miR-210-3p通过自噬调控MC3T3-E1细胞成骨分化的机制研究*
杨玉梅, 林青, 李小云,3, 叶倩云, 张志芬, 朱晓峰,3, 杨丽, 张荣华,3△
miR-210-3p通过自噬调控MC3T3-E1细胞成骨分化的机制研究*
杨玉梅1, 林青2, 李小云1,3, 叶倩云2, 张志芬1, 朱晓峰2,3, 杨丽1, 张荣华1,3△
(1暨南大学药学院,广东 广州 510632;2暨南大学中医学院,广东 广州 510632;3广东省中医药信息化重点实验室,广东 广州 510632)
探讨微小RNA-210-3p(microRNA-210-3p, miR-210-3p)通过自噬对小鼠成骨细胞系MC3T3-E1成骨分化的影响。(1)采用脂质体转染法构建miR-210-3p过表达或沉默的MC3T3-E1细胞模型,设置阴性对照(negative control, NC) mimic组、miR-210-3p mimic组、NC inhibitor组和miR-210-3p inhibitor组,Western blot、RT-qPCR和免疫荧光法检测MC3T3-E1细胞成骨分化相关指标及自噬相关指标的变化,茜素红染色法观察矿化结节形成情况。(2)用雷帕霉素(rapamycin, RAPA)和3-甲基腺嘌呤(3-methyladenine, 3-MA)分别构建自噬激活和抑制模型,设置control组、RAPA组和3-MA组,Western blot、RT-qPCR和免疫荧光法检测自噬激活或抑制后MC3T3-E1细胞成骨分化相关指标的变化,茜素红染色法观察矿化结节形成情况。(3)设置NC mimic组、NC mimic+RAPA组和miR-210-3p mimic+RAPA组,Western blot和RT-qPCR检测各组MC3T3-E1细胞成骨分化相关指标的变化。(1)与NC mimic组相比,miR-210-3p mimic组细胞成骨分化指标的mRNA和蛋白表达水平显著升高(<0.05),细胞内Runx2免疫荧光强度升高,矿化结节数目增加,细胞自噬水平显著降低(<0.05)。与NC inhibitor组相比,miR-210-3p inhibitor组细胞成骨分化指标的mRNA和蛋白表达水平显著降低(<0.05),细胞内Runx2疫荧光强度降低,矿化结节数目减少,细胞自噬水平显著升高(<0.05)。(2)与control组相比,3-MA组细胞成骨分化指标的mRNA和蛋白表达水平显著升高(<0.05),细胞内Runx2免疫荧光强度升高,而RAPA组细胞成骨分化指标的mRNA和蛋白表达水平显著降低(<0.05),细胞内Runx2免疫荧光强度降低。(3)miR-210-3p过表达可逆转RAPA对MC3T3-E1细胞成骨分化的抑制作用(<0.01)。miR-210-3p可通过降低自噬水平促进MC3T3-E1细胞的成骨分化。
骨质疏松症;微小RNA-210-3p;自噬;MC3T3-E1细胞;成骨分化
骨质疏松症(osteoporosis, OP)是临床常见的骨代谢疾病,其特征表现为骨量降低、骨组织受损和骨微结构破坏[1]。现有的研究显示,OP的发生与性激素水平的改变、年龄的增长、遗传因素、营养状况、生活习惯等密切相关[2-4],但其根本原因在于成骨细胞介导的骨形成与破骨细胞介导的骨吸收动态失衡。成骨细胞是骨形成、骨骼发育和生长的关键功能细胞,主要通过合成、分泌和矿化骨基质进行骨重建,其细胞增殖和分化活性是影响骨形成的关键因素[5]。因此,如何促进骨形成来提高骨量对于开展OP的防治研究具有重要意义。
微小RNA(microRNA, miRNA, miR)是一类长约22个核苷酸的高度保守单链非编码小分子RNA,主要通过抑制靶基因蛋白质翻译或促进其mRNA降解,进而调控靶基因表达[6]。研究发现,诸多miRNA通过各种信号传导途径调节干细胞的成骨分化和骨重建过程。miR-196a被证实可通过靶向HDAC9促进小鼠成骨细胞系MC3T3-E1的成骨分化[7]。miR-216a能调节c-Cbl介导的PI3K/AKT通路以增强成骨细胞分化和骨形成[8]。miR-381-3p通过下调MC3T3-E1细胞中的ERK1/2/ETS1信号通路表达水平进而抑制成骨分化[9]。本课题组前期筛查了OP大鼠骨髓间充质干细胞(bone marrow mesenchymal stem cells, BMSCs)外泌体miRNA的表达谱,发现介导自噬信号通路的miR-210-3p能明显促进BMSCs的成骨分化[10-11]。但miR-210-3p对成骨细胞的作用尚未见深入的相关研究。
自噬水平的变化与成骨细胞的功能和活性息息相关。近年来,有研究发现自噬相关基因7(autophagy-related gene 7,)敲除导致小鼠骨体积分数、骨皮质厚度和骨密度明显下降,提示了自噬可能参与了骨代谢的调控[12]。维生素K2能通过自噬诱导刺激MC3T3-E1成骨细胞分化和矿化[13]。Gavali等[14]发现,雌激素能促进成骨细胞分化过程中的自噬,从而提高成骨细胞的活性和矿化能力,证明成骨细胞的功能与自噬有关。同时,miRNA也是一类重要的自噬调节因子,参与调控自噬的不同阶段[15]。且miRBase及TargetScan靶基因分析显示,miR-210-3p与自噬相关蛋白Atg5、Atg7和Atg13的3'非翻译区都存在结合位点,因此miR-210-3p可能调节成骨细胞的自噬。鉴于此,本研究以MC3T3-E1细胞为研究对象,探讨miR-210-3p通过自噬调控MC3T3-E1细胞成骨分化的作用机制。
