An Effective Osteogenesis Cryogel γ-PGA/HEMA/PEG Served as Rabbit Orbital Bone Defects Scaffolds*
XIONG Ke, LIU Chun-Tao, WU Zhao-Ying, ZHANG Wei, ZHANG Chao
(1)School of Biomedical Engineering, Sun Yat-sen University, Shenzhen518107,China;2)Department of Ophthalmology, Nanfang Hospital, Southern Medical University, Guangzhou510515,China;3)Department of Outpatient, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou510080,China)
Abstract Objective Orbital bone fracture has become very common in recent years, and its related treatments and therapies aim at repairing the defects. Mineralized poly (γ-glutamic acid)/2-hydroxyethyl methacrylate/poly(ethylene glycol) (γ-PGA/HEMA/PEG) polymeric cryogel is a new type of scaffolding material with an interconnective porous structure. The study aimed to examine its efficacy in the repair of orbital bone defects. Methods The γ-PGA/HEMA/PEG polymeric cryogel was prepared by the cryogelation technique. Orbital bone defects were prepared on twenty-four New Zealand white rabbit. Three groups were made depending on the implanted materials: (1) blank control group; (2) polymeric cryogel group (Gel group); (3) mineralized polymeric cryogel group (M-gel group). Specimens were taken 8 weeks and 16 weeks after implantation for gross observation, micro-computed tomography (μ-CT) and hard tissue grinding slices and tissue sections were used to observe the osteogenesis outcome.Results Radiographic results showed that the mineralized cryogel could effectively facilitate the repair of the orbital bone defect completely, with the defective area completely replaced by bone tissue. Histological results proved that the mineralized polymeric cryogel scaffolds could increase the expression of runt-related transcription factor 2 (Runx-2), alkaline phosphatase (ALP),osteopontin (OPN), and platelet endothelial cell adhesion molecule-1 (CD31), which indicated the strengthened angiogenesis and osteogenic capability after the mineralized cryogel transplantation. Conclusion The mineralized polymeric cryogel served as a potential engineering scaffold in the repair of orbital bone defects via angiogenesis and osteogenesis.
Key words repair, orbital bone fracture, polymeric cryogel scaffolds, mineralization, osteogenesis
The orbit is a vital structure that not only could protect the eyeball but also help maintain normal eye function. It is composed of seven non-weight-bearing bones: the frontal, lacrimal, ethmoid, sphenoid,zygomatic, maxillary, and palatine. The edge of the orbital bone is irregular in shape, and it participates in shaping the lineament. The application of external stress on the cancellous orbital bone could easily create fracture at the weakest position, leading to the orbital defect. The purpose of the treatment of the orbital fracture is to repair the orbital bone defect[1-2].
At present, the most common method in clinical treatment is to implant the repair materials, such as natural materials or artificial materials[3-4]. Cancellous bone is a commonly used form of autogenous bone granting and it has outstanding performance in terms of osteoconduction, osteoinduction, and osteogenesis.But the autogenous bone also has boundedness, for example, the resources of the autogenous bone are limited, and may have the risk of bleeding, infection,and chronic pain. Allogeneic bone has a wider source and provides good osteoconduction, but bad osteoinduction and osteogenesis. In addition, the allogeneic bone also has the risk of HIV or hepatitis B and the potential of immunological rejection[5-8]. The limited availability of autogenous bone and allogeneic bone has become one of the major obstructions in clinical practice. So there is an urgent need to find a new graft that features well in histocompatibility and biomechanics and has the characteristics of osteoconduction, osteoinduction, and osteogenesis.Synthetic materials are employed as alternative bone substitutes to repair a large-scale range of orbital bone defects, such as hydroxyapatite (HA), high-density polyethylene (HDPE), calcium phosphate cement,etc[9-12].
Synthetic hydrogels have been extensively utilized as scaffolding materials in bone repair/regeneration[13]; however, the widely studied inert hydrogels are not able to induce appreciable mineralization that is essentially important for osteoinduction and osteogenesis. To this end, studies have been performed to improve the binding affinity between hydrogels and minerals[14]. For example, it was reported that the modification of poly(2-hydroxyethyl methacrylate) (pHEMA) with carboxyl groups could effectively increase the deposition of calcium[15]. Substrates containing biomimetic mineral-nucleating amino acid ligands could also effectively control the morphology of the mineral, some studies have indicated that crosslinked poly(γ-glutamic acid) (γ-PGA) was shown to induce the heterogeneous nucleation of hydroxyapatitein vitro[16-17]. In addition, insufficient mass transportation condition of hydrogel, which is characterized by a closed porous structure, may hinder the threedimensional homogenous mineralization inside the hydrogel scaffold; scaffolds with interconnective open-pores may better satiate the need for mineralization as well as cell migration/proliferation.
