基于尿液外泌体的液体活检结合代谢组学在评估糖尿病肾脏病中的应用研究*
石彩凤, 刘丹丹, 何爱琴, 吴小梅, 沈新佳, 朱雪婷, 薛颖, 杨俊伟, 周阳
基于尿液外泌体的液体活检结合代谢组学在评估糖尿病肾脏病中的应用研究*
石彩凤, 刘丹丹, 何爱琴, 吴小梅, 沈新佳, 朱雪婷, 薛颖, 杨俊伟△, 周阳△
(南京医科大学第二附属医院肾脏病中心,江苏 南京 210003)
探讨来源于肾小管的尿液外泌体中的代谢物在评估糖尿病肾脏病(DKD)中的应用价值。采用横断面研究,选择健康人群(13例)、单纯2型糖尿病(T2D)患者(12例)及T2D合并DKD患者(12例)作为研究对象,收集人口学和实验室检查等资料。培养原代肾小管上皮细胞。超速离心提取尿液及细胞培养上清液中的外泌体,免疫磁珠富集表达分化簇13(CD13)的外泌体,透射电镜及纳米颗粒跟踪分析外泌体的形态,Western blot和免疫组化染色检测蛋白质的表达和分布,ELISA检测肾损伤因子1和中性粒细胞明胶酶相关脂质运载蛋白,超高效液相色谱-串联质谱方法靶向定量检测外泌体中的代谢物。采用偏最小二乘判别分析进行多维建模。代谢物的两组间比较采用Wilcox检验,多组间比较采用Kruskal-Wallis检验,Spearman相关分析法分析代谢物与临床指标的相关性,受试者工作特征(ROC)曲线评价诊断效能。尿液外泌体中检测到CD13的表达。肾组织中CD13分布在肾小管的顶端侧。原代肾小管上皮细胞及其外泌体中均检测到CD13。在表达CD13的尿液外泌体中定量检测到144种代谢物。在单纯T2D和T2D合并DKD两组间筛选出差异具有统计学意义的15种代谢物(<0.05),包括赖氨酸、-乙酰丙氨酸、-乙酰丝氨酸、正亮氨酸、-苯乙酰苯丙氨酸、缬氨酸、鹅去氧胆酸、葡萄糖、肉豆蔻脑酸、油酸、辛二酸、十一烷酸、延胡索酸、酮亮氨酸和异戊酸,其中,-乙酰丙氨酸、十一烷酸、酮亮氨酸、赖氨酸和鹅去氧胆酸均与尿蛋白和血尿酸显著相关(<0.05或<0.01)。肉豆蔻脑酸、-乙酰丙氨酸、缬氨酸和正亮氨酸的ROC曲线下面积分别为0.854(95% CI: 0.705~1.000)、0.840(95% CI: 0.677~1.000)、0.812(95% CI: 0.640~0.985)和0.806(95% CI: 0.630~0.982)。单纯T2D与T2D合并DKD的肾小管外泌体中的代谢物存在显著差异,从中筛选出的15种代谢物可作为临床诊断DKD的新途径。
糖尿病肾脏病;外泌体;代谢组学;肾小管
糖尿病肾脏病(diabetic kidney disease, DKD)已成为我国终末期肾衰竭的主要病因[1-2]。然而,目前依据尿白蛋白/肌酐比值(urinary albumin-to-creatinine ratio, UACR)、估算的肾小球滤过率(estimated glomerular filtration rate, eGFR)及肾活检的临床诊断标准仍存在诸多缺陷[3]。尿液外泌体大都来自肾单位各段组织细胞,由复杂的分子系统调控其产生及成分[4],并携带来源细胞的特征[5]。这种主动的分泌过程成为基于尿液外泌体对肾组织进行无创性液体活检的依据。本研究采用代谢组学方法,探讨尿液外泌体中的代谢物在评估DKD中的应用价值。
材料和方法
1 研究对象及方案
研究方案经南京医科大学第二附属医院伦理委员会审批(批准号:2019KY097)。所有研究对象均签署知情同意书。选择年龄、性别匹配的健康人群和确诊2型糖尿病(type 2 diabetes, T2D)的患者为研究对象。排除标准包括肾活检提示非糖尿病性肾脏疾病,曾接受肾脏替代治疗,恶性肿瘤,急性肾损伤及其他脏器的严重病变。健康对照组(13例)为不存在高血压、肥胖、糖尿病、肾脏病、恶性肿瘤等的人群,且无长期用药史。单纯T2D组(12例)为符合美国糖尿病学会2020年诊断标准的T2D患者。T2D合并DKD组(12例)的诊断依据为符合T2D诊断标准,且具备明确的、与尿蛋白和肾功能变化存在因果关系的糖尿病病史,并符合随机尿UACR≥30 mg/g和(或)eGFR<60 mL/(min·1.73 m2),其中UACR需排除感染等其他因素情况下,在6个月内重复检查3次中有两次达到标准[1]。
研究对象在清晨空腹(禁食>10 h)采集静脉血和尿液。由一名研究人员采用标准工具测量体重、身高等,并在静坐休息15 min后,用欧姆龙HEM‑7130血压计在右上臂测量血压和心率3次,每次间隔1 min,取3次平均值为最终的诊室血压和心率,罗氏P800全自动生化分析仪检测血、尿样本。采用基于血肌酐的慢性肾脏病流行病学协作公式计算eGFR(http://www.nkdep.nih.gov)。
2 主要试剂和仪器