材料和方法
1 主要试剂
miRNA第一链cDNA合成(加尾法)试剂盒(B532451-0020)、RT-qPCR引物及miR-210-3p的模拟物(mimic)和抑制物(inhibitor)均购自生工生物工程(上海)股份有限公司;Lipofectamine 2000(52887)购自Invitrogen;Opti-MEM培养液购自Gibco;抗Beclin-1抗体(ab62557)、抗p62抗体(ab91526)和抗微管相关蛋白1轻链3B(microtubule-associated protein 1 light chain 3B, LC3B)抗体(ab48394)均购自Abcam;抗碱性磷酸酶(alkaline phosphatase, Alp)抗体(ab83259)和抗骨形态发生蛋白2(bone morphogenetic protein 2, Bmp2)抗体(ab214821)均购自Abpromise;抗Runt相关转录因子2(Runt-related transcription factor 2, Runx2)抗体(12556S)和抗β-actin抗体(4970S)均购自Cell Signaling Technology;雷帕霉素(rapamycin, RAPA; HY-10219)购自MedChemExpress;茜素红染液(G1450)购于北京索莱宝科技有限公司;3-甲基腺嘌呤(3-methyladenine, 3-MA; S24823)购自上海源叶生物公司;BCA定量试剂盒(23227)购自Thermo Fisher;RIPA裂解液(P0013B)、免疫染色Ⅰ抗稀释液(P0103)、免疫染色洗涤液(P0106C)、免疫染色封闭液(P0102)、免疫荧光染色试剂盒-抗兔Alexa Fluor 488(P0176)、DAPI染色液(C1006)和抗荧光淬灭剂(P0126)均购自碧云天生物技术有限公司。
2 主要方法
2.1细胞培养和脂质体转染MC3T3-E1细胞购自中科院上海细胞库,在含10%胎牛血清(fetal bovine serum, FBS)和1%青霉素-链霉素的Opti-MEM中,于37 ℃、5% CO2条件下培养,0.25%胰蛋白酶消化传代。转染前1 d,将细胞接种于6孔板中,次日当细胞密度达到60%左右时进行转染实验,使用Lipofectamine 2000将miR-210-3p mimic、NC mimic、miR-210-3p inhibitor和NC inhibitor分别转入MC3T3-E1细胞中,转染8 h后,将无血清培养液更换为含10% FBS的培养液继续培养,48 h后检查各指标变化。
2.2实验分组干预(1)miR-210-3p mimic或inhibitor转染细胞的分组如下:NC mimic组和miR-210-3p mimic组;NC inhibitor 组和miR-210-3p inhibitor组。(2)加入自噬抑制剂或自噬激活剂干预细胞的分组如下:control组(正常细胞)、3-MA组(5 mmol/L 3-MA干预24 h)和RAPA组(10 μmol/L RAPA干预24 h)。(3)自噬激活后再过表达miR-210-3p的分组如下:NC mimic组(正常细胞培养24 h后,再转染NC mimic)、RAPA+NC mimic组(10 μmol/L RAPA干预24 h后,再转染NC mimic)和RAPA+miR-210-3p mimic组(10 μmol/L RAPA干预24 h后,再转染miR-210-3p mimic)。
2.3RT-qPCR胰酶消化各组细胞后,使用Trizol试剂提取总RNA。针对mRNA的RT-qPCR检测,取各组细胞1 600 ng总RNA,然后使用cDNA逆转录试剂盒,把它逆转录为cDNA,以β-actin为内参,采用2-ΔΔCt法计算分析各组细胞的骨形成指标Alp、Bgp、Col1a1和Runx2,以及自噬指标p62、Beclin-1和Map1lc3b的mRNA表达。针对miRNA的RT-qPCR检测,采用miRNA第一链cDNA合成(加尾法)试剂盒,将500~1 000 ng的总RNA逆转录为cDNA,以U6为内参照,采用2-ΔΔCt法计算分析各组细胞miR-210-3p的表达。引物序列见表1。

表1 引物序列
F: forward; R: reverse; Alp: alkaline phosphatase; Col1a1: collagen type I alpha 1 chain; Runx2: Runt-related transcription factor 2; Bgp: bone γ-carboxyglutamic acid protein; Map1lc3b: microtubule-associated protein 1 light chain 3B.