Polymeric cryogel is fabricated through the cryogelation technique, and is marked by excellent permeability due to its unique inter-connective porous structure; in addition, polymeric cryogel could be easily functionalized to offer appreciable protein adsorption and cell adhesion, and has demonstrated to be prominent scaffolding materials in the regeneration of bone, cartilage, neuron, and liver,etc[18]. The inter-connective porous structure of polymeric cryogel enables fast transportation of nutrients, oxygen, and metabolite. In terms of bone tissue engineering,physical mixing of the cryogel with bioactive minerals or mineralization of the cryogel may endow polymeric cryogel with osteoinductivity and/or osteoconductivity. In recent years, polymeric cryogel has shown great potential in the repair of defects of limb bone with good osteoinductivity and osteogenic capability[19]. Our previous report has polymeric cryogel can induce osteogenic differentiation of rMSC by activating the BMP/Smad and the FAK-ERK1/2 signaling pathways[20-22].
In this work, we introduced γ-PGA into the hydroxyethyl methacrylate/ethylene glycol(HEMA/PEG) backbone to prepare a co-polymer cryogel,namely, methacrylate poly (γ-glutamic acid)/hydroxyethyl methacrylate/poly(ethylene glycol)diacrylate (γ-PGA/HEMA/PEG) cryogel. Then, the γ-PGA/HEMA/PEG cryogel was mineralizedin vitroto form a mineralized cryogel. To assess the therapeutic potential of mineralized polymeric cryogel scaffolds for orbital bone defect repair, we analyzed its angiogenic and osteogenesis abilityin vitro. In addition, the three dimensional-computed tomography(3D-CT) reconstruction and micro-CT (μCT) analysis were applied to analyze the defect repair efficiencyin vivo. These mineralized polymeric cryogel scaffolds would effectively facilitate the regeneration of criticalsized orbital defects in a rabbit orbital defect model.We hypothesized that the mineralized polymeric cryogel scaffolds could boost the orbital defect repair through the angiogenic and osteogenesis dual-lineage effect (Figure 1) .
