Research on Liposomal Irinotecan in Combination with 5-FU/LV for Metastatic Pancreatic Ductal Adenocarcinoma
Wang Wenjun,Wang Yaoling,Huang Zhe
(1.School of Business Administration,Shenyang Pharmaceutical University,Shenyang 110016,China;2.China National Health Development Research Center,National Health Commission,Beijing 100044,China)
Abstract Objective To systematically review the published clinical and economic research on liposomal irinotecan in combination with 5-FU/LV for metastatic pancreatic ductal adenocarcinoma (mPDAC) at home and abroad.Methods PubMed,Cochrane Library,Embase,CBM,CNKI,Wan Fang data,CRD database and health technology assessment official websites were searched to collect clinical and economic studies on liposomal irinotecan for mPDAC.Results and Conclusion Nine clinical studies and four economic studies were included.The result of clinical studies showed that liposomal irinotecan in combination with 5-FU/LV could extend survival with good drug compliance in patients with mPDAC who progressed on prior gemcitabine-based therapy.This agent represented a new treatment option for second-line chemotherapy in these patients.The results of the economic evaluations failed to reach a consistent conclusion due to different economic levels in various countries.
Keywords: liposomal irinotecan;metastatic pancreatic ductal adenocarcinoma (mPDAC);chemotherapy;clinical research;economic evaluation
Pancreatic ductal adenocarcinoma (PDAC) refers to malignant tumor of the digestive system originated from pancreatic ductal epithelium cells and acinar cells.About 95% of PDAC is ductal adenocarcinoma[1].According to the research of GLOBOCAN,in 2020,there were about 122 000 deaths from PDAC which was the sixth cause of cancer-related death in China[2].Based on the poor prognosis of PDAC,there are more than 60% of the patients present with metastatic disease at initial diagnosis[3],and the median overall survival of locally advanced and metastatic PDAC is only 4 to 5.4 months[4].
The “Guideline for Diagnosis and Treatment of Pancreatic Ductal Adenocarcinoma (2020)”released by Chinese Society of Clinical Oncology(CSCO) recommended gemcitabine-based therapies or fluorouracil-based treatments should be applied in first-line chemotherapy for metastatic pancreatic ductal adenocarcinoma (mPDAC).There have been limited therapeutic options following progression during first-line chemotherapy.For patients with mPDAC that progressed following gemcitabinebased therapy,combination treatments of fiuorouracil/leucovorin (hereinafter referred to as “5-FU/LV”)with irinotecan or platinum are commonly used[5].However,the research of Zaniboni,et al.[6]showed that the median overall survival (mOS) in patients received the combination of irinotecan and 5-FU/LV was only 5 months.In addition,the clinical effectiveness of combination of oxaliplatin and 5-FU/LV was supported by conflict results from clinical phase III trials (CONKO-003 and PANCREOX)[7,8].
Liposomal irinotecan is a new formulation which comprises irinotecan free base encapsulated in liposome nanoparticles.It is an anti-metabolic drug that can prevent DNA replication and inhibit RNA synthesis.Combination of liposomal irinotecan with 5-FU/LV (hereinafter referred to as “liposomal irinotecan combination regimen”) could improve the survival of mPDAC patients who failed in prior gemcitabine-based treatment,which was approved by positive results from the global phase Ⅲ NAPOLI-1 trial (NCT01494506)[9].In that case,liposomal irinotecan combination regimen was supported as a category 1 recommendation for those patients who progressed following gemcitabine-based chemotherapy by the current treatment guidelines from the National Comprehensive Cancer Network(NCCN) and CSCO[10,11].
Economic burden gradually increases caused by the prevalence of PDAC.Therefore,clinical decisions should be not only concerned with the effectiveness and safety,but also with the economics.The purpose of this study is to review the clinical and economic research on liposomal irinotecan combination regimen published at home and abroad.Then,its clinical effectiveness and economic value in the treatment of mPDAC patients can be systematically evaluated to provide evidence for further economic evaluation,clinical decision-making,and medical insurance policies.
1 Materials and methods
1.1 Literature search strategy
CNKI,Wan Fang database,PubMed,Cochrane,Embase,CBM and other electronic databases were searched to collect clinical studies of liposomal irinotecan for treatment of mPDAC,such as randomized controlled trial (RCT),real-world evaluation (RWE) and cost-effectiveness analyses.Search terms comprised PDAC,liposomal irinotecan,randomized controlled trial,cost and cost-effectiveness analysis,etc.
