Magnetic modification of lanthanide-based upconversion nanocrystals for fingerprint information recognition
Yafei Bi ,Fu Zhang ,Yu Bai ,Yao Wang ,Nan Zheng ,Sheng Wang ,Ning Zhang,3,Ran Long,Xinglong Gong,and Yujie Xiong,2
1National Synchrotron Radiation Laboratory, School of Chemistry and Materials Science, Department of Environmental Science and Engineering, Experimental Center of Engineering and Material Sciences, CAS Key Laboratory of Mechanical Behavior and Design of Materials, Department of Modern Mechanics, University of Science and Technology of China, Hefei 230026, China;
2Institute of Energy, Hefei Comprehensive National Science Center, Hefei 230031, China;
3Suzhou Institute for Advanced Research, University of Science and Technology of China, Suzhou 215123, China
Abstract:The development of new magnetic fluorescent materials is of great significance for identification and criminal investigation.Since the photosensitive elements used in conventional cameras have exhibited the highest quantum efficiency in the range of 500-700 nm,lanthanide-based upconversion nanoparticles (UCNPs) with main emission peaks at 507-533 nm,533-568 nm and 637-683 nm are suitable for constructing magnetic fluorescent materials.In this work,we demonstrate a type of magnetic upconversion nanoparticle (MUCNP) of NaGdF4:Yb,Er-Fe3O4 by a ligand-linked method.After optimizing the reaction parameters,the composite particles possess remarkable magnetic properties and upconversion fluorescence intensity and achieve high contrast for latent fingerprint recognition on various substrates.The combination of upconversion luminescence and magnetism contributes to good fingerprint recognition sensitivity and universality.
Keywords:lanthanide-based upconversion nanocrystals;magnetic modification;luminescence;fingerprint recognition
1 Introduction
When the finger touches the surface of the object,the distribution pattern of skin ridges and grooves,combined with secreted sweat,oily substances and foreign contaminants,leaves an impression of the unique fingerprint pattern[1-3].Owing to its invisibility to the naked eye,the pattern is known as a latent fingerprint[4,5].Nonetheless,such latent fingerprints can be recognized through proper chemical or physical treatment.For instance,aluminum flake powder,magnetic powder,iron flake powder and fluorescent powder are widely used for fingerprint enhancement in practical fingerprint detection technologies[6,7].Among them,the magnetic fluorescent composite structure is one of the ideal alternatives for enhancing the visibility of latent fingerprints because magnetism can facilitate the nondestructive development of latent fingerprints and fluorescence can increase the contrast of fingerprint patterns[8].Considering that the photoreceptors in the commonly used camera exhibit the highest quantum efficiency in the range of 500-700 nm,lanthanide upconversion nanocrystals (e.g.,NaGdF4:Yb,Er) with luminescence regions in the range of 507-533 nm,533-568 nm and 637-683 nm are regarded as ideal luminescent material candidates.Ferroferric oxide (Fe3O4) is a well-observed compound with strong magnetism.Taken together,the composite of NaGdF4:Yb,Er and Fe3O4is highly anticipated as a good latent fingerprint recognition probe with high sensitivity.
Nevertheless,the synthesis of NaGdF4:Yb,Er-Fe3O4nanocomposites faces reduced stability due to the high temperature during synthesis (350 °C)[9].The encapsulation approach(i.e.,coating method) in an aqueous phase system is commonly used according to previous reports;that is,NaGdF4:Yb,Er and Fe3O4are simultaneously enclosed in another shell layer[10,11].Unfortunately,this method still confronts the dilemma of ensuring that both materials can be well encased in the same shell structure.Another alternative option is to create Fe3O4nanostructures first as the core and then in situ grow NaGdF4shell layers[12].However,high temperature is typically required during the synthesis of NaGdF4,which is adverse to the magnetic properties of the Fe3O4core.In this regard,it is of high urgency to develop a synthesis method that can reduce the impact on the magnetic and luminescent functional components of composite materials.
In this work,we demonstrate a simple ligand-linked method to synthesize NaGdF4:Yb,Er-Fe3O4nanocomposites based on NaGdF4:Yb,Er nanocrystals doped with luminescent lanthanide ions[13].Taking ethyl orthosilicate (TEOS) as the ligand in n-propanol solvent,we can assemble NaGdF4:Yb,Er and Fe3O4as nanocomposites with good dispersion.Specifically,the upconversion luminescence of NaGdF4:Yb,Er and magnetism of Fe3O4can be well balanced.As a result,the as-prepared NaGdF4:Yb,Er-Fe3O4composite exhibits high contrast and high resolution in the latent fingerprint visualization process.