2.4Western blot实验使用RIPA裂解液裂解各组细胞,然后提取总蛋白,BCA法检测并调整蛋白浓度,进行SDS-PAGE。依次经过电泳、转膜、封闭,4 ℃孵育一抗(1∶1 000稀释)过夜,洗涤后,加入ECL发光液,曝光显影,使用ImageJ软件分析显影条带的灰度值,并对蛋白表达定量。
2.5免疫荧光法将细胞接种于24孔板,按照上述分组及处理后,各组去除培养液,加入PBS洗涤3次,然后加入免疫染色固定液10 min后弃去,免疫染色洗涤液洗涤3次;用免疫染色封闭液封闭1 h后去除,4 ℃孵育一抗过夜;次日,用洗涤液清洗3次,每次5 min;荧光标记二抗避光孵育1 h;取出,并用洗涤液清洗3次,每次5 min,期间注意避光操作;滴加100 μL DAPI染色液10 min,然后用洗涤液清洗3次,每次5 min;加入少量抗荧光淬灭剂,用荧光显微镜观察、拍照。
2.6茜素红染色在α-MEM成骨诱导完全培养液(含10% FBS)条件下培养细胞,每隔3 d换液一次,各组干预28 d,弃培养液,PBS清洗2~3次,10%中性福尔马林固定液固定细胞10 min,PBS清洗2~3次,加入适量的茜素红染液浸没细胞,置于室温放置2 h。PBS洗3次,显微镜下观察并拍照。
3 统计学处理
使用软件GraphPad Prism 8.0分析实验数据。结果用均数±标准差(mean±SD)表示,两组间比较采用检验,多组间比较采用单因素方差分析。以<0.05为差异有统计学意义。
结果
1 转染模型的构建
MC3T3-E1细胞转染48 h后,RT-qPCR检测其miR-210-3p表达的变化。结果显示,与NC mimic组相比,miR-210-3p mimic组的miR-210-3p表达显著上调(<0.01),见图1A;与NC inhibitor组相比,miR-210-3p inhibitor组的miR-210-3p表达显著下调(<0.01),见图1B。
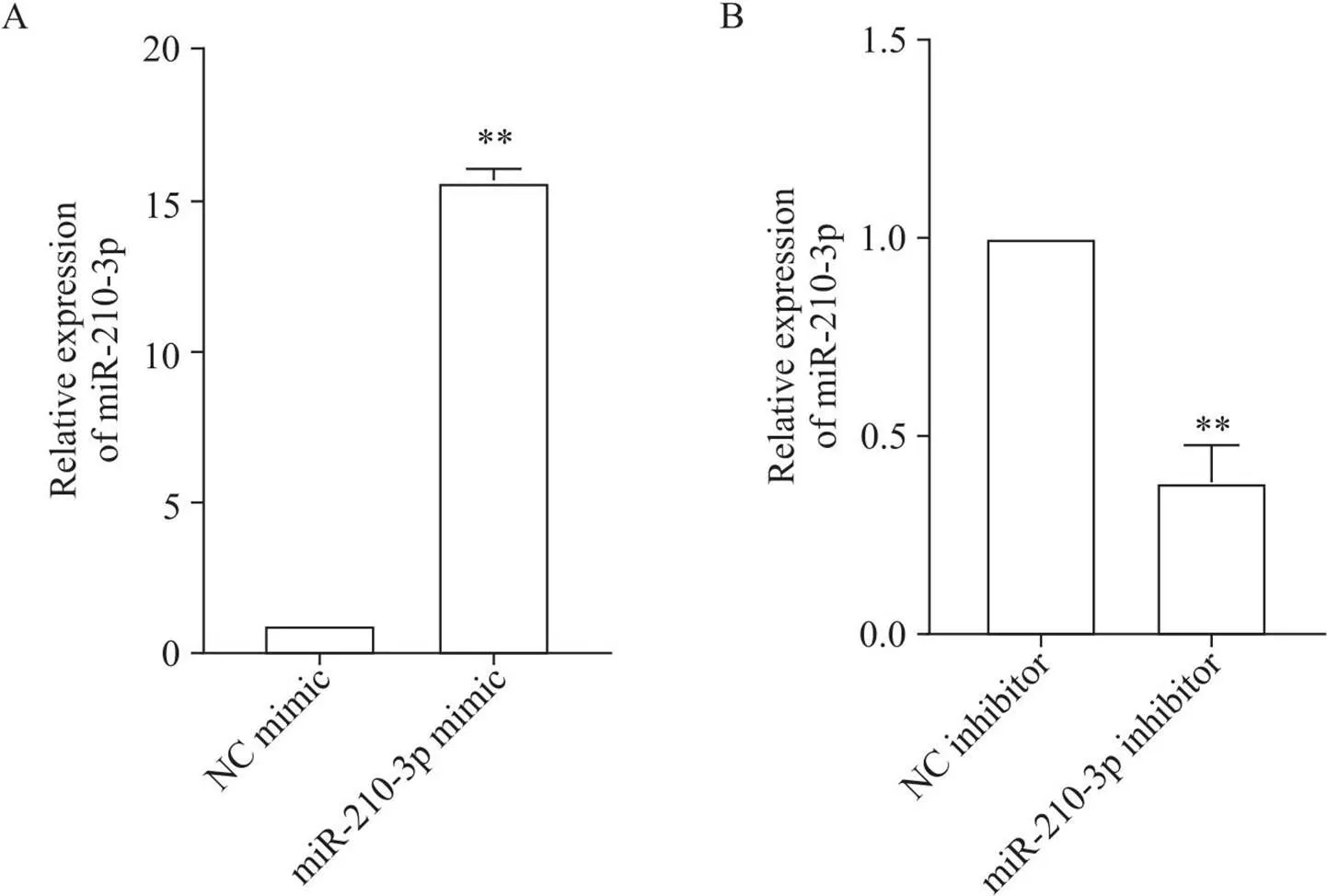
Figure 1. The expression level of miR-210-3p after miR-210-3p mimic (A) or inhibitor (B) transfection. Mean±SD. n=3. **P<0.01 vs NC mimic or inhibitor group.