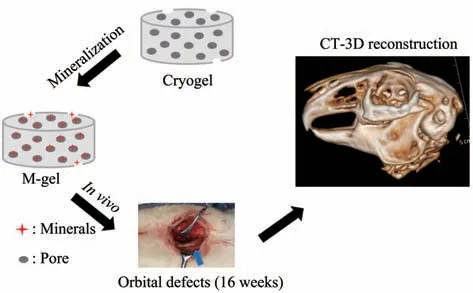
Fig. 1 Schematic illustration of the mineralized polymeric cryogel scaffolds repairs rabbits orbital bone defects
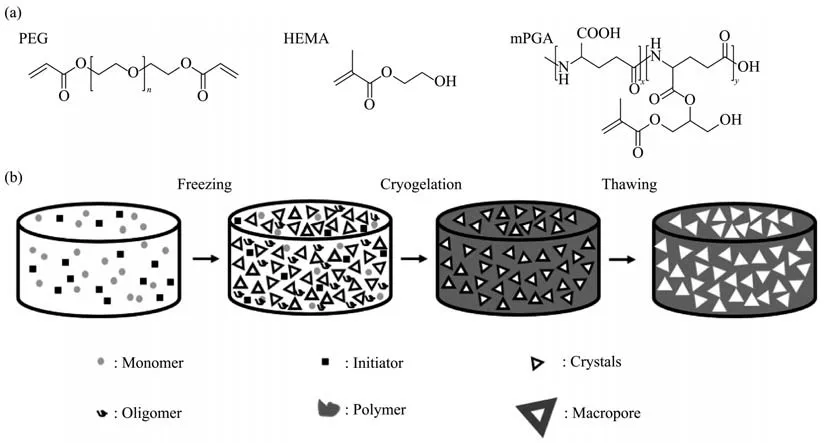
Fig. 2 The structures of PEGDA, HEMA & mPGA (a) and the process of cryogel preparation (b)
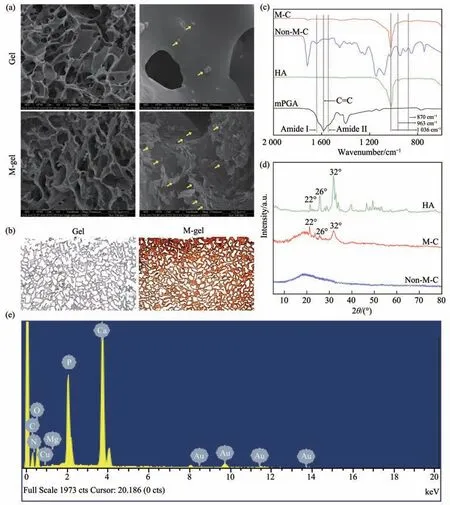
Fig. 3 Characterization of polymeric cryogel scaffolds
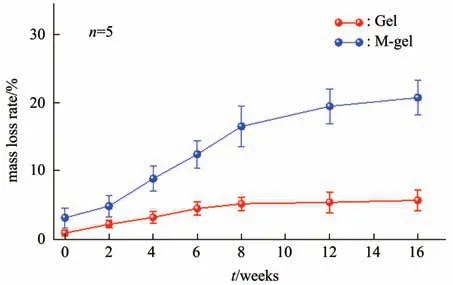
Fig. 4 Mass changes during degradation of cryogel in vitro
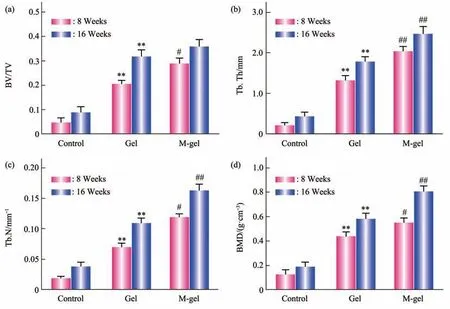
Fig. 5 Microstructure analysis of bone tissue in the defect sites
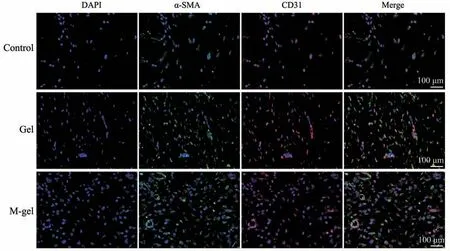
Fig. 6 Immunofluorescence staining of DAPI, CD31, and α-SMA in the orbital bone defect by implantation of Gel and M-gel scaffold for 8 weeks, respectively
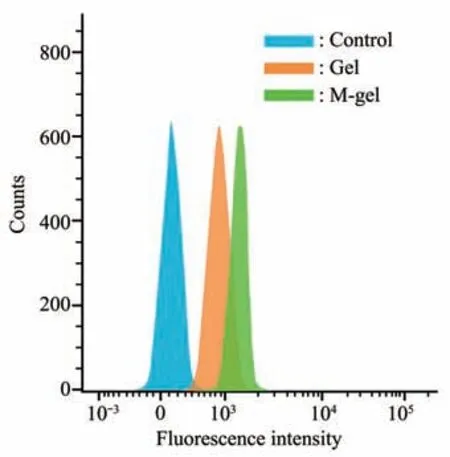
Fig. 7 Quantification fluorescence intensity of APC-conjugated CD31 treated with Gel and M-gel for 8 weeks were analyzed by flow cytometer
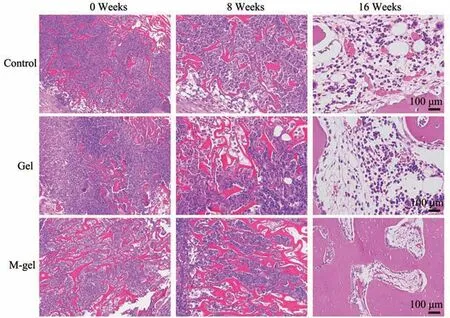
Fig. 8 Hematoxylin and eosin staining of orbital defect tissue sections after 0, 8, and 16 weeks implantation of the materials
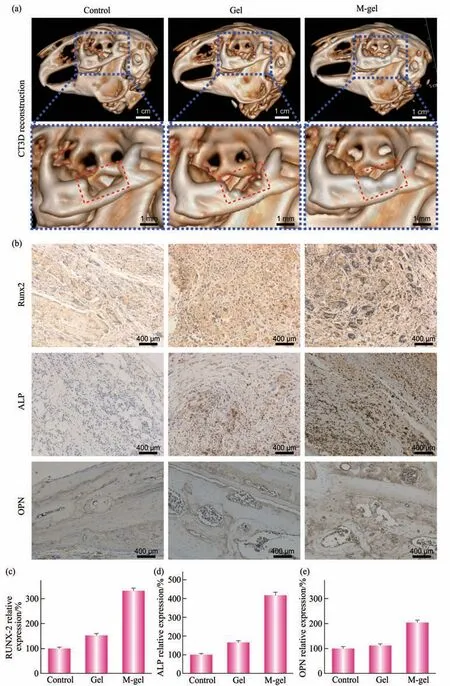
Fig. 9 M-gel promoted the repair of the orbital bone defects
1 Materials and methods
1.1 Materials
Methacrylate poly (γ-glutamic acid) (mPGA,molar mass 1.0×106g/mol, 10.0% of degrees of substitution of methacryloyl groups), and poly(ethylene glycol) diacrylate (PEGDA, molar mass 3 400 g/mol, degrees of substitution of acryloyl groups of 97.5%) were synthesized following previous reports[23-24]. Phosphate-buffered saline(PBS), simulated body fluid (SBF), and 40 mmol/L Ca2+/24 mmol/L HPO42-immersion solution were prepared according to the established protocols[17,25].Tetramethylethylenediamine (TEMED, Aladdin Industrial Co.), 2-hydroxyethyl methacrylate (HEMA,98%, Aladdin Industrial Co.), and ammonium persulphate (APS, Guangzhou Chemical Reagent Factory, China) were used as received. Other chemicals in this study were of analytical grade and were applied without further purification.