1.2 Inclusion and exclusion criteria
1.2.1 Clinical research
Inclusion criteria: (1) Population (P) included advanced/metastatic PDAC patients after failure of gemcitabine treatment.(2) Intervention group (I) was liposomal irinotecan in combination with 5-FU/LV;Control group (C) included 5-FU/LV regimen and oxaliplatin in combination with 5-FU/LV.(3) Primary outcomes (O) were mOS,median progression free survival (mPFS),and the incidence of grade 3-4 adverse reactions,etc;Secondary outcomes were objective response rate (ORR),etc.(4) Studies (S)included RCT,real-world research and retrospective analysis,etc.Exclusion criteria: (1) Repeatedly published literatures;(2) Comments and letters;(3)Full text inaccessible literatures;(4) Literatures with incomplete or missing data or documents whose data cannot be extracted,etc.
1.2.2 Economic research
Inclusion criteria: (1) Population (P) included advanced/metastatic PDAC patients after failure of gemcitabine treatment.(2) Intervention group (I) was liposomal irinotecan in combination with 5-FU/LV;Control group (C) included 5-FU/LV regimen and oxaliplatin in combination with 5-FU/LV regimen.(3) Primary outcomes (O) included incremental costeffectiveness ratio (ICER),incremental cost and incremental effect,etc.(4) Studies (S) included cost utility analysis (CUA),cost effectiveness analysis(CEA),and health technology assessment (HTA)report,etc.Exclusion criteria: (1) Literatures with repeatedly published data;(2) Comments and letters,etc.
2 Results
As to clinical research,2 009 relevant literatures were collected in initial screening,715 duplicated literatures were deleted,1 239 irrelevant literatures were excluded after reading titles and abstracts,and 55 literatures were included.Then 46 articles were excluded after reading the full texts,and only 9 articles were finally included (Fig.1).
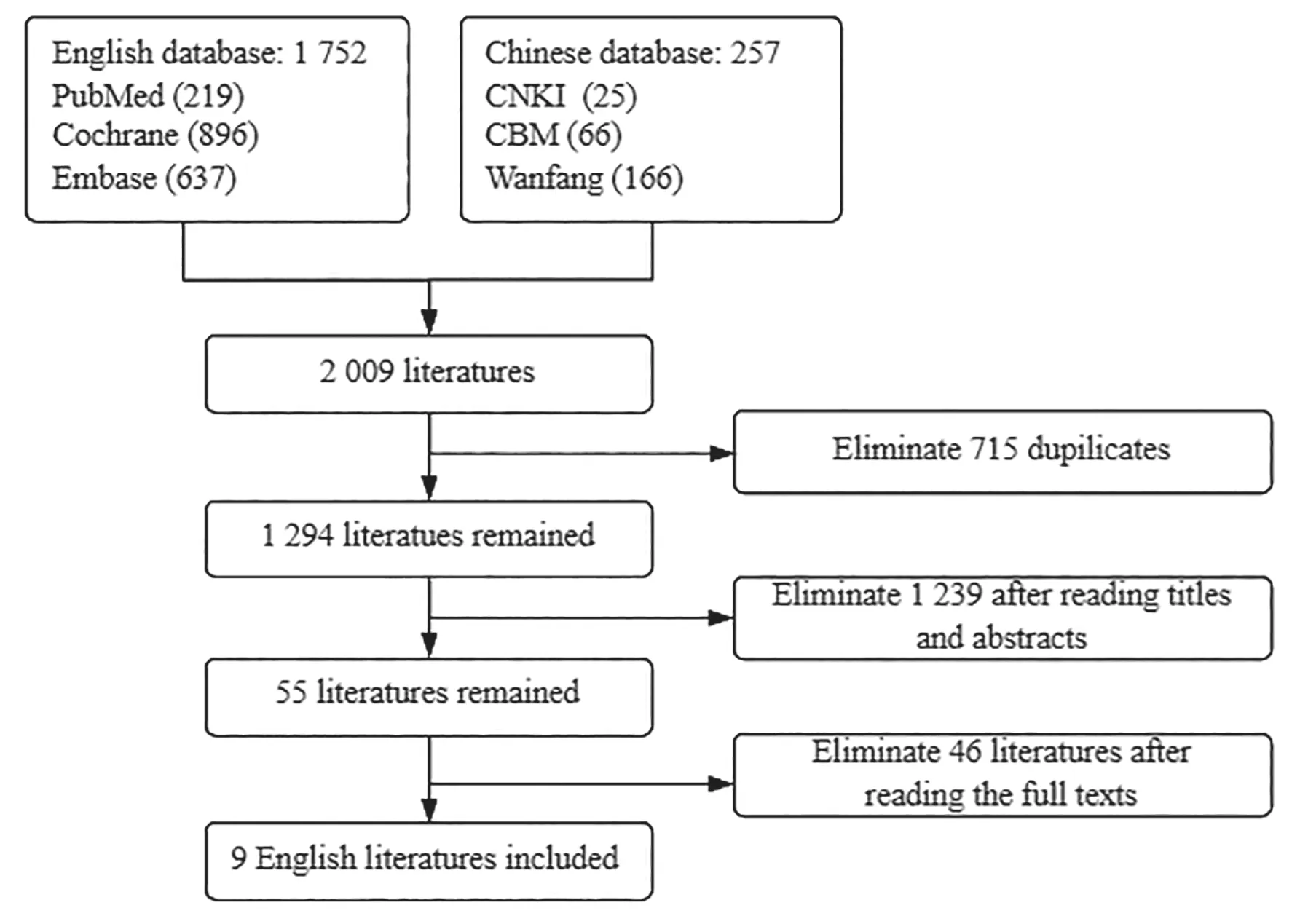
Fig.1 Clinical research selection process