2 Materials and methods
Synthesis of NaGdF4:Yb,Er.1600 μL of gadolinium trichloride solution (1 mol/L),360 μL of ytterbium trichloride solution (1 mol/L),400 μL of erbium trichloride solution (0.1 mol/L),26 mL of ultrapure water and 7.2 mL of sodium hydroxide solution (1 mol/L) were added into a beaker.After stirring for 1 h,the solution was centrifuged and washed three times to obtain the precipitate.The precipitate was transferred to a three-necked flask and redispersed in 16 mL ultrapure water through ultrasonication.Then,4.2 mL of sodium hydroxide solution (1 mol/L) was added into the threenecked flask.After stirring for 10 min,14 mL oleic acid was further introduced into the flask.The solution was then heated to 100 °C,and the heating was maintained until no bubbles were generated and the solution became clear and transparent.After the temperature was reduced to room temperature,25.6 mL of 1-octadecene was added with stirring for 15 min.Then,1.46 mL of trifluoroacetic acid was added with stirring for an additional 30 min.Under the sealing condition of 100 °C,the inside of the flask was evacuated to a vacuum,then under the protection of N2,the temperature was raised to 350 °C,and the reaction was continued for 40 min.After the reaction was completed,the solution was cooled to room temperature,and the product was obtained by washing and centrifugation 5 times.
Synthesis of NaGdF4:Yb,Er-Fe3O4.30 mg of aspurchased Fe3O4was dispersed in 30 mL of ethanol solution to make a suspension of 1 mg/mL for further use.Then,72 mL of npropanol,72 mg of NaGdF4and 24 mL of Fe3O4ethanol suspension were added into a 500-mL round-bottomed flask and stirred by a mechanical stirring table with a Teflon stirring bar.After dispersing for 30 min,360 μL of NaOH solution(2 mol/L) and 60 μL of TEOS were added and stirred in an oil bath at 70 °C for 18 h in a sealed state.Finally,the obtained product was collected by centrifugation,washed with absolute ethanol and then dried in a vacuum drying oven.
3 Results and discussion
According to previous reports[14-16],NaGd(80%)F4:Yb(18%),Er(2%) was synthesized as the luminescent upconversion counterpart.The characterization results (Figs.S1-S3) of the as-synthesized sample demonstrate that the upconversion material has a hexagonal morphology with an edge length of approximately 60 nm with optimized fluorescence intensity.Furthermore,using a ligand-linked approach (TEOS as a ligand),we synthesized a NaGdF4:Yb,Er-Fe3O4composite structure based on the as-synthesized NaGdF4:Yb,Er nanocrystals with Fe3O4as the magnetic counterpart.Fig.1a depicts the accumulation of Fe3O4with comparable size surrounding the origina lNaGdF4:Yb,Er hexagona lnanoparticles .Energydispersive X-ray spectroscopy (EDS) (Fig.S3b) shows the presence of Na,Yb,Fe and other elements.Fig.1b illustrates that the elemental mapping distributions of the F,O and Fe elements are similar,indicating that the as-obtained sample is composed of NaGdF4:Yb,Er and Fe3O4.The emerging X-ray diffraction (XRD) diffraction peak (Fig.1c) is compatible with the diffraction pattern of hexagonal NaGdF4(PDF#27-0699) and cubic Fe3O4(PDF#99-0073),indicating that the product is highly crystalline.The infrared spectrum peaks of O-H stretching and Si-O-Si asymmetric/symmetric stretching vibration (Fig.S4) in the NaGdF4:Yb,Er-Fe3O4structure indicate the presence of TEOS ligands.These structural characterizations demonstrate that the NaGdF4:Yb,Er-Fe3O4composite is successfully prepared.The fluorescence spectra of the composite (Fig.1d) show that the ratio between 520 nm and 540 nm is balanced compared to the bare NaGdF4:Yb,Er,and the fluorescence property of our NaGdF4:Yb,Er-Fe3O4composite is still acceptable even if there is a significant decline.We then explored the effects of the reactant feed ratio and reaction time on the fluorescence properties of the products.Fluorescence spectra (Fig.S5 and Table S2)showed that the peak intensity was the highest when the feeding ratio was 4.5∶1 with an 18 h reaction time.