1.2 Fabrication of polymeric cryogel scaffolds
γ-PGA/HEMA/PEG cryogel was fabricated according to previously reported methods[21], using phosphate-buffered saline containing 1.0 mol/L of sodium chloride as the reaction media. Briefly, a solution of 0.1% (w/v) of mPGA, 5% (w/v) of PEGDA3.4k, and 10% (w/v) of HEMA in the reaction media were chilled to 4℃. To this solution, 0.1%(v/v) of TEMED and 0.5% (w/v) of APS were orderly added and vortexed. A certain amount of the mixture solution was quickly transferred to a plastic straw as the mold and polymerized at -20℃ for 8 h, and then at -16℃ for 72 h. After the cryogelation, the product was thawed in deionized water and placed in the shaker (100 r/min) at 25℃ for 24 h, the deionized water was for three times to remove the residual monomer, precursor, and initiator. Forin vitromineralization, the cryogel was autoclaved and equilibrated in sterile phosphate-buffered saline for 24 h; then the materials were immersed in 40 mmol/L Ca2+/24 mmol/L HPO42-solution for 3 h, followed by incubation in simulated body fluid for 16 h, and then in fresh simulated body fluid for another 16 h. The mineralized cryogel in this study were the products after two immersion processes. The mineralized polymeric cryogel scaffolds were presented as M-gel,and the non-mineralized polymeric cryogel scaffolds were termed Gel in the following context. The final products were storage at normal temperature(Figure 2) .
1.3 Characterization of polymeric cryogel scaffolds
The chemical structures of the cryogels or mineralized cryogels were confirmed by Fourier transform infrared (FT-IR) spectroscopy. The materials were homogenized and lyophilized. The spectra were recorded over a range of 4 000-400 cm-1with the Bruker Vertex 70 spectrometer (Bruker,Germany). The acquired dry powder was compressed into a thin film and analyzed with the Rigaku X-ray diffractometer (Cu Kα1) (energy: 36 kV, current:30 mA, rotating rate:4°/min, and angular range (2θ):5°-80°).
Equilibrium water content (EWC) of the polymeric cryogel scaffolds was measured gravimetrically using the following equation[22]:
WhereMsandMdare the weight of the swollen cryogel and the dry cryogel respectively.
The porosity of the cryogel was calculated according to Archimedes’ principle using a gravity bottle. Briefly, first, the dry weight of cryogel sample was recorded, followed by soaking the cryogel sample in cyclohexane filled in a specific gravity glass bottle and recording the submerged weight of the cryogel sample. The cryogel was then taken out and the weight of the cryogel (containing cyclohexane in the void volume) was recorded. The values taken at each step were substituted in the given equation to calculate the porosity of the cryogel.
WhereMwis cyclohexane saturated cryogel.Mdis the dry mass of the cryogel andMsubis the submerged mass of the cryogel.
Freeze-dried cryogels (n=4) with known mass(Md) were immersed in PBS in a water bath at 37℃under mild shaking, and the solution was changed every 48 h. Samples were taken out at a predetermined time points (0, 2, 4, 6, 8, 12, and 16 weeks), washed with ultra-pure water 10 times to remove the residual enzymes and degradation products, air-dried, and weighed (Mdt). The mass loss rate was calculated according to the following equation:
The cross-sections of the cryogel were observed,briefly, and the paraffin sections of cryogel (thickness:10 μm) were prepared using a microtome, stained with Alizarin red, and photographed digitally under a light microscope (×7; Olympus, Tokyo, Japan). The pore size of the cryogel was measured manually using image analyzing software (Image-Pro plus 6.0, Media Cybernetic, Silver Springs, MD, USA).