As to economic evaluations,237 relevant literatures were collected in initial screening,79 duplicated literatures were deleted,130 irrelevant literatures were excluded after reading titles and abstracts,28 literatures were included.Then 24 articles were excluded after reading the full texts,and only 4 articles were finally included (Fig.2).
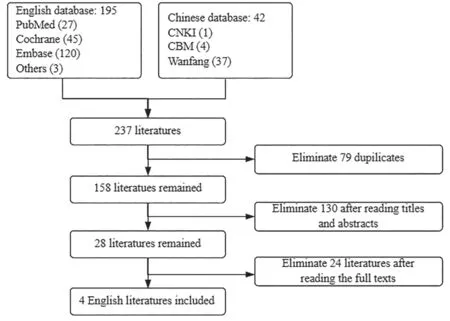
Fig.2 Economic research selection process
2.1 Clinical research status of liposomal irinotecan combination regimen
Nine clinical studies were finally included,including 3 phase III clinical trials[9,12,13],1 phase II clinical trial[14],and 5 real-world studies[15-19](Table 1).

2.1.1 Clinical effectiveness
In 2013,the phase II clinical trial carried out by Ko,et al.[20]showed that the median overall survival of patients treated with liposomal irinotecan therapy was 5.2 months,which proved that liposomal irinotecan could bring better clinical benefits to these people.Based on this result,many experts continued to conduct phase II/III clinical trials or real-world studies of liposomal irinotecan in the treatment of mPDAC,demonstrating the clinical efficacy of liposomal irinotecan combination regimen from different perspectives.
Wang-Gillam,et al.[9]reported the results of a global,multicenter,phase III,randomized controlled clinical trial NAPOLI-1,which was conducted in adult patients with mPDAC who had been previously treated with gemcitabine-based therapy.In this trial,417 patients from 14 countries were randomized to three treatments and the combination treatment with liposomal irinotecan and 5-FU/LV demonstrated better survival compared with 5-FU/LV regimen,which represented a new standard of care for secondline chemotherapy.It demonstrated that liposomal irinotecan combination regimen could prolong mOS by 1.9 months (6.1vs4.2 months,P=0.012),mPFS by 1.6 months (3.1vs1.5 months,P=0.0001),and ORR by 15.4% (16%vs1%) in target patients[21].