Fig.1.(a) Transmission electron microscopy (TEM) image,(b) mapping image,(c) XRD pattern and (d) fluorescence spectra of NaGdF4:Yb,Er-Fe3O4.
Reducing the sacrifice of fluorescence intensity,enhancing the saturation magnetization of the material,and seeking the relative balance of fluorescence and magnetism are conducive to the construction of fingerprint powders with excellent performance.As shown in Fig.2,the saturation magnetization intensities of the two NaGdF4:Yb,Er-Fe3O4composite nanomaterials decrease slightly relative to that of Fe3O4but still remain on the same order of magnitude.In particular,the NaGdF4:Yb,Er-Fe3O4(N∶F=4∶1) sample improved the magnetic characteristics of the upconversion material by approximately half while maintaining the fluorescence properties.
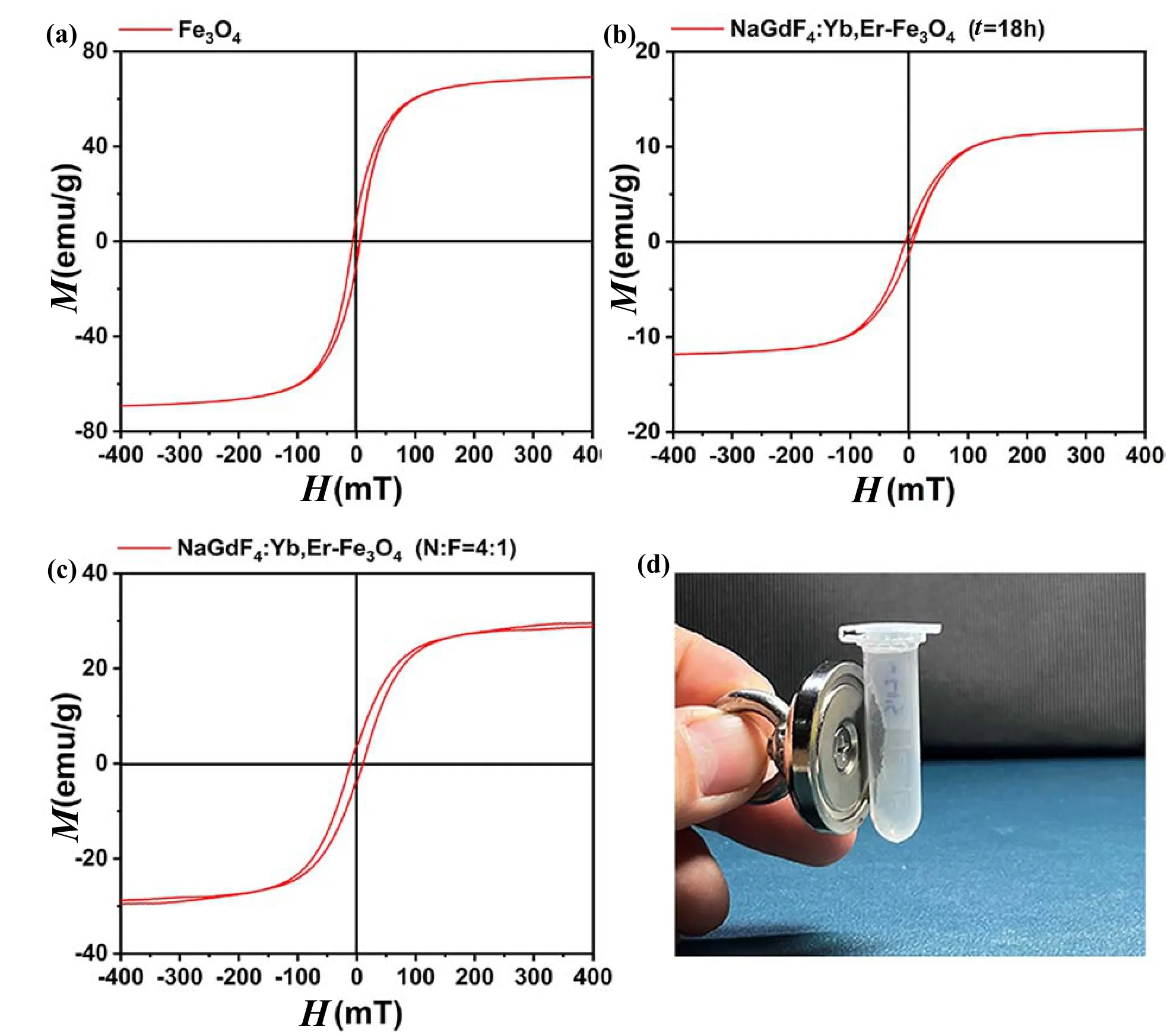
Fig.2.Magnetic characterizations.(a-c) The hysteresis loop of (a) Fe3O4,(b) NaGdF4:Yb,Er-Fe3O4 (t=18 h) and (c) NaGdF4:Yb,Er-Fe3O4 (N∶F=4∶1).(d) Photograph of the NaGdF4:Yb,Er-Fe3O4 (N∶F=4∶1) sample attracted by the magnet.
