A Novel O2-conotoxin Tx7.29 That Inhibits Calcium Currents and Presents Analgesic Activity*
WU Yun, YANG Man-Yi2), ZHANG Wei, ZHOU Mao-Jun4), CAO Kun
(1)Guangdong Provincial Key Laboratory of Medical Molecular Diagnostics, The First Dongguan Affiliated Hospital,Guangdong Medical University, Dongguan523808,China;2)Department of Hepatobiliary and Pancreatic Surgery, NHC Key Laboratory of Nanobiological Technology, Xiangya Hospital,Central South University, Changsha410008,China;3)Institute of High Energy Physics, Chinese Academy of Sciences, Beijing100049,China;4)Department of Oncology, NHC Key Laboratory of Cancer Proteomics, State Local Joint Engineering Laboratory for Anticancer Drugs,Xiangya Hospital, Central South University, Changsha410008,China)
Abstract Objective The venom of carnivorous cone snails provides a valuable source of biologically active peptides, which are composed of a complex mixture of disulfide-rich neurotoxins, commonly known as conotoxin. In this work, a novel O2 superfamily conotoxin Tx7.29 was reported, and through functional research, it is expected to discover a new analgesic drug candidate.Methods The cDNA sequence of Tx7.29 was obtained from the venom duct cDNA library of the molluscivorous Conus textile collected from the South China Sea. The mature peptide Tx7.29 with modified amino acids and disulfide bonds was synthesized and identified by mass spectrometry. Patch clamp and animal experiments were used to determine the biological function of Tx7.29.Results The cDNA of Tx7.29 encodes a 68 amino acid residues conotoxin precursor, which consists of 19 residues in the signal peptide, 28 residues in the pro-region and 22 residues in the mature peptide. Circular dichroism (CD) spectra showed that β-turn and antiparallel sheet structures were dominant contents in Tx7.29. Patch clamp experiments on the rat DRG neurons showed that Tx7.29 could significantly inhibit calcium currents, but it had no obvious effects on the sodium and potassium currents. Tx7.29 increased the hot plate latency from 0.5 to 4 h in a dose dependent manner in the mice hot plate assay and had low toxicity to ND7/23 cells.Conclusion This novel conotoxin Tx7.29 may be a useful tool for analgesic drug development and could expand our visions of the molecular targets of O2-conotoxins.
Key words conotoxin, O2 superfamily, Tx7.29, Conus textile, calcium currents, analgesic activity
Conotoxins or conopeptides, secreted by the marine mollusk cone snails, are small peptides typically comprising 10-50 amino acids and 1-5 disulfide bridges[1-2]. Because of their high efficiency and specificity on targeting ion channels or neurotransmitter receptors, conotoxins are promising neuropharmacology tools and drug candidates[3]. The ω-conotoxin GVIA was the first O superfamily conotoxin with definite pharmacological activity,reported in 1984[4]. And another O-conotoxin ω-MVIIA (Ziconotide) was approved by USA Food and Drug Administration (FDA) for treating intractable chronic pain in 2004[5]. Up to now, 2 986 nucleic acid sequences, 8 362 protein sequences and 232 structures of conotoxins have been collected by ConoServer, a database of conotoxins[6].
Based on the conserved signal peptide sequence in the precursor, conotoxins can be divided into 30 gene superfamilies[6]. The O gene superfamily was first named in 1995, which mainly include the cysteine framework VI/VII[7]. While in 2006, O superfamily was divided into 3 new superfamilies(O1, O2, O3) according to their different signal peptides[8]. Unlike the multifunctional O1 superfamily, there are only four O2-conotoxins’functions have been identified. O2-PnVIIA and O2-TxVIIA induced depolarization and increased firing of action potentials in some molluscan neuronal systems[9-10]. These two peptides have been classified as γ-family, because of their acting as the agonists of neuronal pacemaker cation currents. O2-Lt7a inhibited the voltage-sensitive sodium channel currents in rat dorsal root ganglion (DRG) neurons[11].O2-PiVIIA produced a significant increase in the Ca2+currents in the µmol/L range, without significantly modifying other currents[12].
In our previous work, a novel O2-conotoxin Tx7.29 (GenBank number: JX293454) was cloned from the cDNA library ofConus textileusing primers designed based on the signal peptide region and the 3'untranslated region (3'UTR) elements conserved in O2 superfamily[1]. The precursor of Tx7.29 comprises 69 amino acid residues, including a mature peptide of 22 amino acid residues (CSVWGPCTVNAECCSGDCHETC). In this work, we reported the synthesis,identification, and physiological functions of this O2-conotoxin. Tx7.29 was synthesized by solid-phase polypeptide synthesis and identified by mass spectrum and circular dichroism. Patch clamp on rat DRG cells showed that Tx7.29 could significantly inhibit the calcium currents. Moreover, Tx7.29 had showed analgesic effects in the mice hot plate assay. This novel conotoxin expands our visions of O2-conotoxins and their potential molecular targets.