After that,the subgroup analysis of Asian population in NAPOLI-1 trial conducted by Bang,et al.[13]showed that liposomal irinotecan combination regimen had higher clinical advantage in Asian patients.The study showed that compared with 5-FU/LV regimen,liposomal irinotecan combination regimen prolonged mOS by 5.2 months (8.9vs3.7 months,P=0.025),mPFS by 2.6 months (4.0vs1.4 months,P=0.011),and ORR by 8.8% (8.8%vs0,P=0.114)in target patients.Studies conducted on patients in China’s Taiwan region,Korea and Japan found that liposomal irinotecan combination regimen can prolong mOS among 6.6 months to 9.4 months[14,17,18].Patients with good performance status (Eastern cooperative oncology group performance status,ECOG PS 0-1) had longer mOS (7.8vs2.7 months)than patients with poor performance status (ECOG PS ≥ 2).These studies further confirmed the results of NAPOLI-1 Asian patient subgroup analysis and found that patients with good physical fitness can obtain higher clinical benefits.Real-world study conducted by Kieler,et al.[16]in Vienna in 2019 found that liposomal irinotecan combination regimen could improve mPFS(4.49vs3.44 months,HR 0.47,P=0.007) and mOS(7.41vs6.16 months,HR 0.68,P=0.181) in mPDAC patients compared with combined treatment with oxaliplatin and fiuorouracil,demonstrating liposomal irinotecan combination regimen also had higher clinical benefits than oxaliplatin combination regimen.
2.1.2 Safety
Patients with mPDAC have lower quality of life after first-line chemotherapy,and often discontinue their therapies for intolerance of chemotherapies induced by adverse reactions.Therefore,adverse event is an important factor in comparing secondline chemotherapy regimens.The clinical literatures included in this article indicated that liposomal irinotecan combination regimen can be well tolerated in chemotherapy for patients with mPDAC.
The most common treatment-emergent adverse events of 3-4 grades in these patients included neutropenia,diarrhea,and vomiting[9].A routine review assessed cases of serious thromboembolic events reported in patients receiving liposomal irinotecan in England.In the cumulative review(October 2015-April 2018),23 serious reports of thromboembolic events were identified,including pulmonary embolism,vena cava thrombosis,deep vein thrombosis and so on[22].Therefore,it is suggested that doctors should identify the potential risk factors and inform patients of the potential risk of thromboembolism when using liposomal irinotecan in the treatment of mPDAC.A retrospective analysis conducted by Park,et al.[19]in 2021 found the incidence of grade 3-4 neutropenia (47.2%vs35%,P=0.033) and peripheral neuropathy (5.9%vs1.0%,P=0.049) was higher in patients treated with FOLFIRINOX (oxaliplatin combined with irinotecan and 5-FU/LV) than with liposomal irinotecan combination regimen,showing that liposomal irinotecan combination regimen had higher safety than FOLFIRINOX .
2.2 Research status of economic evaluation of liposomal irinotecan combination regimen
The economic research of liposomal irinotecan combination scheme commonly evaluated the cost effectiveness of liposomal irinotecan combination regimen compared with 5-FU/LV or oxaliplatin in combination with 5-FU/LV.The factors affected the evaluation results mainly include drug price,cost calculation method,and model data resource,etc.
Four pharmacoeconomic studies were included in this study,one was cost-effectiveness study and three were HTA reports.Research countries included Greece,Australia,the United Kingdom and Canada.These research commonly were from the perspective of medical insurance payers or health service system.The target population included in each study was mPDAC patients who progressed following gemcitabine-based treatment.The evaluation method was cost utility analysis.Each study chose the partitional survival model to predict the distribution of individuals across health states.The time horizon ranged from 3 to 10 years.Only direct medical costs were included in model as cost input.The primary outcomes mainly included quality adjusted life years(QALYs) and life years (LYs).The characteristics of each study are shown in Table 2.