2 miR-210-3p对MC3T3-E1细胞成骨分化的影响
RT-qPCR结果显示,与NC mimic组相比,miR-210-3p mimic组Col1a1、Alp和Bgp的mRNA表达水平显著升高(<0.01),Runx2的mRNA表达无显著变化(>0.05),见图2A;与NC inhibitor组相比,miR-210-3p inhibitor组Col1a1、Alp、Bgp和Runx2的mRNA表达水平显著降低(<0.05),见图2B。

Figure 2. The mRNA expression of osteogenic differentiation factors in MC3T3-E1 cells after miR-210-3p mimic (A) or inhibitor (B) transfection. Mean±SD. n=3. *P<0.05, **P<0.01 vs NC mimic or inhibitor group.
Western blot结果显示,与NC mimic组相比,miR-210-3p mimic组Bmp2和Runx2蛋白表达水平显著升高(<0.01),Alp蛋白无显著变化(>0.05),见图3A;与NC inhibitor组相比,miR-210-3p inhibitor组Alp、Bmp2和Runx2蛋白表达水平显著降低(<0.01),见图3B。

Figure 3. The protein expression of osteogenic differentiation factors in MC3T3-E1 cells after miR-210-3p mimic (A) or inhibitor (B) transfection. Mean±SD. n=3. **P<0.01 vs NC mimic or inhibitor group.
免疫荧光检测miR-210-3p过表达或沉默后MC3T3-E1细胞中Runx2的表达及分布,结果显示,与NC mimic组相比,miR-210-3p mimic组Runx2免疫荧光强度明显增强;与NC inhibitor组相比,miR-210-3p inhibitor组Runx2免疫荧光强度明显降低,见图4。

Figure 4. Immunofluorescence detection of Runx2 in MC3T3-E1 cells after miR-210-3p mimic or inhibitor transfection.
茜素红染色结果显示,与NC mimic组相比,miR-210-3p mimic组矿化结节数目明显增加;与NC inhibitor组相比,miR-210-3p inhibitor组矿化结节数目明显减少,见图5。
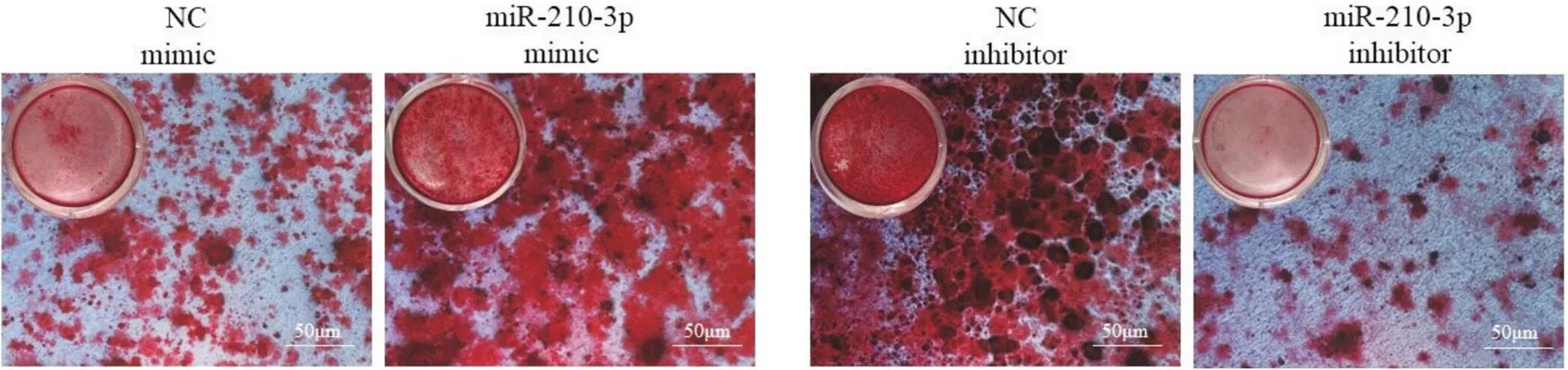
Figure 5. Effect of miR-210-3p on mineralized nodules of MC3T3-E1 cells.
3 miR-210-3p对MC3T3-E1细胞自噬的影响
RT-qPCR结果显示,与NC mimic组相比,miR-210-3p mimic组Beclin-1的mRNA表达水平显著降低(<0.01),p62的mRNA表达水平显著升高(<0.05),Map1lc3b的mRNA表达显著降低(<0.01),见图6A;与NC inhibitor组相比,miR-210-3p inhibitor组Map1lc3b和Beclin-1的mRNA表达水平均显著升高(<0.05),p62的mRNA表达水平显著降低(<0.01),见图6B。

Figure 6. The mRNA expression of autophagy-related factors in MC3T3-E1 cells after miR-210-3p mimic (A) or inhibitor (B) transfection. Mean±SD. n=3. *P<0.05, **P<0.01 vs NC mimic or inhibitor group.
Western blot结果显示,与NC mimic组相比,miR-210-3p mimic组Beclin-1蛋白表达水平显著降低(<0.01),p62蛋白表达水平显著升高(<0.05),LC3-II/LC3-I比值无显著变化(>0.05),见图7A;与NC inhibitior组相比,miR-210-3p inhibitor组LC3-II/LC3-I比值和Beclin-1蛋白表达水平显著升高(<0.05),p62蛋白表达水平显著降低(<0.01),见图7B。

Figure 7. The protein expression of autophagy-related factors in MC3T3-E1 cells after miR-210-3p mimic (A) or inhibitor (B) transfection. Mean±SD. n=3. *P<0.05, **P<0.01 vs NC mimic or inhibitor group.