胎牛血清购自Thermo Fisher;叠氮化钠(S2002)、苯甲基磺酰氟(P7626)、亮抑肽酶(L8511)和二硫苏糖醇(D9779)均购自Sigma-Aldrich;磁珠(10608D)购自Invitrogen;硝酸纤维膜购自Amersham;生物素化的CD13抗体(5160823270)购自MiltenyiBiotec;抗肿瘤易感基因101(tumor susceptibility gene 101, Tsg101)抗体(ab30871)、抗CD63抗体(ab213090)和抗CD13抗体(Ab108310)均购自Abcam;抗CD9抗体(sc-13118)和抗CD13抗体(sc-166105)均购自Santa Cruz;肾损伤因子1(kidney injury molecule-1, KIM-1)ELISA试剂盒(DKM100)和中性粒细胞明胶酶相关脂质运载蛋白(neutrophil gelatinase-associated lipocalin, NGAL)ELISA试剂盒(DLCN20)均购自R&D Systems;靶向代谢组学的标准品购自Sigma‑Aldrich、Steraloids和TRC Chemicals。
DynaMag™-2磁力架(12321D; Thermo Fisher);Optima X Series低温超速离心机(Beckman);JEM 1011透射电子显微镜(JEOL);NS 300系统(NanoSight);Xevo TQ‑S超高效液相色谱‑串联质谱联用(ultraperformance liquid chromatography-tandem mass spectrometry, UPLC‑MS)仪(Waters Corp)。
3 主要方法
3.1原代肾小管上皮细胞培养采用110 000×离心90 min去除胎牛血清中的外泌体后,用于培养细胞。应用改良的胶原酶消化-密度梯度离心法分离原代肾小管上皮细胞[6]:2~3周龄小鼠,处死后无菌取肾,剥离外膜去除髓质后将皮质剪碎,分别经80、100和200目不锈钢筛网过滤,收集200目网上物,经0.25%胰蛋白酶消化,吸取高密度层细胞,用含10%胎牛血清的DMEM/F12培养,细胞贴壁后定期换液,传代3~4代后获得形态稳定的细胞并鉴定纯度。
3.2外泌体的提取及富集用超速离心法提取细胞培养上清液或尿液中的外泌体[5],步骤简述如下:用去除外泌体的胎牛血清培养细胞,收集细胞培养上清液,4 ℃、500×离心15 min,10 000×离心30 min,110 000×离心90 min,沉淀细胞的外泌体。新鲜收集24 h尿液中加入防腐剂和蛋白酶抑制剂,4 ℃、500×离心15 min,10 000×离心30 min,110 000×离心90 min,沉淀物中加入二硫苏糖醇,95 ℃加热2 min去除Tamm-Horsfall蛋白,再次110 000×离心90 min沉淀外泌体。取1 × 107个磁珠,用无菌分选缓冲液(含0.1% 胎牛血清的磷酸缓冲盐溶液)清洗,置于DynaMag™-2磁力架上1 min,去除上清,加入4 μg生物素化的CD13抗体,室温60 min,用分选缓冲液清洗,置于磁力架上1 min,弃上清,制成CD13免疫磁珠。取20 000个CD13免疫磁珠与含200 μg蛋白的外泌体,4 ℃孵育18~22 h,用无菌磷酸缓冲盐溶液清洗,置于磁力架上1 min,弃上清,进行后续实验或-80 ℃暂存。
3.3透射电子显微镜外泌体置于2.5%戊二醛中4 ℃固定过夜,磷酸缓冲盐溶液洗3次,室温1%四氧化锇固定60 min。梯度乙醇脱水后,室温浸润在Epon树脂(Ted Pella):环氧丙烷(1∶1)溶液中过夜。第2天置于新鲜Epon中60 ℃包埋过夜。用Leica EM UC7超微切片,收集在甲醛涂层格上,醋酸铀酰和柠檬酸铅染色,在80 kV下观察和拍摄。
3.4纳米颗粒跟踪分析(nanoparticle tracking analysis, NTA)用配置有488 nm激光器和高灵敏度sCMOS相机的NanoSight NS 300系统[7]。外泌体以5 g/L蛋白浓度重悬于磷酸缓冲盐溶液,稀释100~500倍,以达到每帧20~100个物体。室温下手动注入样品室,相机设置13下一式三次测量,采集时间30 s,检测阈值7,每个视频至少分析200个完整音轨。用NTA分析软件2.3 版本进行数据采集和分析。
3.5Western blot裂解细胞或外泌体后制备成相同蛋白浓度的样本,取10 μg蛋白样本在聚丙酰胺凝胶分离后,转至硝酸纤维膜上,封闭后,与Ⅰ抗、Ⅱ抗分别孵育,采用美国国立卫生研究院凝胶图像分析程序分析信号强度。
3.6免疫组化染色选取DKD的肾活检组织,对照为病理上仅表现为肾小球轻度系膜增生的肾活检组织。将石蜡包埋组织切成 3 μm薄片,去石蜡化,二甲苯、乙醇和纯水再水化后,室温封闭 30 min,加入抗CD13抗体4 ℃过夜,Ⅱ抗室温1 h,封片,用配备有DS-Ril(Nikon)数码相机的Nikon Eclipse 80i显微镜观察并拍照。