On account of the dimensions of these cryogel reductions after drying, lyophilization was performed for purpose of obtaining the correct morphological information. The cryogel samples were prepared from the monoliths, which were vacuum-dried at -50℃using a lyophilizer (Martin Christ GmbH, Germany).The gold coating of the cryogel was performed by mounting them on the base plate of a sputter coater(Vacuum Tech, Bangalore, India). The topographical structure of the cryogel was examined by scanning electron microscope (SEM, Thermal field emission environmental SEM-EDS-EBSD, Quanta 400 FEG,FEI/OXFORD/HKL, France) at a high vacuum(20 kV) with a spot size of 3.5-4.5 mm varied from sample to sample.
Minerals in the mineralized cryogel were measured by Scanning electron microscopy-energy dispersive spectroscopy (SEM-EDS). Calcium deposition in cryogel was also stained with Alizarin Red S and analyzed under the optical microscope. The swollen cryogels (n=6) were trimmed into a disc shape with diameter of 6 mm and height of 4 mm for uniaxial static compression test at a strain rate of 0.1 mm/min on an LR10KPlus mechanical analyzer(LLOYD, UK)[22].
1.4 Rabbit model establishment of repair of orbital bone defects
In the present study, a total of 24 five-month-old New Zealand white rabbits (bodyweight 2.0-3.0 kg,Center of Experimental Animals, Southern Medical University, Guangzhou, China) were randomized into three groups (n=8 in each group)[26]. To build the orbital bone defect, the rabbits were anesthetized with intramuscular droperidol (0.25 mg/kg), intravenous pentobarbital (20 mg/kg), and general 2% isoflurane(v/v), in a lateral decubitus position. When skin preparation and draping were completed, muscle along the inferior margin eyelid was cut horizontally.The operation region was marked with the methylene blue pen, then the bone tissue (12 mm) along the inferior margin of the orbit was cut off with an electrosaw. Because the inferior margin of the orbit is mainly composed of the zygomatic bone, the bone defect model of zygomatic bone (length: 12 mm, width:6 mm, and thickness: 2 mm) was built on all of the 24 rabbits and then randomized into three groups: Group A, self-control group without any treatment (Control group); Group B, which was the treatment group with non-mineralized polymeric cryogel scaffolds (Gel group); Group C, which was the treatment group with mineralized polymeric cryogel scaffolds (M-gel group). After bleeding was stopped with a gelatin sponge, corresponding prepared implants were implanted into the orbital defect sites to fill the whole area. Subsequently, a 5/0 suture was employed to suture the incision on the skin. The three groups of rabbits were kept in different cages in the SPF laboratory. There is no material pull-out, migration, or incision infection during the period of feeding. The protocols of animal study were authorized by the Committee on Ethics of Animal Experiments of Southern Medical University and carried out according to the institutional guidelines (No.NFYY-2019-73).
1.5 Micro-CT analysis
Experimental rabbits were sacrificed at 8 and 16 weeks postoperatively. The separated cranium was scanned with μCT. The specimens were harvested and fixed in 4% of paraformaldehyde for 48 h. The bone microstructure of orbital bone was analyzed using μCT (SkyScan 1176 μ-CT system, Bruker, USA)(resolution: 18 μm, tube voltage: 80 kV, and a tube current: 313 μA). Bone morphometric parameters,including the bone volume to sample volume (BV/TV), the number of bone trabeculae per mm of tissue(Tb. N), the trabecular thickness (Tb. Th), and the bone mineral density (BMD) were analyzed and calculated based on the region of interest (ROI) of the orbital bone defect. The technician conducting the scan analysis was unaware of the treatments associated with the specimens[27-28].
1.6 CT 3D reconstruction
To investigate the gross morphologies, the samples were scanned using CT and reconstructed into 3D images. Orbital CT scan was performed under anesthesia before sacrifice at 16 weeks with Philips Brilliance scanners (Philips, Netherlands). CT images were reconstructed with a slice thickness of 1 mm and a 512×512 matrix. CT images were preprocessed via the bone window setting (W: 1 500 HU,L: 450 HU)and resampling resample to isotropic 1 mm voxels.The 3D reconstruction technique was used to visualize the degree of orbital defect repair.