免疫荧光检测miR-210-3p过表达或沉默后MC3T3-E1细胞中LC3B、Beclin-1和p62的表达和分布,结果显示,与NC mimic组相比,miR-210-3p mimic组p62免疫荧光强度明显增强,Beclin-1和LC3B免疫荧光强度明显降低;与NC inhibitor组相比,miR-210-3p inhibitor组p62免疫荧光强度明显降低,Beclin-1和LC3B免疫荧光强度明显增强,见图8。

Figure 8. Immunofluorescence detection of autophagy-related factors in MC3T3-E1 cells after miR-210-3p mimic or inhibitor transfection. A: LC3B; B: p62; C: Beclin-1.
4 自噬抑制剂3-MA和自噬激活剂RAPA浓度的选择
用不同浓度的自噬抑制剂和自噬激活剂干预MC3T3-E1细胞后,Western blot结果显示,与control组相比,5 mmol/L 3-MA组Beclin-1蛋白表达水平明显降低,p62蛋白表达水平明显升高,而10 μmol/L RAPA组Beclin-1蛋白表达水平明显升高,p62蛋白表达水平明显降低,见图9。因此,选择5 mmol/L 3-MA和10 μmol/L RAPA进行后续实验。

Figure 9. Selection of concentrations of autophagy inhibitor 3-MA (A) and autophagy activator RAPA (B).
5 自噬抑制或激活对MC3T3-E1细胞成骨分化的影响
RT-qPCR结果显示,与control组相比,3-MA组Alp、Col1a1和Runx2的mRNA表达水平显著升高(<0.01),而PAPA组Alp、Col1a1和Runx2的mRNA表达水平显著降低(<0.01),见图10。

Figure 10. Effect of autophagy on the mRNA expression of osteogenic differentiation factors in MC3T3-E1 cells. A: Alp; B: Runx2; C: Col1a1. Mean±SD. n=3. **P<0.01 vs control group.
Western blot结果显示,与control组相比,3-MA组Alp、Runx2和Bmp2蛋白表达水平显著升高(<0.05),而RAPA组Alp和Runx2蛋白表达水平显著降低(<0.01),Bmp2蛋白表达则无显著变化(>0.05),见图11。

Figure 11. Effect of autophagy on the protein expression levels of osteogenic differentiation factors in MC3T3-E1 cells. Mean±SD. n=3. **P<0.01 vs control group.
免疫荧光检测3-MA或RAPA干预后MC3T3-E1细胞中Runx2的表达和分布,结果显示,与control组相比,3-MA组Runx2免疫荧光强度明显增加,而RAPA组Runx2免疫荧光强度明显降低,见图12。

Figure 12. Immunofluorescence detection of Runx2 in MC3T3-E1 cells after autophagy inhibition or activation.
6 miR-210-3p过表达对自噬激活后MC3T3-E1细胞成骨分化的影响
RT-qPCR结果显示,与NC mimic组相比,RAPA+NC mimic组Alp、Runx2和Col1a1的mRNA表达水平显著降低(<0.01);与RAPA+NC mimic组相比,RAPA+miR-210-3p mimic组Alp和Runx2的mRNA表达水平显著升高(<0.005),Col1a1的mRNA表达无显著变化(>0.05),见图13。
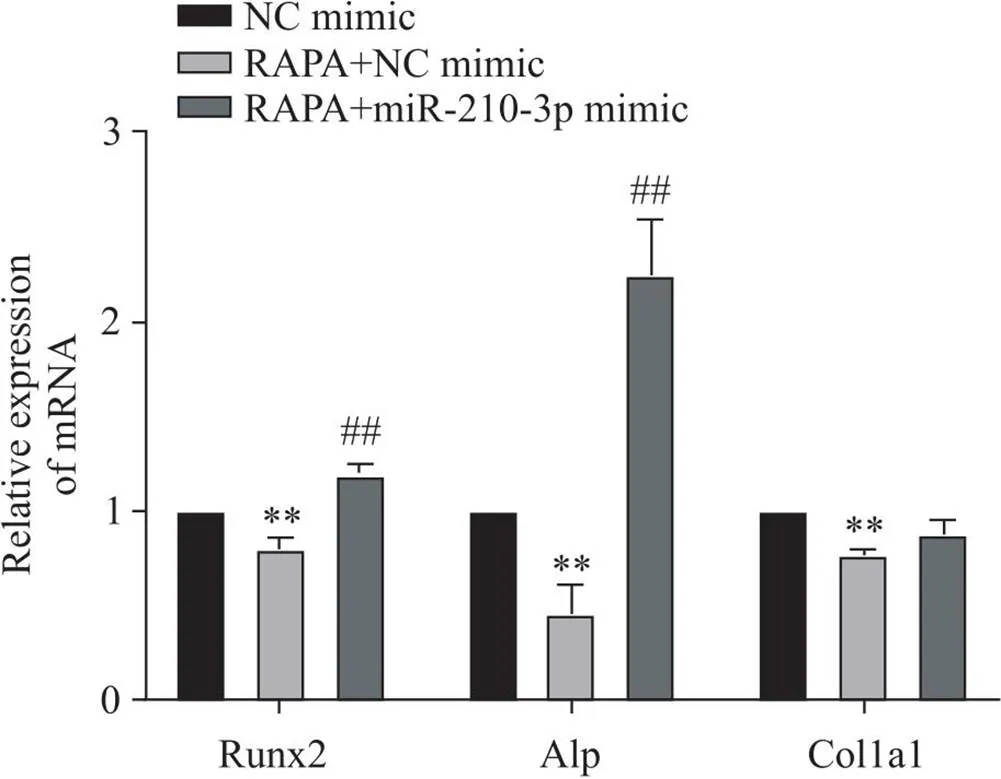
Figure 13. Effect of miR-210-3p overexpression on the mRNA expression of osteogenic differentiation factors in MC3T3-E1 cells after autophagy activation. Mean±SD. n=3. **P<0.01 vs NC mimic group; ##P<0.01 vs RAPA+NC mimic group.