3.7ELISA采用ELISA检测尿液KIM-1和NGAL:在包被有捕获抗体的检测孔中,分别加入50 μL梯度稀释的标准品、尿液样本及对照,室温2 h后,去除液体并充分洗涤,每孔加入200 μL检测抗体4 ℃ 2 h后,再次去除液体并充分洗涤,每孔加入200 μL底物反应液,室温30 min,加入50 μL 2 mol/L硫酸溶液,450 nm读数,根据标准曲线计算浓度。
3.8代谢组学采用UPLC‑MS靶向定量检测代谢物[8]。CD13+外泌体中加入氧化锆珠和25 μL去离子水匀浆,加入150 μL含内标的甲醇溶液再次匀浆,18 000×离心20 min后将上清移入96孔板。后续步骤在Eppendorf epMotion工作站(Eppendorf Inc.)进行,每孔加入20 μL衍生化试剂,加入50%甲醇,4 ℃下4 000×离心30 min,吸取上清液与内标混合,用于UPLC‑MS分析。用MassLynx 4.1软件处理原始数据,用外泌体蛋白总量校正代谢物的定量。
4 统计学处理
采用SPSS 25.0和R软件进行统计分析。采用bartlett检验确定变量分布特征。正态分布的连续性变量以均数±标准差(mean±SD)表示,两组间比较采用检验,多组间比较采用方差分析。非正态分布的连续性变量用(1,3)描述,两组间比较采用Mann-Whitney U检验,多组间比较采用秩和检验。分类变量采用频数和百分数表示,组间比较采用2检验。代谢物用(1,3)描述,两组间比较采用Wilcox检验,多组间比较采用Kruskal-Wallis检验。Spearman相关分析法评估代谢物与临床指标的相关性。用iMAP 1.0平台(Metabo Profile)分析代谢物。偏最小二乘判别分析(partial least squares discriminant analysis, PLS-DA)进行多维建模,进行999次permutation置换检验评估模型的过拟合风险。采用受试者工作特征(receiver operating characteristic, ROC)曲线评价诊断效能。以<0.05为差异有统计学意义。
结果
1 研究对象的一般特征
如表1所示,三组研究对象的年龄、性别构成比、体重指数、血红蛋白、总胆固醇、甘油三酯、高密度脂蛋白胆固醇、低密度脂蛋白胆固醇、NGAL均无统计学意义(>0.05)。与对照组相比,单纯T2D组和(或)T2D合并DKD组的收缩压、舒张压、空腹血糖、糖化血红蛋白、UACR及KIM-1均显著增高,血白蛋白显著降低(<0.05)。与单纯T2D组相比,T2D合并DKD组的血清肌酐、尿酸及UACR均显著增高,eGFR显著降低(<0.05),糖尿病病程、血糖、血压、血白蛋白及KIM-1均无统计学意义(>0.05)。

表1 研究对象的临床特征
DOT: duration of type 2 diabetes (T2D); BMI: body mass index; SBP: systolic blood pressure; DBP: diastolic blood pressure; Hb: hemoglobin; Alb: albumin; FBG: fast blood glucose; HbA1c: hemoglobin A1c; TC: total cholesterol; TG: triglyceride; HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol; SCr: serum creatinine; UA: uric acid; eGFR: estimated glomerular filtration rate; UACR: urinary albumin-to-creatinine ratio; KIM-1: kidney injury molecule-1; NGAL: neutrophil gelatinase associated lipocalin. 1 mmHg=0.133 kPa.*<0.05healthy control group;#<0.05simple T2D group.
2 外泌体的形态及CD13的表达
透射电镜下超速离心的沉淀物为直径50~100 nm的双层膜结构囊泡(图1A),NTA分析粒径分布表明囊泡的直径约100 nm(图1B),符合外泌体的形态和大小。Western blot表明三组研究对象的尿液外泌体均表达标志蛋白Tsg101、CD63和CD9,同时还表达CD13(图1C)。免疫组化染色表明CD13在对照及DKD的肾组织中均有表达,且都分布在肾小管顶端侧(图1D),肾小球和肾间质中几乎检测不到。原代肾小管上皮细胞及其外泌体中均检测到CD13及外泌体标志蛋白Tsg101、CD63和CD9(图1E)。由此可见,健康对照及T2D的尿液中均存在表达CD13并来源于肾小管的外泌体。