1 Materials and methods
1.1 Specimen collection, cDNA cloning and sequence analysis
The specimen collection and cDNA cloning of Tx7.29 were performed as previously described[1]. To amplify the coding sequences of O2 superfamily conotoxins ofConus textile, PCR primers were designed to recognize conserved signal sequence and 3'UTR regions (forward primer: 5'-ATGGAGAAACTGACAATYCTGC-3'; reverse primer: 5'-GCCTTGAAGACTCTGAAGAGGA-3').
Gene superfamilies, signal peptides, and cleavage sites of conotoxins were predicted using the ConoPrec tool in ConoServer (http://www.conoserver.org) and the SignalP algorithm (http://www.cbs.dtu.dk/services/SignalP). Nucleotide and amino acid multiple alignments were generated using ClustalW and refined manually.
1.2 Peptide synthesis of Tx7.29
The mature peptide of conotoxin Tx7.29 was synthesized on a Rink amide resin using a standard Fmoc-strategy according to previously reported methods[13]. The three disulfide bridges are protected by Trt, Acm, and Dbs, respectively. After 3 steps oxidation, the mature peptide was purified by reversephase high-performance liquid chromatography(RP-HPLC) and the molecular mass was confirmed by mass spectrometry analysis. After purification by HPLC, the purity of synthetic Tx7.29 was more than 98%.
1.3 Circular dichroism measurement
Circular dichroism (CD) spectra were measured by a Chirascan spectropolarimeter instrument(Applied Photophysics, England). The purified Tx7.29 was dissolved in PBS buffer to a final concentration of 0.1 g/L. The spectra were recorded over a 180-260 nm range at 20°C using an average of 5 scans (scan speed 100 nm/min). The percentages of protein secondary structure were estimated using a Kohonen neural network with a 2-dimensional output layer by DicroProt[14].
1.4 Whole-cell patch clamp for DRG cells
Acutely separated DRG cells were isolated as previously described[15]. SD rats (30 d old) were purchased from Guangzhou University of Chinese Medicine Experimental Animal Center (No. SYXK(Yue) 2018-0182). All animal procedures were carried out according to the approved protocol(GDY2002208) of the Institutional Animal Care and Use Committee at the Guangdong Medical University.The rats were euthanized and the dorsal root ganglia tissue was removed quickly and cut into small pieces.The ganglia were treated with 0.1% collagenase and 0.05% trypsin. After centrifugation, the DRG cells were suspended in essential DMEM with 10% (v/v)fetal bovine serum.
For recording sodium currents, the intracellular solution contained the following composition:10 mmol/L CsCl, 5 mmol/L NaCl, 10 mmol/L HEPES, 2 mmol/L Mg-ATP, 135 mmol/L CsF,5 mmol/L EGTA, pH=7.2 (CsOH), and the extracellular solution contained the following composition: 22 mmol/L NaCl, 110 mmol/L cholinechloride, 5 mmol/L D-glucose, 10 mmol/L HEPES, 0.8 mmol/L MgCl2, 1.8 mmol/L CaCl2, pH=7.4 (NaOH). Peptide was administrated by continuous perfusion and 100 μmol/L CdCl2was used to inhibit calcium currents. To acquire current-voltage (I-V)relationships of sodium channels in DRG cells, test potentials ranged from -120 to +100 mV in 5 mV steps from a holding potential of -120 mV using EPC-10 (HEKA, Germany).
For recording potassium currents, the intracellular solution contained the following composition: 120 mmol/L KCl, 1 mmol/L MgCl2,5 mmol/L EGTA, 14 mmol/L phoshocreatine disodium salt, 5 mmol/L Na2-GTP, pH=7.2 (KOH),and the extracellular solution contained the following composition: 1.8 mmol/L CaCl2, 135 mmol/L cholinechloride, 10 mmol/L D-glucose, 10 mmol/L HEPES, 1 mmol/L MgCl2, 4.5 mmol/L KCl, pH=7.4(KOH). To acquire current-voltage (I-V) relationships of potassium channels in DRG cells, test potentials ranged from -80 to +80 mV in 5 mV steps from a holding potential of -80 mV using EPC-10.