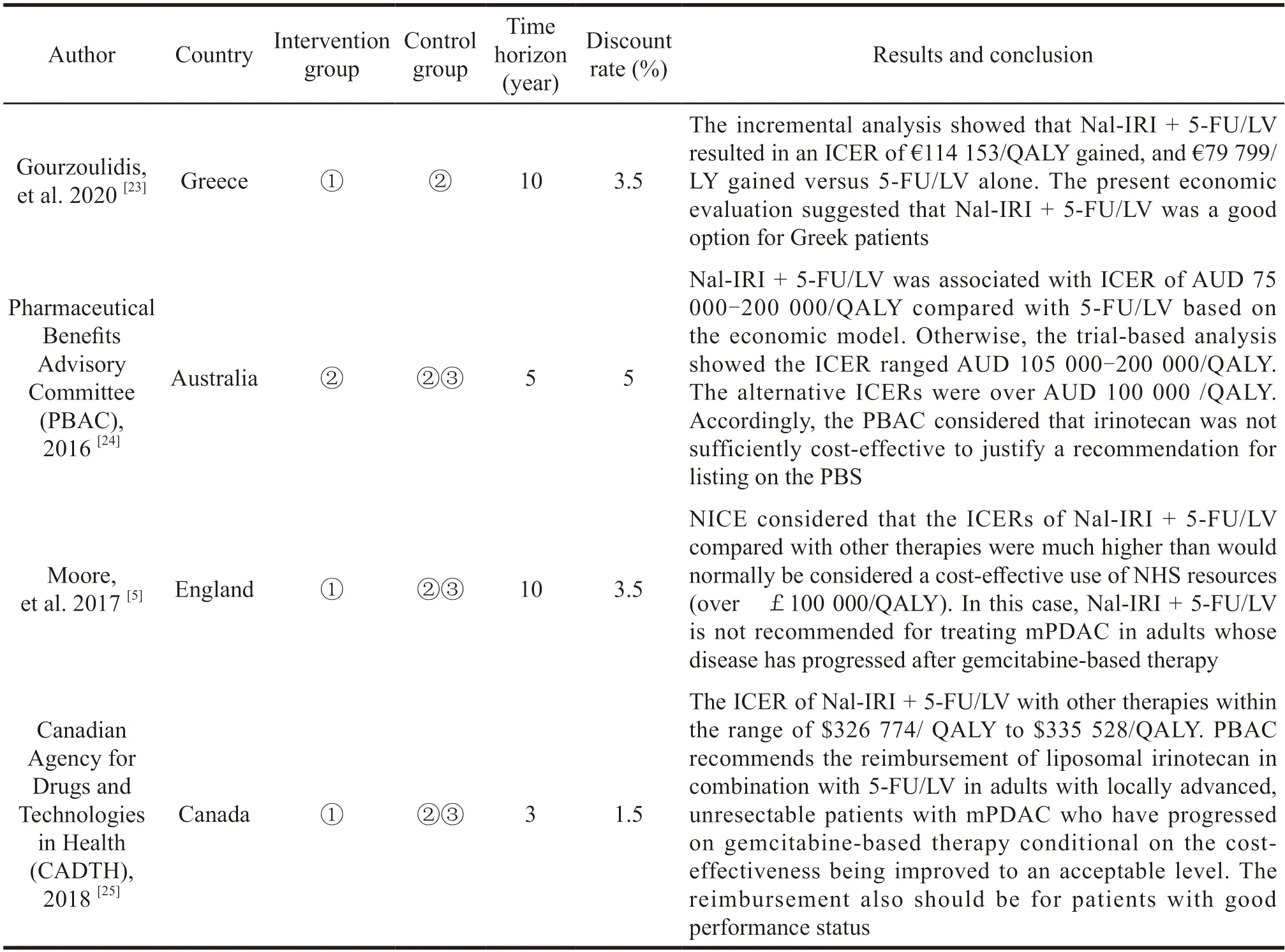
Table 2 Characteristic of included economic studies
Due to the differences in the economic level of various countries,the evaluation results of various countries have not reached a consistent conclusion.The analysis conducted by Gourzoulidis,et al.[23]approved that liposomal irinotecan combination regimen could provide survival benefits to patients with mPDAC after disease progression following gemcitabine-based treatment,demonstrating it had better clinical value and social value of the treatment for Greek patients.It also showed the most infiuential parameter on the model was utility values assigned to the pre-progression state.Canadian Agency for Drugs and Technologies in Health (CADTH)[25]recommended the reimbursement of liposomal irinotecan in combination with 5-FU/LV in specific patients conditional on the cost-effectiveness being improved to an acceptable level.However,both the National Institute of Health and Clinical Excellence(NICE)[5]and the Pharmaceutical Benefits Advisory Committee (PBAC)[24]announced that the liposomal irinotecan combination regimen was not economic for mPDAC patients with the submitted price,and they did not recommend the listing of liposomal irinotecan in the reimbursement list for the time being.Remarkably,all HTA institutions considered that the indirect comparison of liposomal irinotecan combination regimen with oxaliplatin plus 5-FU/LV regimen was not appropriate for heterogeneities in the clinical trials.Clinical effectiveness results of oxaliplatin plus 5-FU/LV regimen in phase III clinicals trials (CONKO-003 and PANCREOX) were inconsistent.The role of oxaliplatin in patients with mPDAC that progressed on first-line gemcitabinebased therapy was therefore controversial.It is not reliable for using this controversial clinical data in cost-effectiveness analysis.