Figure 1. Expression of CD13 in kidney tissue and exosomes. A: representative image showed the structure and size of isolated exosomes from urine assessed by transmission electron microscopy (arrows indicate exosomes; scale bar=100 nm); B: size distribution demonstrated by finite track length adjustment concentration (left) and intensity (right) of isolated exosomes from urine assessed by NTA (n=3); C: the protein expression in isolated urinary exosomes from subjects as indicated was assessed by Western blot (n=12); D: representative images showed the expression of CD13 in the brush border of kidney tubules from control individuals and patients with DKD assessed by immunohistochemical staining (scale bar=100 μm); E: the protein expression in primarily cultured tubular cells and isolated cellular exosomes was assessed by Western blot (n=3).
3 CD13+外泌体中代谢物的差异变化
免疫磁珠富集表达CD13的外泌体(CD13+外泌体),靶向代谢组学定量其中的代谢物,测得144种代谢物的含量。PLS-DA表明单纯T2D组与T2D合并DKD组的代谢物存在显著差异(图2A),提示以CD13+外泌体中的代谢物评估T2D是否合并DKD是可行的。从单纯T2D组与T2D合并DKD组间筛选出15种差异具有统计学意义(<0.05)的代谢物,比较它们在三组间的差异(表2),并按照分类绘制的浓度中位数热图(图2B)均显示,与单纯T2D组相比,T2D合并DKD组CD13+外泌体中仅-乙酰丝氨酸增多,其余14种代谢物均减少(<0.05);与健康对照组相比,单纯T2D组CD13+外泌体中-乙酰丙氨酸、正亮氨酸、葡萄糖、肉豆蔻脑酸、酮亮氨酸增多,仅-乙酰丝氨酸减少(<0.05);健康对照组和T2D合并DKD组的CD13+外泌体中,上述15种代谢物的差异均无统计学意义(>0.05)。相关性网络图表明15种代谢物之间存在广泛的相关关系(图2C)。
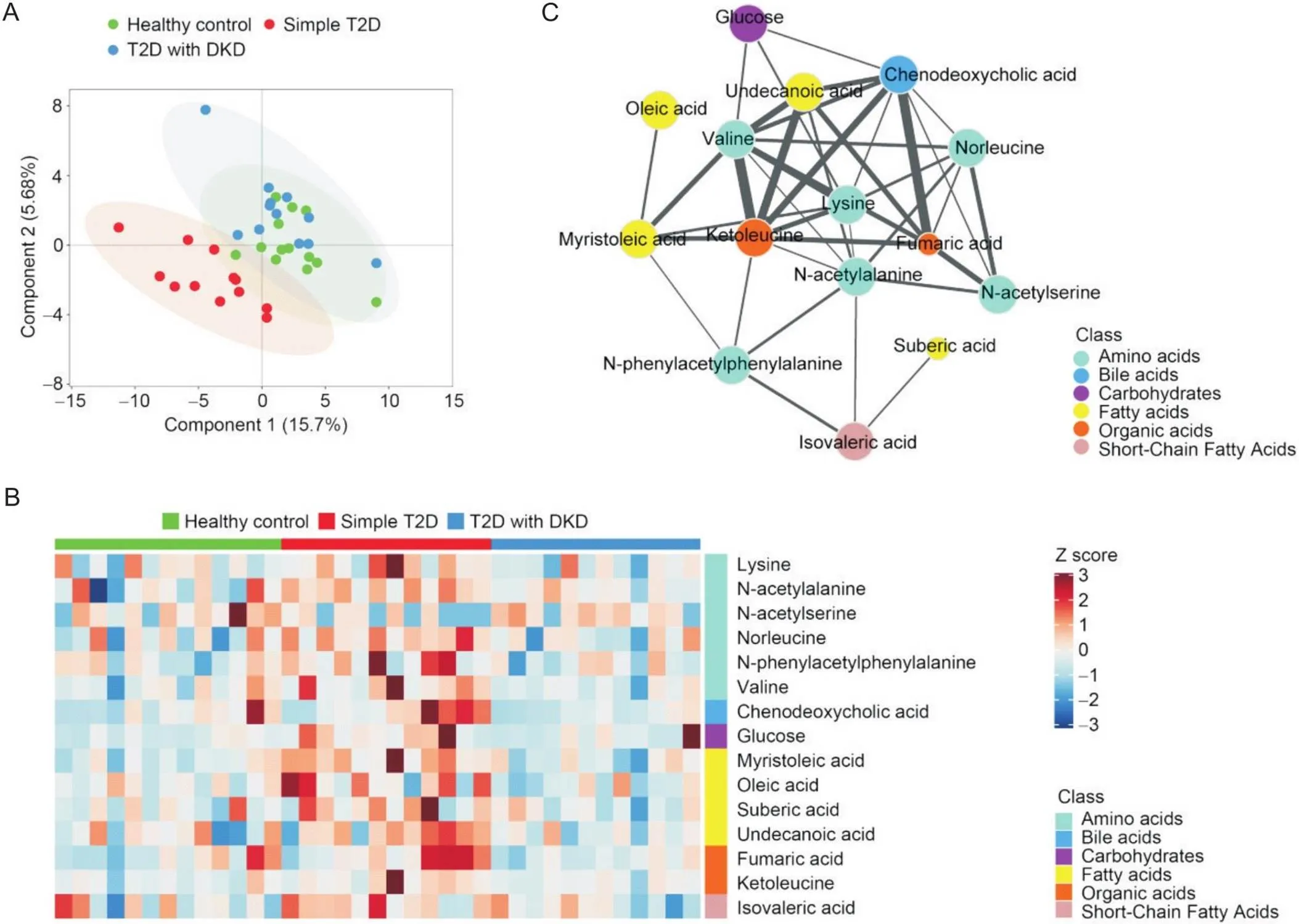
Figure 2. Identification of potential metabolite biomarkers in urinary CD13+ exosomes. A: PLS-DA score plot for healthy control individuals (n=13), patients with simple T2D (n=12) and patients with DKD (n=12); B: heatmap classification of samples based on the 15 significantly altered metabolites between the simple T2D group and the T2D with DKD group; C: correlation network of significantly altered metabolites. Each node represents a metabolite, and each edge represents the strength of the correlation between two compounds. The size of each circle represents the significance of the compound in the metabolic network.
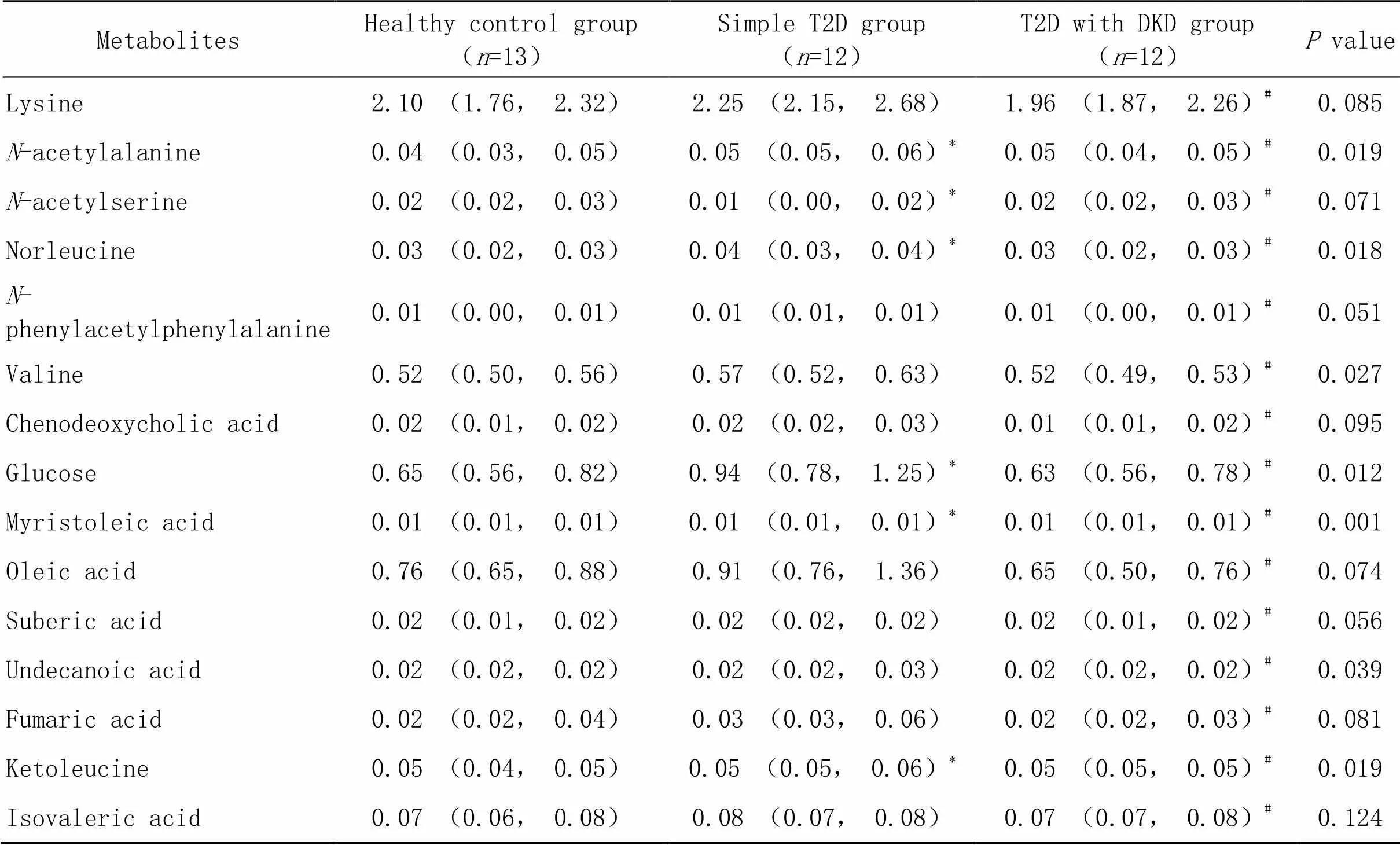
表2 研究对象尿液CD13+外泌体中15种代谢物水平的比较结果
DKD: diabetic kidney disease; T2D: type 2 diabetes.*<0.05healthy control group;#<0.05simple T2D group.