1.7 Histological analysis
The isolated orbital bone of each group was processed for histology and immunohistochemistry.Hematoxylin and eosin (H&E) staining of histological slices was carried out at room temperature to observe the new bone regeneration conditions. Tissue samples were fixed in 4% paraformaldehyde and stored for 24 h, then decalcified with 10% EDTA solution for 3 weeks at room temperature and embedded in paraffin before being sectioned, and sliced into 5-μmthick transverse sections following the standard method using a rotary microtome (RM2255, Leica,Hamburg, Germany). ALP is a marker for early osteocytic differentiation, in addition, Runx-2 and OPN are important expressions of osteogenesisrelated proteins. Then the slices were incubated with primary antibodies against Runx-2, ALP, and OPN at 4℃ overnight according to established protocol. After staining, the histological sections were viewed on the optical microscope (DM5000B, Leica, Germany)under a bright field.
1.8 Immunofluorescence double staining
The sections assessed by immunofluorescence were blocked by incubation with 5% (w/v) BSA,incubated with primary antibodies for Anti-CD31 antibody (Abcom, ab7388), SMA skeletal muscle actin monoclonal antibody (Thermo Fisher, AB_10984949), incubated with corresponding secondary antibodies conjugated to Alexa Fluor®647 and Alexa Fluor 488 fluorescent dye (Abcom, ab150167, and Thermo Fisher, AB_2534069). Immunofluorescence staining of CD31 and α-SMA was used to assess the angiogenesis of tissue. Sections were visualized with the fluorescent microscope (IX71 Olympus, Tokyo,Japan).
For the analysis of CD31 expression in the orbital bone defect area, defect area tissues were collected from sacrificed experimental rabbits by removing the tissue around the bone. To obtain a single-cell suspension, the whole samples were digested for 30 min with collagenase at 37℃. The cells were counted and incubated at 4℃ for 45 min after filtration and washing. And then, cells were washed and further incubated with APC-conjugated CD31 (R&D Systems, FAB3628A) antibodies for 45 min at 4℃. The acquisition was performed on a FACScan cytometer (BD Immunocytometry Systems,USA) for demarcating and analyzing of CD31 positive cells.
1.9 Statistical analysis
One-way ANOVA tests were used to detect distinctions between groups. AP-value of less than 0.05 (P<0.05) was considered statistically significant.Data were analyzed using SPSS 22.0 statistical software (IBM, USA) and presented as mean±SD.Significance level was presented as either*P<0.05 or**P<0.01.
2 Results
2.1 Characterization of polymeric cryogel scaffolds
The fabrication and physicochemical characterization of the polymeric cryogel scaffolds has been reported in terms of EWC, porosity, pore diameter, and compressive modulus (Table 1). After mineralization, the EWC of mineralized polymeric cryogel scaffolds reduced to (87.3±0.5)% compared with that of the non-mineralized group (89.0±0.9)%;and the porosity of mineralized polymeric cryogel scaffolds (66.2±1.7)% was slightly lower than the non-mineralized group (68.6±0.3)% ; no obvious changes in the pore diameter between mineralized(53.0±35.6) μm and non-mineralized (62.6±45.6) μm polymeric cryogel scaffolds was observed. Moreover,due to the minerals deposition, the compressive modulus of mineralized polymeric cryogel scaffolds largely increased to (42.4±1.6) kPa, which was almost twice as much as that of the non-mineralized group(21.1±0.1) kPa.

Table 1 Physical properties of cryogels
SEM scanning displayed the pore structure of the polymeric cryogel scaffolds. Compared with nonmineralized polymeric cryogel scaffolds, the interconnectivity of mineralized polymeric cryogel scaffolds was maintained with a small variation in the structure of the pores. The highly inter-connective structure was retained after the mineralization of cryogel under SEM. Abundant cells were observed adhered to the surface of the mineralized polymeric cryogel scaffolds, compared with the non-mineralized group (Figure 3a).
In the mineralized polymeric cryogel scaffold group, the whole section was stained red. It indicated the minerals were deposited homogeneously in the polymeric cryogel scaffolds. Alizarin red S staining confirmed the deposition of minerals inside the pores of the scaffolds after the mineralization process(Figure 3b).
In the Fourier tranform infrared spectroscopy(FTIR) spectra image, we can find the disappearance of the peak for the C=C bond (~1 600 cm-1) and the appearance of the amide peaks (~1 650 cm-1and~1 550 cm-1). It indicated that γ-PGA was successfully incorporated into the matrix of cryogelviaa covalent bond. After mineralization, the intensity of peaks(~963 cm-1and ~1 036 cm-1) form the backbone of the polymeric matrix scaffolds diminished greatly,while the bands of PO43-groups appeared. Moreover,CO32-groups had been also successfully incorporated into the minerals (~870 cm-1) (Figure 3c).