Based on our literature review,the primary methods and the parameters in the economic evaluation process of liposomal irinotecan combination regimen were determined,and logical framework of pharmacoeconomic evaluation was formed.Then,the evaluation system was built up.The evaluation process is as follows.Firstly,we should identify the target population,intervention and comparator treatments for economic evaluation based on PDAC diagnosis guidelines and published clinical trials.Secondly,the perspective of cost effectiveness analysis is determined according to the research purpose,and the method of pharmacoeconomic evaluation is determined based on the disease characteristics of mPDAC.Then,we can determine the type of model to simulate the disease transition of patient health status and refine the important parameters such as costs and utility involved in the evaluation process according to the characteristics of disease and treatments.Finally,the incremental cost-effectiveness is calculated to evaluate the economy of drugs.Sensitivity analyses are conducted in model input parameters to evaluate the uncertainty of pharmacoeconomic evaluation.The logical framework is shown in Fig.3.

Fig.3 Logical framework of pharmacoeconomic evaluation
3 Discussion and prospect
Clinical studies included in this paper confirmed the clinical advantages of liposomal irinotecan combination regimen compared with other regimens in patients with mPDAC who have failed to gemcitabine-based treatment.The adverse reactions caused by liposomal irinotecan combination regimen had no negative effect on health-related quality of life of patients[5,25],and the combination had a good drug compliance.In terms of economic evaluation,various studies at abroad have not reached a consistent conclusion,which may be affected by different economic levels of various countries and the high price of liposomal irinotecan.
The small sample size of clinical studies included in this paper brings limitations to the clinical conclusion.Moreover,the clinical research and pharmacoeconomic evaluations included are conducted in Western developed countries.In that case,the research conclusions are not fully applicable in China which belongs to developing countries.
The appearance of liposomal irinotecan provided a new choice for second-line treatment of mPDAC,which is beneficial to prolong the survival time and improve the quality of life for these patients.However,the research on liposomal irinotecan are at an early stage at present,there are still many problems to be solved.(1) There are few controlled clinical studies related to liposomal irinotecan,so more larger sample size clinical studies are needed to compare the clinical efficacy of this regimen with other second-line chemotherapy regimens in the future.(2) Most of the clinical studies included in this paper are carried out for patients in Western countries.In that case,there is a lack of further clinical research on efficacy and toxicity of liposomal irinotecan combination regimen in patients in mainland of China.At present,few clinical trials and bioequivalence studies of liposomal irinotecan for mPDAC have been conducted in several pilot cities in China.Therefore,it will be feasible to evaluate the clinical benefit of liposomal irinotecan regimen in Chinese patients in the future.(3) The incidence rate of PDAC is increasing in China,which brings heavy financial burden to patients.Therefore,it is suggested to conduct economic evaluation of liposomal irinotecan regimen according to the actual medical situation in China,which can assist doctors and patients to choose more cost-effective treatment plan.
- 亚洲社会药学杂志的其它文章
- Enlightenment and Suggestions on the Construction of Information Platform and Processing Mechanism of Drug Shortages in Developed Countries to China
- Investigation on the Current Situation of China’s DTP Pharmacy and Suggestions for Its Development
- Study on the Management of Chronic Diseases in American and British Community Pharmacy
- lnfluence and Suggestions on Trial lmplementation Measures for Early Settlement Mechanism of Drug Patent Disputes
- Enlightenment of COVID-19 Treated by Botanical Drugs on the Development of Drugs for Rare Diseases in China
- Present Situation and Thinking of the Evaluation and Management of Biomedical Projects in Shenzhen Industrial Park of Shenzhen-Hong Kong Cooperation Zone