4 代谢物与临床指标的相关性
如图3所示,Spearman相关分析表明,葡萄糖、-乙酰丙氨酸、正亮氨酸、肉豆蔻脑酸、缬氨酸、十一烷酸、酮亮氨酸、赖氨酸、鹅去氧胆酸和延胡索酸均与尿蛋白负相关;-乙酰丝氨酸与尿蛋白正相关(<0.05);-乙酰丙氨酸、十一烷酸、酮亮氨酸、赖氨酸、鹅去氧胆酸和-苯乙酰苯丙氨酸均与血尿酸负相关(<0.05)。此外,葡萄糖与糖化血红蛋白正相关,与血肌酐负相关(<0.05);酮亮氨酸与低密度脂蛋白胆固醇正相关(<0.05);赖氨酸与体重指数负相关(<0.05);鹅去氧胆酸与低密度脂蛋白胆固醇及总胆固醇均正相关(<0.05);仅延胡索酸与年龄正相关(<0.05);仅-苯乙酰苯丙氨酸与性别有关(<0.05)。

Figure 3. Heatmap based on the correlations between the 15 significantly altered metabolites and clinical features of subjects with T2D. Alb: albumin; BMI: body mass index; DBP: diastolic blood pressure; DOT: duration of T2D; eGFR: estimated glomerular filtration rate; FBG: fasting blood glucose; Hb: hemoglobin; HbA1c: hemoglobin A1c; HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol; KIM-1: kidney injury molecule-1; NGAL: neutrophil gelatinase-associated lipocalin; SBP: systolic blood pressure; SCr: serum creatinine; TC: total cholesterol; TG: triglyceride; UA: uric acid; UACR: urinary albumin-to-creatinine ratio. *P<0.05; #P<0.01.
5 ROC曲线评估代谢物在DKD诊断中的应用价值
如图4所示,15种代谢物ROC曲线下面积(area under the curve, AUC)的中位数均高于0.700;其中,肉豆蔻脑酸、-乙酰丙氨酸、缬氨酸和正亮氨酸的AUC分别为0.854 (95% CI: 0.705~1.000)、0.840 (95% CI: 0.677~1.000)、0.812 (95% CI: 0.640~0.985)和0.806 (95% CI: 0.630~0.982),表明上述代谢物可作为诊断DKD的新标志物。
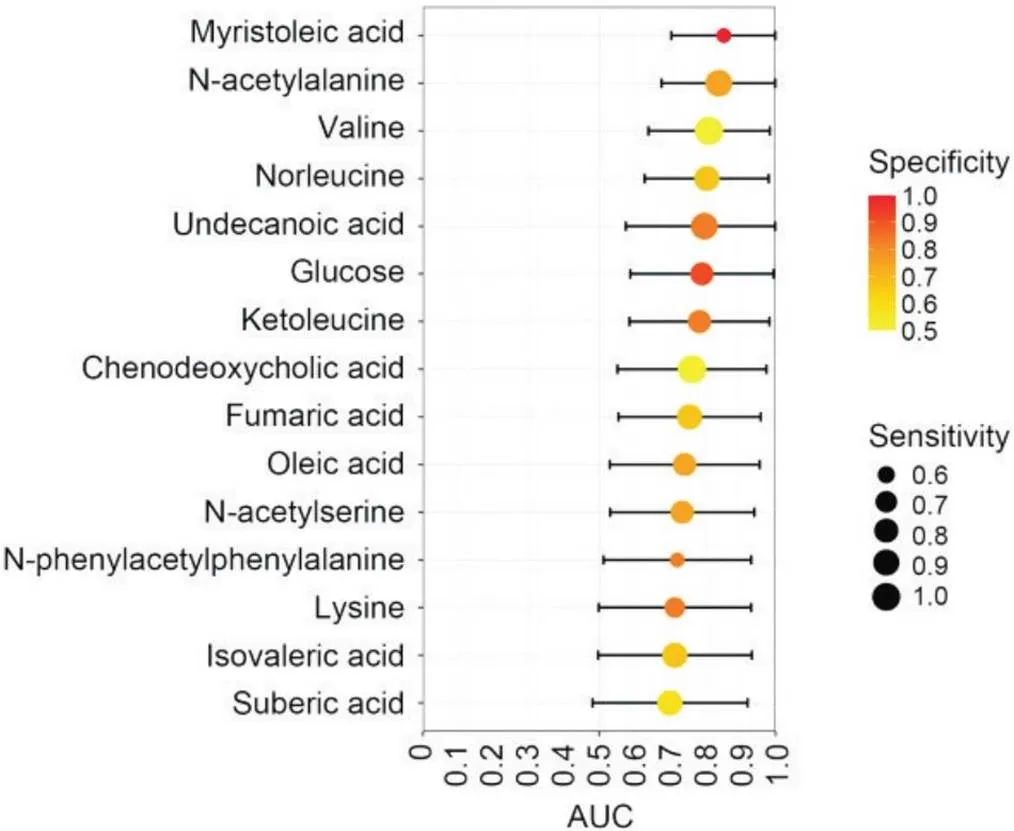
Figure 4. Fifteen significantly altered metabolites discriminating T2D with DKD from simple T2D samples. AUC: area under the receiver operating characteristic curve. The position of each node corresponds to the AUC on the horizontal axis. The black lines on either side of the node represent the 95% confidence interval of the AUC. The colour of the node indicates the specificity, and the size of the circle indicates the sensitivity.