In the X-ray diffraction (XRD) patterns, the prominent intensity of three peaks (22°, 26°, and 33°)in the mineralized polymeric cryogel scaffolds has similar to hydroxyapatite (Figure 3d). The presence of Ca and P in the minerals was identified by SEM-EDS,the Ca/P ratios on the surface of the mineralized polymeric cryogel scaffolds were 1.57, which was close to that of hydroxyapatite (1.67) (Figure 3e).
The mass changes of the cryogel scaffolds during degradation are shown in Figure 4, where the nonmineralized polymeric cryogel scaffolds and the mineralized scaffolds were slowly degraded with immersion time. At 16 weeks, the mass loss rate of the non-mineralized cryogel scaffolds was (5.7±1.6)% ;and that of the mineralized cryogel scaffold was(20.8±2.6)%, which is much higher than the nonmineralized ones.
2.2 Microstructure analysis of the new bone tissue
The microstructure analysis was carried out on the newly formed bones in the orbital defect sites using the CTAn software (V1.14.4) (Figure 5). The BV/TV, Tb. N, Tb. Th, and BMD of newly formed bones in the polymeric cryogel scaffold groups(Group B & C) was significantly increased, compared with the control blank group (P<0.05). It indicated that polymeric cryogel scaffolds promoted bone regeneration in bone defect repair. Additionally,compared with the non-mineralized cryogel group, the bone volume of newly formed bones in the mineralized cryogel group was further increased (P<0.05). It indicated that mineralization enhanced bone regeneration in the orbital bone defect.
2.3 Angiogenesis in vivo
To further probe the changes in the orbital bone defect model microenvironment after implant surgery,we detected the expression of CD31 and α-SMA to evaluate the formation of new blood vessels. The expression of CD31 and α-SMA was less in the bone defect repair by the control group and the nonmineralized polymeric cryogel group (Figure 6).However, the co-localization of CD31 and α-SMA positive staining treated by the mineralized polymeric cryogel scaffolds group was significantly more than the other groups. The results demonstrated that the mineralized polymeric cryogel scaffolds had a significant effect on promoting angiogenesis during the bone defect repair process.
The FACS analysis suggested that CD31 protein expression was significantly increased in the mineralized cryogel group compared with the nonmineralized group, indicating that the mineralized cryogel scaffolds have a positive regulatory role during angiogenesis. Obviously, the results suggest that the mineralized polymeric cryogel scaffolds can promote endothelial cell overexpression of CD31 protein and consequently promote angiogenesis in the orbital defect region (Figure 7).
2.4 CT 3D reconstruction of the orbital defect
The morphology of newly formed bone is an important feature that is used to identify bone formation. The conditions of newly formed bones in the orbital bone defect site were detected with the CT 3D reconstruction technique. The 3D images (Figure 9a) described the different reparative effects of the three groups. No obvious bone formation was seen in the blank control group. Rabbits in Group B,implanted with the non-mineralized polymeric cryogel scaffolds in the defect site, depicted bits of scattered newly formed bones at the edge of orbit.Rabbit in Group C in the defect site presented an obvious increase in newly formed bones compared with the other groups, which almost filled the whole orbital bone defect site.
2.5 H&E staining and immunohistochemical results
In order to detect the host response to the implant and the new bone formation, H&E staining and immunohistochemical staining were carried out to look into the bone regeneration conditions in the orbital bone defect sites. The H&E staining results showed that when compared with the control blank group (Group A), the polymeric cryogel scaffold groups (Group B, C) could significantly induce bone formation and matrix mineralization. In the orbital bone defect sites from the three groups, Group C showed regeneration of bone tissues, and even staining of newly formed bone tissues was noted. A certain amount of erythrocytes were observed inside and surrounding newly formed bones and scattered in new blood vessels. Group B displayed minimal regeneration of bone tissues except for some bone cells, and Group A showed no regeneration of bone tissue. While Group C displayed an even stronger mineralization ability than Group B. It was also noticed that the defect area was significantly reduced after 16 weeks (Figure 8).
In addition, we also examined the expression of osteogenic proteins of each group and found that positive staining of Runx-2, OPN, and ALP in cryogel scaffold groups increased notably as compared with the control blank group. It’s worth noting that the mineralized polymeric cryogel scaffolds further enhanced the ability (Figure 9b-e). It indicates that the mineralized polymeric cryogel scaffolds may promote bone regeneration during the repair of bone defects.
3 Discussion
The frontal, zygomatic, maxillary, sphenoid,ethmoid, palatine and lacrimal bones contribute to the structure of the orbit. Orbital bone fractures include fractures of these bones. With the increasing occurrence rates of the traffic accidents and industrial injuries in recent years, the incidence of orbital bone fracture defects also rises as well. The orbital bone defect is a common disease in ophthalmology clinics and a challenge in bone repair treatment as it is difficult to regenerate bone loss and reconstruct bone function. Although bone autograft and allograft have been extensively used in bone repair, there is the urge for the development of biomaterials that could not only overcome the shortcomings of autograft and allograft but also facilitate the regeneration/reconstruction of new bone tissue[1,3,9].