To explore the application performance of the assynthesized NaGdF4:Yb,Er-Fe3O4composite nanomaterials for latent fingerprint detection,we carried out three classic crafts,including the powder spraying technique,residual powder adsorption and infrared excitation (Fig.S6)[17].The visualization of latent fingerprints mainly includes three processes:powder adhesion,blowing,and excitation.First,press the finger on the substrate to obtain invisible latent fingerprints,and then use a magnetic fingerprint brush to carry out the process of fingerprint adsorption and detachment.The above process was repeated,and excess powder was blown off to obtain visible latent fingerprints.Under the irradiation of 980 nm infrared light,we can observe the green emitting fingerprint pattern through the charge-coupled device (CCD)imaging system.The feasibility of fingerprint detection is highly dependent on the accuracy of identifying the latent fingerprint level 1,2,and 3 features[18],which are regarded as absolutely critical quantitative criteria in practice.The unique ridge features of fingerprints on smooth metal surfaces can be viewed and photographed using NaGdF4:Yb,Er-Fe3O4nanomaterials as photoreceptors under 980 nm infrared (IR) laser irradiation,and all three levels of fingerprint features can be clearly recognizable (Fig.3).The primary pattern shows a dustpan core (Fig.3b),which can be used to initially determine the fingerprint type and ridge orientation.The secondary pattern reflects the resolution of fingerprint ridges,including bifurcations (Fig.3c),ridge ends (Fig.3d) and bridges(Fig.3e),which are defined as small dots and are mainly used for personal identification.More intriguingly,the latent fingerprints using magnetic fluorescent nanoparticles can clearly show the level 3 pattern with islands (Fig.3f) and display more complex detail points and additional personal characteristics,which is highly beneficial to improve the reliability and accuracy of identifying latent fingerprints in the analysis.
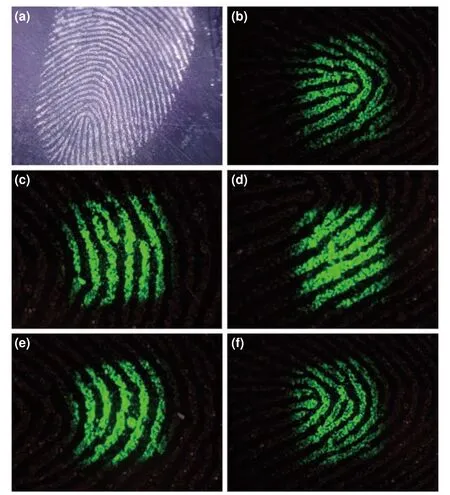
Fig.3.Detailed characteristic images of fingerprints developed by NaGdF4:Yb,Er-Fe3O4 nanomaterials under a 980 nm infrared laser on metal substrates.(a) Complete fingerprint without IR light,(b) dustpan,(c) bifurcation,(d) ridge ends,(e) bridges and (f) islands.
To further demonstrate the advantages of NaGdF4:Yb,Er-Fe3O4in practical applications,we compared the development of latent fingerprints on two surfaces with dark complex backgrounds from magnetic powders,fluorescent powder and the composites.The results show that the fingerprint patterns using the NaGdF4:Yb,Er-Fe3O4composite material (Fig.S7) have high contrast and high resolution,and relatively fine structures can still be observed.In contrast,only low-contrast gray fingerprints were developed using magnetic powder,and structures were difficult to identify on dark backgrounds.In general,the composite material integrates the advantages of magnetic powder and fluorescent powder,greatly improving the resolution and contrast of fingerprints under the premise of nondestructive construction of fingerprints.