讨论
1 肾小管与DKD的临床诊断
我国是世界上糖尿病患病人数最多的国家[9],其中20%~40%并发DKD[10-11],不仅成为终末期肾衰竭的主要病因,而且显著增高心血管事件和死亡风险[12]。然而,DKD的诊断几乎停滞不前,仍依据UACR、eGFR和肾活检。个体尿蛋白的排泄变异系数近40%[13],运动、发热、感染等均造成尿蛋白增高,甚至有超过半数的已出现肾功能损害的T2D患者却始终无临床意义的蛋白尿[14]。肌酐水平同样受运动、饮食、肌肉总量等影响,采用计算公式估计肾小球滤过率的准确性也备受争议[15]。肾活检对不典型的DKD存在漏诊风险,其在糖尿病患者中的适应症还存在争议[16-17]。视网膜病变仅可作为诊断依据,但并非必要条件,也与DKD的发生发展并不平行[18]。学者们一直致力于探索新的诊断标志物,但其临床应用价值尚缺乏有力证据[19-22]。
UACR和eGFR主要反映肾小球滤过功能,DKD的病理特征也侧重于肾小球的变化。近年来的遗传学研究表明绝大多数eGFR相关基因在肾小管而非肾小球中表达,提示肾小管才是决定肾小球滤过功能的关键[23]。肾小管病变促成DKD早期的高滤过状态和细胞肥大,晚期eGFR下降与肾小管萎缩有关[24]。然而尚缺乏特异性指标评估DKD的肾小管损伤[25]。肾小管代谢模式转变及代谢中间产物蓄积成为DKD的关键机制[26],血液及尿液中发生相应变化的代谢物已成为诊断DKD的潜在标志物[8, 27-28]。循环中代谢物的来源广泛,与肾脏病变之间难以建立直接的病理生理联系;由于组织结构的高度异质性及尿液产生过程的复杂性,尽管尿液代谢物与肾脏关系紧密,但仍然无法精准的反映DKD的病变部位,因而在现阶段的临床研究中代谢组学尚未展现出足够的优越性[29]。本研究聚焦肾小管,立足于糖尿病引发代谢改变的角度,旨在寻找诊断DKD的新策略。
2 基于尿液外泌体靶向肾小管的液体活检
尿液外泌体主要来源于泌尿道上皮细胞,如足细胞、肾小管上皮细胞等。外泌体的形成经历一系列复杂调控,也使其携带来源细胞的特征标志,并实时反映该细胞的生理和病理状态[5]。CD13,又称氨基肽酶N,是肾脏近端小管细胞和肠道粘膜细胞刷缘膜的主要成分[30],在子宫内膜、脾脏、脑组织及免疫细胞中也有表达。既往研究表明尿液外泌体表达CD13[31-34],并将其作为肾小管外泌体的标志蛋白[35]。本研究进一步证实原代肾小管上皮细胞及其外泌体均表达CD13,首次借助CD13从尿液中筛选肾小管外泌体,并采用高通量的代谢组学技术定量其中的代谢物。与尿蛋白、尿酸等功能学指标和外泌体代谢物之间的广泛关联相比,KIM-1和NGAL这类肾脏损伤指标与代谢物之间并不存在相关性,可能与肾小管主动的产生外泌体与其被动的遭受损伤破坏分别属于不同的生物学过程有关,推测前者在反映肾小管病变上的价值更高。ROC曲线证实了肾小管外泌体中代谢物的诊断效能。因此,探索肾小管来源外泌体中的代谢物可能成为寻找DKD诊断标志物的新方向。
3 DKD未来研究方向的启发
单纯T2D肾小管外泌体中的代谢物大都较健康对照显著增高的变化趋势并未在T2D合并DKD时更加显著,反之,T2D合并DKD的代谢物较单纯T2D却显著减少。DKD是否存在肾小管中外泌体的产生、分泌及成分筛选调控的变化仍不清楚。与尿蛋白、血尿酸显著相关的代谢物中,-乙酰丙氨酸和赖氨酸属于氨基酸类,前者包含的-乙酰基参与蛋白质的翻译后修饰,血中-乙酰丙氨酸的水平与eGFR及肾脏病有关[36]。采用尿液赖氨酸等多种代谢物构建的代谢分数模型能够预测盐皮质激素受体拮抗剂缓解T2D患者白蛋白尿的疗效[37]。酮亮氨酸为有机酸,是亮氨酸分解代谢的中间产物,血清酮亮氨酸及亮氨酸的增高能预测T2D的发生[38]。十一烷酸和鹅去氧胆酸分别属于脂肪酸类和胆汁酸类,两者与T2D或DKD的关联尚未见报道。此外,外泌体中显著性改变的代谢物在肾小管中如何变化,潜在的临床意义为何、是否参与糖尿病肾小管病变的发生发展,以及能否成为临床诊治的新靶标[39]等,均有待深入研究。
本研究采用病例对照研究设计,因而无法得出因果关系的结论。但筛选出的具有统计学意义的代谢物及其与临床特征之间的关联是可靠的,因此可以作为未来探讨DKD发病机制的实验室研究及寻找早期诊断标志物的前瞻性研究的基础。
综上所述,肾小管外泌体中的肉豆蔻脑酸、-乙酰丙氨酸、缬氨酸、正亮氨酸、十一烷酸、葡萄糖、酮亮氨酸、鹅去氧胆酸、延胡索酸、油酸、-乙酰丝氨酸、-苯乙酰苯丙氨酸、赖氨酸、异戊酸和辛二酸可作为诊断T2D合并DKD的新途径。
[1]中华医学会糖尿病学分会微血管并发症学组. 中国糖尿病肾脏病防治指南(2021年版)[J]. 中华糖尿病杂志, 2021, 13(8):762-784.
Microvascular Complications Group of Chinese Diabetes Society. Clinical guideline for the prevention and treatment of diabetic kidney disease in China (2021 edition) [J]. Chin J Diabetes Mellitus, 2021, 13(8):762-784.