For recording calcium currents, the intracellular solution contained the following composition:120 mmol/L CsCl, 1 mmol/L MgCl2, 10 mmol/L HEPES, 4 mmol/L Mg-ATP, 0.3 mmol/L Na2-GTP,10 mmol/L EGTA, pH=7.2 (CsOH). The extracellular solution contained the following composition:140 mmol/L TEA-Cl, 2 mmol/L MgCl2, 5 mmol/L D-glucose, 10 mmol/L HEPES, 10 mmol/L CaCl2,pH=7.4 (NaOH). To acquire current-voltage (I-V)relationships of calcium channels in DRG cells, test potentials ranged from -60 to +40 mV in 5 mV steps.
1.5 Analgesic activity bioassays
Female Kunming mice (body mass 18-22 g) were purchased from Guangzhou University of Chinese Medicine Experimental Animal Center (No. SYXK(Yue)2018-0182). All animal procedures were carried out according to the approved protocol(GDY2002208) of the Institutional Animal Care and Use Committee at the Guangdong Medical University.50 Kunming mice were randomly divided into 5 groups. Each mouse was intrathecally injected with 10 μl Tx7.29 (1, 10, 100 μmol/L), pethidine (positive control, 10 mmol/L), or 0.9% saline (negative control). Mice were placed on a hot plate (55°C) and the time until the mouse jumped or licked either of its hind paws was recorded as hot plate latency[16]. Hot plate latency was tested at 12 h before drug administration and 0.5, 1, 2, 3, 4 h after drug administration. Hot plate latency increment percentage= (hot plate latency after administration-hot plate latency before administration)/(hot plate latency before administration)×100%.
1.6 MTT cytotoxicity assay
The conotoxin Tx7.29 was examined for cytotoxic activities against ND7/23 cell lines, which were obtained from National Collection of Authenticated Cell Cultures (Shanghai, China).ND7/23 cells were grown in DMEM high glucose growth medium supplemented with 10% fetal bovine serum, 1% glutamax, 50 U/ml penicillin and 50 mg/L streptomycin at 37° C with a 5% CO2/95% air humidified atmosphere. Cytotoxicity assay was carried outin vitrousing MTT staining according to the procedures described by Al-Allaf and Rashan[17].The peptide concentrations used were 0, 0.01, 0.1, 1,10, and 100 μmol/L for each well, respectively. Three separate experiments were carried out, and six replicated wells were used to determine each point.After 48 h incubation, the cells were stained by MTT and placed in a BIO-RAD model 680 microplate reader to determine the absorbance at 490 nm.
1.7 Statistical analysis
Pvalues were calculated with the Student’st-test.P<0.01 was considered to be statistically significant andPvalues are designated as follows:*P<0.01,**P<0.001. All error bars in graphs represent the standard error of the mean calculated from at least 3 replicates.
2 Results
2.1 Sequence identification of the O2-conotoxin Tx7.29
The precursor peptide of Tx7.29 comprises 69 amino acid residues, including 19 amino acids in the signal peptide, 28 amino acids in the pro-region and 22 amino acids in the mature peptide (Figure 1a). The alignment of Tx7.29 with other O2-conotoxins showed that they all had a conserved motif-E(γ)CCS-(the glutamate before the third cysteine was carboxylated) (Figure 1b). According to the analysis of other conotoxins in O superfamily, the amino acid sequence of Tx7.29 should have the same disulfide connectivity as that of I-IV, II-V and III-VI.

Fig. 1 The precursor sequence of conotoxin Tx7.29 and the clustal alignment of eight O2 superfamily conotoxins

Fig. 2 Purification and identification of the synthetic Tx7.29

Fig. 3 Effects of Tx7.29 on DRG sodium,potassium and calcium currents

Fig. 4 Analgesic effect of Tx7.29 tested by the mice hot plate assay
2.2 Synthesis and identification of Tx7.29
The conotoxin Tx7.29 was synthesized on a Rink amide resin using a standard Fmoc-strategy.According to the sequence analysis of the known O2-superfamily conotoxins, we replaced three original amino acid residues of Tx7.29 with modified residues(Figure 1b). 6P (proline in the sixth position) was replaced by hydroxyproline (O); while 12E and 20E were replaced by γ-carboxyglutamate (γ). The final synthetic sequence of Tx7.29 is CSVWGOCTVNAγCCSGDCHγTC (Figure 2a). The oxidized peptide was purified by RP-HPLC (Figure 2b) and the molecular mass was confirmed by mass spectrometry (Figure 2c). Mass of the oxidized peptide was 2 522.2 u, which was consistent with the expected mass. CD spectra of Tx7.29 showed a V-shaped curve, with a distinct trough at 200 nm. The calculating data revealed that β-turn and antiparallel sheet structures were dominant contents in Tx7.29(seen in the table below in Figure 2d).