To evaluate the universality of the as-developed NaGdF4:Yb,Er-Fe3O4latent fingerprints under 980 nm infrared irradiation,fingerprints were created simultaneously on various permeable and impermeable substrates.Highresolution photographs of fingerprints (Fig.4) indicate that the synthetic magnetic phosphor has excellent adherence to fingerprint ridges on impervious surfaces (e.g.,metal,glass,ceramic and acrylic);smooth-surfaced impermeable substrates (e.g.,bank cards,nail clippers,mobile phone screens and tin foil);and permeable substrates (e.g.,A4 paper,envelopes,vouchers and playing cards).All these results demonstrate the versatility of bifunctional nanomaterials for latent fingerprint detection applications.

Fig.4.Photographs of latent fingerprints developed by NaGdF4:Yb,Er-Fe3O4 on various substrates.(a) Metal,(b) glass,(c) ceramic,(d) acrylic,(e) bank card,(f) nail clipper,(g) mobile phone screen,(h) tin foil,(i) A4 paper,(j) envelope,(k) voucher and (l) playing card.
4 Conclusions
In conclusion,we developed a NaGdF4:Yb,Er-Fe3O4upconversion-magnetic composite structure using a ligand-linked method and investigated its resolution as a latent fingerprint visualizer.The NaGdF4:Yb,Er-Fe3O4composite exhibits outstanding adherence on fingerprint ridges toward developing fingerprints and realizing the most basic latent fingerprint visualization material function.Multiple sets of fingerprints generated on permeable and impermeable substrates demonstrate that NaGdF4:Yb,Er-Fe3O4can cope with a wide range of material substrates.Compared to commercial magnetic powders,the fingerprints generated by NaGdF4:Yb,Er-Fe3O4on substrates with dark and complex backgrounds had superior identification and resolution.When partially obscured by dark text or lines,commercial magnetic powder fingerprints are barely detectable,indicating the truncated essence of the fingerprints.In contrast,our NaGdF4:Yb,Er-Fe3O4fingerprints are virtually detectable,showcasing the continuous nature of the fingerprints.Our developed NaGdF4:Yb,Er-Fe3O4composite nanomaterials have excellent resolution and a broad application in latent fingerprint development,as well as the ability to cope with dark and complex background substrates,making them promising as latent fingerprint developers with broad application prospects.
Supporting information
The supporting information for this article can be found online at https://doi.org/10.52396/JUSTC-2022-0147.The supporting information includes seven figures and two tables.
Acknowledgments
This work was financially supported in part by the National Key R&D Program of China (2020YFA0406103),the National Natural Science Foundation of China (21725102,22122506,91961106,22075267,22109148),Strategic Priority Research Program of the Chinese Academy of Sciences(XDPB14),the Youth Innovation Promotion Association of the Chinese Academy of Sciences (2019444),the Hundred Talents Program of the Chinese Academy of Sciences,and Fundamental Research Funds for the Central Universities(WK2060000039).The authors thank the support from the USTC Center for Micro-and Nanoscale Research and Fabrication.
Conflict of interest
The authors declare that they have no conflict of interest.
Biographies
Yafei Bireceived his master’s degree in Chemistry from the University of Science and Technology of China under the supervision of Prof.Yujie Xiong and Prof.Ran Long.His research mainly focuses on lanthanide-based upconversion nanocrystals.
Ning Zhangreceived his Ph.D.degree in Chemistry from the University of Science and Technology of China.He is currently a Research Professor at the University of Science and Technology of China.His research focuses on the sustainable energy-driven molecular transformation.
Ran Longreceived her Ph.D.degree in Chemistry from the University of Science and Technology of China.She is currently a Professor at the University of Science and Technology of China.Her research focuses on the controlled synthesis and catalytic applications of nanocrystals.
Yujie Xiongreceived his Ph.D.degree in Chemistry from the University of Science and Technology of China.He is currently a Chair Professor at the University of Science and Technology of China.His research mainly aims to discover and engineer the nature’s catalytic processes,achieving artificial cycles of elements and energy toward ecosystem reconstruction.
- 中国科学技术大学学报的其它文章
- Recent progress on diaCEST MRI for tumor imaging
- The mystery of Li2O2 formation pathways in aprotic Li-O2 batteries
- Mercaptopropane-assisted synthesis of graphitic carbonsupported Pt nanoparticles for enhancing fuel cell start-stop performance
- Amorphous TiO2 ultrathin nanosheet for stable high-rate lithium storage
- Mechanism of nickel-catalyzed hydroalkylation of branched 1,3-dienes
- Polymerized small molecular dyes providing nanoparticles with stable photosensitivity for eradicating cancer cells