To this end, polymeric cryogel with a unique interconnective porous structure and desired functionality may be harnessed as scaffolding materials in bone regeneration[15]. The polymeric cryogel could be modified by various methods to acquire specific functions,i.e., osteoinductivity and osteoconductivity. Mineralization has been a demonstrably effective and convenient method to introduce bioactivity and osteoinductivity to microporous scaffold materials. Previousin vitrostudies[17]have shown mineralized polymeric cryogel promotes osteogenic differentiation of mesenchymal stem cells (MSCs). The adsorption, entrapment, and concentration of vascular endothelial growth factor(VEGF) and bone morphogenetic protein 2 (BMP-2)in the matrices may participate in the process of angiogenesis and osteogenesisin vivo.
In this study, the polymeric cryogel maintains appreciable porosity and compressive modulus as well after mineralization. In addition, the higher mass loss rate of the mineralized cryogel scaffolds was evidenced, and the minerals deposited in the polymeric cryogel contain hydroxyapatite, carbonated apatite, and calcium phosphate, suggesting such the mineralized polymeric cryogel could be used as a potential bone tissue engineering material. Further studiesin vivoare needed to prove thein vitrofindings, the tests were performed by implanting the polymeric cryogel scaffolds in rabbit orbital bone defects sites. Our study has confirmed that the implantation of the mineralized polymeric cryogel could promote osteogenesis in the animal orbital bone defect model at the studied time point. As for the nonmineralized polymeric cryogel scaffolds group, the microstructure analysis showed that much more new bones were detected in the mineralized polymeric cryogel scaffolds group, as confirmed by the quantificational results of the BV/TV, Tb. N, Tb. Th and BMD, indicating the mineralized cryogel scaffolds could repair the bone defect effectively. We observed that the new bone was almost filled the orbital bone defect in the mineralized polymeric cryogel scaffolds group in the image of CT 3D reconstruction. Angiogenesis is essential for the repair of bone defect. The results of immunofluorescence staining and flow cytometry demonstrated that the mineralized polymeric cryogel scaffolds could considerably accelerate the new bone formation.Histological analysis showed that in the control group,even though there was the minimum formation of new bone, the bony connection could not be detected and the defect area was filled by connective tissue,indicating the failure of repair of the defect. In comparison, the mineralized cryogel group achieved the significant formation of new bone tissue. In the meantime, the elevated staining of Runx-2, OPN, and ALP in the mineralized polymeric cryogel scaffolds group compared to the other groups unambiguously suggested the enhanced expression of osteogenic factorsin vivo. The mineralized polymeric cryogel scaffolds used in the present study displayed admirable and positive histocompatibility and osteoinductivity, which could markedly enhance novel bone formation and bone regeneration when implanted into the orbital bone defect sites.
In the present study, the mineralized polymeric cryogel scaffolds were implanted into defect sites in rabbit models of orbital bone defects. Whereafter, the regeneration and volume of newly formed bones were demonstrated to be obviously increased compared with the control groups and non-mineralized cryogel groups. These analyses presented that mineralized polymeric cryogel had more extensivein vivobone formation compared with non-mineralization.Multiple factors endow these positive outcomes. First,polymeric cryogel is characterized by appreciable protein adsorption and cell adhesion as a result of its unique inter-connective porous structure, recruiting more osteoblasts to accelerate bone regeneration.What’s more, mineralization played an indispensable role in osteogenic differentiation, which facilitated the new bone regenerationin vivo.
4 Conclusion
In brief, we developed the mineralized polymeric cryogel scaffolds as novel orbital bone repair biomaterials. The material had an inter-connective porous structure that enables fast transportation of nutrients, oxygen, and metabolite. The mineralized polymeric cryogel scaffolds had excellent biocompatibility. As compared with the nonmineralized polymeric cryogel scaffolds, the expression of osteogenic-related genes (Runx-2,OCN, and ALP) was remarkably up-regulated by the mineralized polymeric cryogel scaffolds. Additionally,the mineralized polymeric cryogel scaffolds accelerated bone regeneration and angiogenesis in the rabbit orbital defect sites. This study has demonstrated that mineralized polymeric cryogel scaffolds could be harnessed in the repair of orbital bone defects effectively, providing great clinical significance, and the novel material maybe has broader application in clinical practice.

