressing dendrite formation in lithium metal batteries
- Advances in carbon-based composite anodes with gradients of lithiophilicity and conductivity used for stable lithium metal batteries
- Recent advances in Mxene-based nanomaterials as hosts for high-performance lithium metal anodes and as additives for improving the properties of solid electrolytes
- Understanding the process of lithium deposition on a graphite anode for better lithium-ion batteries
- Advances in the use of carbonaceous scaffolds for constructing stable composite Li metal anodes