Western blot结果显示,与NC mimic组相比,RAPA+NC mimic组Alp、Runx2和Bmp2蛋白表达水平显著降低(<0.01);与RAPA+NC mimic组相比,RAPA+miR-210-3p mimic组Alp、Runx2和Bmp2蛋白表达水平显著升高(<0.01),见图14。

Figure 14. Effect of miR-210-3p overexpression on the protein expression of osteogenic differentiation factors in MC3T3-E1 cells after autophagy activation. Mean±SD. n=3. **P<0.01 vs NC mimic group; ##P<0.01 vs RAPA+NC mimic group.
讨论
OP是一种常见的慢性疾病,主要特点为骨量减少,骨脆性增加,易发生骨折。临床上OP患者主要表现为腰背疼痛和活动受限,往往伴随有变矮和驼背等并发症。随着人口老龄化趋势的迅速发展,OP的发病率逐年上升,在我国的患病人数已达9 000万,其女性约7 000万[16]。目前临床上治疗OP的药物以双膦酸盐类抗骨吸收抑制剂为主等,这些药物虽然对OP具有一定的治疗作用,但是也存在许多副作用,如引发非典型股骨骨折、下颌骨坏死、心脑血管疾病等不良反应[17-18]。因此,探索相关的骨形成药物可能是一重要突破口。在本研究中,将调节骨形成作为出发点来探寻OP的防治手段。近年来,miRNA作为骨代谢相关靶点的调节剂而被重点关注。随着表观遗传学的深入研究,越来越多的证据表明miRNA在骨骼发育和细胞的成骨分化过程中扮演着重要角色[19]。研究证实miR-1260a下调HDAC7和COL4A2的表达促进BMMSCs成骨分化[20]。miR-135-5p通过靶向HIF1AN促进MC3T3-E1细胞成骨分化[21]。金丝桃苷(hyperin)通过上调MC3T3-E1细胞中的miR-7031-5p而促进成骨分化[22]。本实验室前期筛查OP大鼠BMSCs外泌体miRNA表达谱发现,miR-210-3p能明显促进成骨分化。另外,本研究中,我们发现miR-210-3p过表达后MC3T3-E1细胞成骨分化能力增加,miR-210-3p沉默后MC3T3-E1细胞成骨分化能力降低,提示miR-210-3p与MC3T3-E1细胞成骨分化正相关。所以,miR-210-3p能提高骨形成能力可作为其能防止OP发生发展的重要手段。
自噬是细胞质成分(如功能失调的细胞器)被包裹形成自噬体的过程,然后将后者转运到溶酶体进行降解,以便营养物质可以再利用[23],这种分解代谢过程在所有细胞中都处于基本水平,以促进细胞在营养剥夺或缺氧下的存活[24]。近年来大量文献证实,自噬信号通路介导了成骨细胞的分化,从而维持骨稳态。Cai等[25]发现白藜芦醇通过促进自噬诱导MC3T3-E1细胞的增殖和分化。山柰酚通过诱导自噬促进MC3T3-E1细胞分化和矿化[26]。Weng等[27]发现,敲除了的成骨细胞,不仅其增殖和分化受到了抑制,且更容易受到氧化应激而导致细胞凋亡。所以,针对自噬信号途径可能是诊治OP疾病的有效策略。紧接着,我们通过补充验证自噬对MC3T3-E1细胞的成骨分化作用,结果发现自噬抑制对MC3T3-E1细胞的成骨分化有促进作用,自噬激活则抑制MC3T3-E1细胞成骨分化。
此外,miRNA参与自噬调控的不同阶段,自噬的发生受miRNA的调节。研究发现,过表达miR-20a可以显著下调自噬相关因子LC3及p62等表达[28]。在退化的髓核细胞中,miR-210通过沉默来抑制自噬,从而促进细胞外基质降解[29]。miR-27a通过PI3K/AKT/mTOR信号传导促进骨关节炎中IL-1β处理关节软骨细胞的自噬和凋亡[30]。且前期实验筛查出的miR-210-3p在参与调控成骨分化过程中也证实与自噬调节有关,所以这里进一步体外探究miR-210-3p与自噬之间的关系。RT-qPCR、Western blot和免疫荧光结果显示,miR-210-3p和自噬相关指标表达呈负相关关系。因此我们猜测,miR-210-3p对成骨细胞的调控可能与自噬有关。进一步探究miR-210-3p对自噬激活状态下MC3T3-E1细胞的影响,发现miR-210-3p能逆转自噬激活状态下MC3T3-E1细胞成骨分化抑制的趋势。
综上所述,本研究发现miR-210-3p可通过降低自噬水平促进MC3T3-E1细胞的成骨分化,挖掘了MC3T3-E1细胞成骨分化的新机制,可为OP的研究提供参考。
[1] Lorentzon M, Cummings SR. Osteoporosis: the evolution of a diagnosis[J]. J Intern Med, 2015, 277(6):650-661.
[2] Yang TL, Shen H, Liu A, et al. A road map for understanding molecular and genetic determinants of osteoporosis[J]. Nat Rev Endocrinol, 2020, 16(2):91-103.