[2]中华医学会肾脏病学分会专家组. 糖尿病肾脏疾病临床诊疗中国指南[J]. 中华肾脏病杂志, 2021, 37(3):255-304.
Chinese Medical Association. Expert Group of Chinese Society of Nephrology. Chinese guidelines for diagnosis and treatment of diabetic kidney disease[J]. Chin J Nephrol, 2021, 37(3):255-304.
[3] Elsayed NA, Aleppo G, Aroda VR, et al. 11. Chronic kidney disease and risk management: standards of care in diabetes-2023[J]. Diabetes Care, 2023, 46(Suppl 1):S191-S202.
[4] Colombo M, Raposo G, Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles[J]. Annu Rev Cell Dev Biol, 2014, 30:255-289.
[5] Erdbrugger U, Blijdorp CJ, Bijnsdorp IV, et al. Urinary extracellular vesicles: a position paper by the urine task force of the international society for extracellular vesicles [J]. J Extracell Vesicles, 2021, 10(7):e12093.
[6] Terryn S, Jouret F, Vandenabeele F, et al. A primary culture of mouse proximal tubular cells, established on collagen-coated membranes[J]. Am J Physiol Renal Physiol, 2007, 293(2):F476-F485.
[7] Felicetti F, De Feo A, Coscia C, et al. Exosome-mediated transfer of miR-222 is sufficient to increase tumor malignancy in melanoma[J]. J Transl Med, 2016, 14:56.
[8]石彩凤, 周阳, 何爱琴, 等. 新型尿液代谢标志物在糖尿病肾脏病诊断中的价值[J]. 中华糖尿病杂志, 2022, 14(5):456-464.
Shi CF, Zhou Y, He AQ, et al. Novel urinary metabolite biomarkers in diagnosis of diabetic kidney disease[J]. Chin J Diabetes Mellitus, 2022, 14(5):456-464.
[9] Li Y, Teng D, Shi X, et al. Prevalence of diabetes recorded in mainland China using 2018 diagnostic criteria from the American Diabetes Association: national cross sectional study[J]. BMJ, 2020, 369:m997.
[10] Zhang XX, Kong J, Yun K. Prevalence of diabetic nephropathy among patients with type 2 diabetes mellitus in China: a meta-analysis of observational studies[J]. J Diabetes Res, 2020, 2020:2315607.
[11] Johansen KL, Chertow GM, Foley RN, et al. US renal data system 2020 annual data report: epidemiology of kidney disease in the United States[J]. Am J Kidney Dis, 2021, 77(4 Suppl 1):A7-A8.
[12] Fox CS, Matsushita K, Woodward M, et al. Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: a meta-analysis[J]. Lancet, 2012, 380(9854):1662-1673.
[13] Kdoqi. KDOQI clinical practice guidelines and clinical practice recommendations for diabetes and chronic kidney disease[J]. Am J Kidney Dis, 2007, 49(2 Suppl 2):S12-154.
[14] Oshima M, Shimizu M, Yamanouchi M, et al. Trajectories of kidney function in diabetes: a clinicopathological update [J]. Nat Rev Nephrol, 2021, 17(11):740-750.
[15] Elsayed NA, Aleppo G, Aroda VR, et al. 2. Classification and diagnosis of diabetes: standards of care in diabetes-2023[J]. Diabetes Care, 2023, 46(Suppl 1):S19-S40.
[16] Bermejo S, Pascual J, Soler MJ. The current role of renal biopsy in diabetic patients[J]. Minerva Med, 2018, 109(2):116-125.
[17] Gonzalez Suarez ML, Thomas DB, Barisoni L, et al. Diabetic nephropathy: is it time yet for routine kidney biopsy?[J]. World J Diabetes, 2013, 4(6):245-255.
[18] He F, Xia X, Wu XF, et al. Diabetic retinopathy in predicting diabetic nephropathy in patients with type 2 diabetes and renal disease: a meta-analysis[J]. Diabetologia, 2013, 56(3):457-466.
[19] Ye X, Luo T, Wang K, et al. Circulating TNF receptors 1 and 2 predict progression of diabetic kidney disease: a meta-analysis[J]. Diabetes Metab Res Rev, 2019, 35(8):e3195.
[20] Coca SG, Nadkarni GN, Huang Y, et al. Plasma biomarkers and kidney function decline in early and established diabetic kidney disease[J]. J Am Soc Nephrol, 2017, 28(9):2786-2793.
[21] Looker HC, Colombo M, Hess S, et al. Biomarkers of rapid chronic kidney disease progression in type 2 diabetes[J]. Kidney Int, 2015, 88(4):888-896.
[22] Niewczas MA, Pavkov ME, Skupien J, et al. A signature of circulating inflammatory proteins and development of end-stage renal disease in diabetes[J]. Nat Med, 2019, 25(5):805-813.
[23] Qiu C, Huang S, Park J, et al. Renal compartment-specific genetic variation analyses identify new pathways in chronic kidney disease[J]. Nat Med, 2018, 24(11):1721-1731.
[24] Vallon V, Thomson SC. The tubular hypothesis of nephron filtration and diabetic kidney disease[J]. Nat Rev Nephrol, 2020, 16(6):317-336.
[25] Satirapoj B, Pooluea P, Nata N, et al. Urinary biomarkers of tubular injury to predict renal progression and end stage renal disease in type 2 diabetes mellitus with advanced nephropathy: a prospective cohort study[J]. J Diabetes Complications, 2019, 33(9):675-681.
[26] Tanaka S, Sugiura Y, Saito H, et al. Sodium-glucose cotransporter 2 inhibition normalizes glucose metabolism and suppresses oxidative stress in the kidneys of diabetic mice[J]. Kidney Int, 2018, 94(5):912-925.
[27] Wu IW, Tsai TH, Lo CJ, et al. Discovery of a biomarker signature that reveals a molecular mechanism underlying diabetic kidney disease via organ cross talk[J]. Diabetes Care, 2022, 45(6):e102-e104.
[28] Kwan B, Fuhrer T, Zhang J, et al. Metabolomic markers of kidney function decline in patients with diabetes: evidence from the chronic renal insufficiency cohort (CRIC) study[J]. Am J Kidney Dis, 2020, 76(4):511-520.