[3]朱晓峰, 张荣华. 高盐摄入与骨代谢[J]. 中国病理生理杂志, 2016, 32(2):371-376.
Zhu XF, Zhang RH. High salt intake and bone metabolism[J]. Chin J Pathophysiol, 2016, 32(2):371-376.
[4] Xu F, Li W, Yang X, et al. The roles of epigenetics regulation in bone metabolism and osteoporosis[J]. Front Cell Dev Biol, 2021, 8:619301.
[5]张荣华, 朱晓峰, 蔡宇, 等. 益骨胶囊含药血清对大鼠成骨细胞IGF-I mRNA及其蛋白表达的影响[J].中国病理生理杂志, 2004, 20(7):1222-1225.
Zhang RH, Zhu XF, Cai Y, et al. Effect of YIGU capsule on IGF-I mRNA and protein expression in rat osteoblasts[J]. Chin J Pathophysiol, 2004, 20(7):1222-1225.
[6] Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function[J]. Cell, 2004, 116(2):281-297.
[7]李华峰, 张广凤, 张旭, 等. MicroRNA-196a靶向调节HDAC9对MC3T3-E1细胞成骨分化的影响[J]. 中国骨质疏松杂志, 2022, 28(9):1303-1309.
Li HF, Zhang GF, Zhang X, et al. Effect of microRNA-196a targeted regulation of HDAC9 on osteogenic differentiation of MC3T3-E1 cells [J]. Chin J Osteoporos, 2022, 28(9):1303-1309.
[8] Pan JM, Wu LG, Cai JW, et al. Dexamethasone suppresses osteogenesis of osteoblastthe Pl3K/Akt signaling pathwayand[J]. J Recept Signal Transduct Res, 2019, 39(1):80-86.
[9]陈锦成, 朱国涛, 秦晓飞, 等. miR-381-3p通过靶向ERK1/2/ETS1信号通路影响MC3T3-E1细胞成骨分化[J].中国骨质疏松杂志, 2022, 28(3):325-331.
Chen JC, Zhu GT, Qin XF, et al. miR-381-3p affects the osteogenic differentiation of MC3T3-E1 cells by targeting the ERK1/2/ETS1 signaling pathway [J]. Chin J Osteoporos, 2022, 28(3):325-331.
[10] 孙珂焕, 朱晓峰, 杨丽, 等. 骨髓间充质干细胞外泌体在骨质疏松中的研究进展[J]. 中国骨质疏松杂志, 2019, 25(3):393-415.
Sun KH, Zhu XF, Yang L, et al. Research progress of bone marrow mesenchymal stem cell exosomes in osteoporosis [J]. Chin J Osteoporos, 2019, 25(3):393-415.
[11] Li XY, Peng BJ, Zhu XF. et al. MiR-210-3p inhibits osteogenic differentiation and promotes adipogenic differentiation correlated with Wnt signaling in ERa-deficient rBMSCs[J]. J Cell Physiol, 2019, 234(12):23475-23484.
[12] Xu A, Yang Y, Shao Y, et al. Activation of cannabinoid receptor type 2-induced osteogenic differentiation involves autophagy induction and p62-mediated Nrf2 deactivation[J]. Cell Commun Signal, 2020, 18(1):9.
[13] Li W, Zhang S, Liu J, et al. Vitamin K2 stimulates MC3T3-E1 osteoblast differentiation and mineralization through autophagy induction[J]. Mol Med Rep, 2019, 19(5):3676-3684.
[14] Gavali S, Gupta MK, Daswani B, et al. Estrogen enhances human osteoblast survival and function via promotion of autophagy[J]. Biochim Biophys Acta Mol Cell Res, 2019, 1866(9):1498-1507.
[15] Shen G, Ren H, Shang Q, et al. Autophagy as a target for glucocorticoid-induced osteoporosis therapy[J]. Cell Mol Life Sci, 2018, 75(15):2683-2693.
[16] 章振林. 原发性骨质疏松症诊疗指南(2022)[J]. 中国全科医学, 2023, 26(14):1671-1691.
Zhang ZL. Guidelines for the diagnosis and treatment of primary osteoporosis (2022)[J]. Chin Gen Pract, 2023, 26(14):1671-1691.
[17] Whitaker M, Guo J, Kehoe T, et al. Bisphosphonates for osteoporosis: where do we go from here?[J]. N Engl J Med, 2012, 366(22):2048-2051.
[18] Hofbauer LC, Schoppet M. Clinical implications of the osteoprotegerin/RANKL/RANK system for bone and vascular diseases[J]. JAMA, 2004, 292(4):490-495.
[19] 阮文东. miRNA在骨重建中的作用[J]. 中国矫形外科杂志, 2016, 24(8):722-726.
Ruan WD. The role of miRNA in bone remodeling[J]. Chin J Orthop Surg, 2016, 24(8):722-726.
[20] Wu D, Chang X, Tian J, et al. Bone mesenchymal stem cells stimulation by magnetic nanoparticles and a static magnetic field: release of exosomal miR-1260a improves osteogenesis and angiogenesis[J]. J Nanobiotechnol, 2021, 19(1):209.
[21] Yin N, Zhu L, Ding L, et al. MiR-135-5p promotes osteoblast differentiation by targeting HIF1AN in MC3T3-E1 cells[J]. Cell Mol Biol Lett. 2019, 24:51.
[22] Qian D, Chen Y, Qiu X, et al. Hyperin up-regulates miR-7031-5P to promote osteogenic differentiation of MC3T3-E1 cells[J/OL]. Histol Histopathol, 2022 (2022-12-30) [2023-05-01]. https://www.hh.um.es/Abstracts/Vol_/_/__18579.htm. DOI: 10.14670/HH-18-579.
[23] Kroemer G, Mariño G, Levine B. Autophagy and the integrated stress response[J]. Mol Cell, 2010, 40:280-293.
[24] Pantovic A, Krstic A, Janjetovic K, et al. Coordinated time-dependent modulation of AMPK/Akt/mTOR signaling and autophagy controls osteogenic differentiation of human mesenchymal stem cells[J]. Bone, 2013, 52:524-531.
[25] Cai W, Sun B, Song C, et al. Resveratrol induces proliferation and differentiation of mouse pre-osteoblast MC3T3-E1 by promoting autophagy[J]. BMC Complement Med Ther, 2023, 23(1):121.
[26] Kim IR, Kim SE, Baek HS, et al. The role of kaempferol-induced autophagy on differentiation and mineralization of osteoblastic MC3T3-E1 cells[J]. BMC Complement Altern Med, 2016, 16(1):333.
[27] Weng YM, Ke CR, Kong JZ, et al. The significant role of ATG5 in the maintenance of normal functions of MC3T3-E1 osteoblast[J]. Eur Rev Med Pharmacol Sci, 2018, 22:1224-1232.
[28] Sun KT, Chen MY, Tu MG, et al. MicroRNA-20a regulates autophagy related protein-ATG16L1 in hypoxia-induced osteoclast differentiation [J]. Bone, 2015, 73:145-153.
[29] Wang C, Zhang ZZ, Yang W, et al. MiR-210 facilitates ECM degradation by suppressing autophagy via silencing of ATG7 in human degenerated NP cells[J]. Biomed Pharmacother, 2017, 93:470-479.
[30] Cai C, Min S, Yan B, et al. MiR-27a promotes the autophagy and apoptosis of IL-1β-treated articular chondrocytes in osteoarthritis through PI3K/AKT/mTOR signaling[J]. Aging (Albany NY), 2019, 11(16):6371-6384.
miR-210-3p regulates osteogenic differentiation of MC3T3-E1 cells through autophagy
YANG Yumei1, LIN Qing2, LI Xiaoyun1,3, YE Qianyun2, ZHANG Zhifen1, ZHU Xiaofeng2,3, YANG Li1, ZHANG Ronghua1,3△
(1,,510632,;2,,510632,;3,510632,)
To investigate the effect of microRNA-210-3p (miR-210-3p) on osteogenic differentiation of mouse osteoblastic cell line MC3T3-E1, and to explore its mechanism related to autophagy.(1) Lipofection was used to construct MC3T3-E1 cell model with overexpression or silencing of miR-210-3p, and negative control (NC) mimic group, miR-210-3p mimic group, NC inhibitor group and miR-210-3p inhibitor group were set up, Western blot, RT-qPCR and immunofluorescence were used to detect the changes of osteogenic differentiation- and autophagy-related factors in MC3T3-E1 cells, and alizarin red staining was used to observe the mineralized nodules. (2) Rapamycin (RAPA) or 3-methyladenine (3-MA) was used to construct autophagy activation or inhibition model, and control group, RAPA group and 3-MA group were set up. Western blot, RT-qPCR and immunofluorescence were used to detect the changes of osteogenic differentiation factors in MC3T3-E1 cells after autophagy activation or inhibition, and alizarin red staining was used to observe the mineralized nodules. (3) The cells were divided into NC mimic group, NC mimic+RAPA group and miR-210-3p mimic+RAPA group, and Western blot and RT-qPCR were used to detect the changes of osteogenic differentiation factors in each group.(1) Compared with NC mimic group, the mRNA and protein expression levels of osteogenic differentiation factors were increased in miR-210-3p mimic group (<0.05), the immunofluorescence intensity of Runx2 was increased in the cells, the number of mineralized nodules was increased, and the level of autophagy was decreased (<0.05). Compared with NC inhibitor group, the mRNA and protein expression levels of osteogenic differentiation factors were decreased in miR-210-3p inhibitor group (<0.05), the immunofluorescent intensity of Runx2 was decreased in the cells, the number of mineralized nodules was reduced, and the level of autophagy was increased in the miR-210-3p inhibitor group (<0.05). (2) Compared with control group, the mRNA and protein expression levels of osteogenic differentiation factors were increased in 3-MA group (<0.05), and the immunofluorescence intensity of Runx2 was increased in the cells. By contrast, the mRNA and protein expression levels of osteogenic differentiation factors were decreased in RAPA group (<0.05), and the immunofluorescence intensity of Runx2 was decreased in the cells. (3) Overexpression of miR-210-3p reversed the inhibitory effect of RAPA on osteogenic differentiation of MC3T3-E1 cells (<0.01).miR-210-3p promotes osteogenic differentiation of MC3T3-E1 cells by inhibiting autophagy.
osteoporosis; microRNA-210-3p; autophagy; MC3T3-E1 cells; osteogenic differentiation
1000-4718(2023)07-1253-12
2023-05-08
2023-06-26
020-85220007; E-mail: tzrh@jnu.edu.cn
R363.2; R681
A
10.3969/j.issn.1000-4718.2023.07.012
[基金项目]国家自然科学基金项目(No. 82074287; No. 82274232);国家重点研发计划项目(No. 2018YFC2002501);广东省中医药信息化重点实验室(No. 2021B1212040007)
(责任编辑:卢萍,罗森)