[29] Kammer M, Heinzel A, Willency JA, et al. Integrative analysis of prognostic biomarkers derived from multiomics panels helps discrimination of chronic kidney disease trajectories in people with type 2 diabetes[J]. Kidney Int, 2019, 96(6):1381-1388.
[30] Olsen J, Kokholm K, Noren O, et al. Structure and expression of aminopeptidase N[J]. Adv Exp Med Biol, 1997, 421:47-57.
[31] Pisitkun T, Shen RF, Knepper MA. Identification and proteomic profiling of exosomes in human urine[J]. Proc Natl Acad Sci U S A, 2004, 101(36):13368-13373.
[32] Gonzales PA, Pisitkun T, Hoffert JD, et al. Large-scale proteomics and phosphoproteomics of urinary exosomes[J]. J Am Soc Nephrol, 2009, 20(2):363-379.
[33] Raj DA, Fiume I, Capasso G, et al. A multiplex quantitative proteomics strategy for protein biomarker studies in urinary exosomes[J]. Kidney Int, 2012, 81(12):1263-1272.
[34] Oeyen E, Van Mol K, Baggerman G, et al. Ultrafiltration and size exclusion chromatography combined with asymmetrical-flow field-flow fractionation for the isolation and characterisation of extracellular vesicles from urine[J]. J Extracell Vesicles, 2018, 7(1):1490143.
[35] Zhang W, Zhou X, Zhang H, et al. Extracellular vesicles in diagnosis and therapy of kidney diseases[J]. Am J Physiol Renal Physiol, 2016, 311(5):F844-F851.
[36] Sekula P, Goek ON, Quaye L, et al. A metabolome-wide association study of kidney function and disease in the general population[J]. J Am Soc Nephrol, 2016, 27(4):1175-1188.
[37] Mulder S, Perco P, Oxlund C, et al. Baseline urinary metabolites predict albuminuria response to spironolactone in type 2 diabetes [J]. Transl Res, 2020, 222: 17-27.
[38] Zeng Y, Mtintsilana A, Goedecke JH, et al. Alterations in the metabolism of phospholipids, bile acids and branched-chain amino acids predicts development of type 2 diabetes in black South African women: a prospective cohort study[J]. Metabolism, 2019, 95:57-64.
[39] 王艳丽, 刘春花, 潘洁, 等. 基于细胞代谢组学的药物研究方法及应用[J]. 中国病理生理杂志, 2022, 38(12):2258-2267.
Wang Y, Liu C, Pan J, et al. Methods and application of cell metabolomics in drug research[J]. Chin J Pathophysiol, 2022, 38(12):2258-2267.
Application of urinary exosome-based liquid biopsy combined with metabolomics in clinical diagnosis of diabetic kidney disease
SHI Caifeng, LIU Dandan, HE Aiqin, WU Xiaomei, SHEN Xinjia, ZHU Xueting, XUE Ying, YANG Junwei△, ZHOU Yang△
(,,210003,)
To investigate the application of metabolites in urinary exosomes from kidney tubules in the assessment of diabetic kidney disease (DKD).Healthy individuals (=13), simple type 2 diabetes (T2D) patients (=12) and T2D patients with DKD (=12) were enrolled in this cross-sectional study. Demographic and laboratory data were collected. Primary renal tubular epithelial cells were cultured. Exosomes were extracted by ultracentrifugation. Exosomes expressing cluster of differentiation 13 (CD13) were enriched by immunomagnetic beads. The morphology of exosomes was tracked by transmission electron microscopy and nanoparticle tracking analysis. Western blot and immunohistochemical staining were applied. Kidney injury molecule-1 and neutrophil gelatinase-associated lipocalin were detected by ELISA. Metabolites in exosomes were quantified by ultraperformance liquid chromatography coupled to tandem mass spectrometry. Partial least squares discriminant analysis was used for multidimensional modelling. The Wilcox test was used for comparison of metabolite data between two groups, and the Kruskal-Wallis test was used for comparison between multiple groups. Spearman correlation analysis was used to analyse the correlation between metabolites and clinical indicators. The receiver operating characteristic (ROC) curve was used to evaluate diagnostic efficacy.Expression of CD13 was detected in urinary exosomes. Immunostaining showed that CD13 was located in the apical membrane of kidney tubules. Moreover, CD13 was expressed in primary renal tubular epithelial cells and their exosomes. A total of 144 metabolites were detected in urinary exosomes expressing CD13 by targeted quantitative metabolomics. Fifteen metabolites with statistical significance were selected between the simple T2D group and the T2D with DKD group (<0.05). These metabolites included lysine,-acetylalanine,-acetylserine, norleucine,-phenylacetylphenylalanine, valine, chenodeoxycholic acid, glucose, myristoleic acid, oleic acid, suberic acid, undecanoic acid, fumaric acid, ketoleucine, and isovaleric acid.-acetylalanine, undecanoic acid, ketoleucine, lysine and chenodeoxycholic acid in urinary CD13+exosomes were all significantly correlated with urinary albumin and serum uric acid levels in T2D patients (<0.05 or<0.01). The areas under the ROC curves of myristoleic acid,-acetylalanine, valine, and norleucine were 0.854 (95% CI: 0.705~1.000), 0.840 (95% CI: 0.677~1.000), 0.812 (95% CI: 0.640~0.985), and 0.806 (95% CI: 0.630~0.982), respectively.There are significant differences in metabolites encapsulated in kidney tubular cell-derived exosomes between simple T2D patients and T2D patients with DKD. The 15 metabolites with statistical significance could become novel biomarkers for the clinical diagnosis of DKD.
diabetic kidney disease; exosome; metabolomics; kidney tubules
1000-4718(2023)07-1244-09
2023-05-12
2023-06-26
025-58509741; E-mail: zhouyang@njmu.edu.cn(周阳); jwyang@njmu.edu.cn(杨俊伟)
R363; R587
A
10.3969/j.issn.1000-4718.2023.07.011
[基金项目]国家自然科学基金资助项目(No. 82270760);江苏省自然科学基金资助项目(No. BK20201497)
(责任编辑:宋延君,李淑媛)