2.3 Effects of Tx7.29 on DRG sodium,potassium and calcium currents
Tx7.29 was tested its effects on sodium,potassium and calcium currents in the acute isolated rat DRG neurons using patch clamp. For the sodium currents, perfusion of 10 μmol/L Tx7.29 (n=5) had no obvious effects on the amplitude (Figure 3a), the current-voltage relationship (Figure 3b), activation(Figure 3c), inactivation (Figure 3d) and recovery(Figure 3e) of the sodium currents in rat DRG neurons. For the potassium currents, 10 μmol/L Tx7.29 (n=5) had little inhibitory effects (Figure 3f)with a (11.22±3.41)% reducing of the peak potassium currents, and did not induce a shift in the currentvoltage relationship (Figure 3g).
For the calcium currents, 10 μmol/L Tx7.29(n=5) could significantly inhibit the amplitude of calcium currents (Figure 3h) and the peak currents were reduced (55.33±2.61)% (Figure 3i). Tx7.29 did not induce a shift in the current-voltage relationship(Figure 3i). TheIC50value of Tx7.29 on calcium currents in rat DRG neurons was (8.53±1.32) μmol/L(Figure 3j).
2.4 The analgesic activity of Tx7.29
The analgesic activity of Tx7.29 was evaluated by the mice hot plate assay, which was tested at 0.5, 1,2, 3 and 4 h after intrathecal injection (Figure 4).Pethidine was used as positive control in this experiment. In the pethidine group, the analgesic effects reached maximum at 0.5 h, with the hot plate latency increasing 125.46% and then decreasing over time (Figure 4). All the three doses of Tx7.29 obviously increased the hot plate latency from 0.5 h to 4 h (Figure 4a). The analgesic effect of the high dose group of Tx7.29 (100 μmol/L) reached maximum at 2 h and the hot plate latency increased 137.48%(Figure 4b). At 0.5 h and 1h, pethidine showed better analgesic effects than Tx7.29; while at 2, 3 and 4 h,Tx7.29 (10 μmol/L and 100 μmol/L) showed better analgesic effects than pethidine.
(a) The hot plate latency of the mice. (b) The relationship between test time and the increased percentage of hot plate latency (%). Values marked with asterisks are significantly different from the saline group.*P<0.01,**P<0.001.
2.5 The cytotoxicity of Tx7.29
To determine the cytotoxicity of Tx7.29, cell viability of ND7/23 cells incubated with different concentrations of Tx7.29 was measured by MTT(Table 1). The cell viability values were more than 96% at all detected concentrations (0.01, 0.1, 1, 10,and 100 μmol/L), indicating that Tx7.29 had no significant cytotoxicity against ND7/23 cells up to 100 μmol/L (P>0.01).
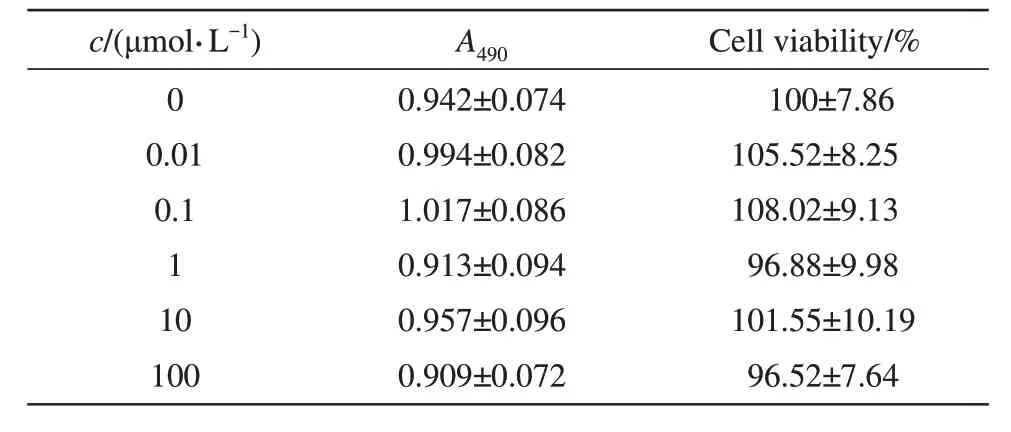
Table 1 Cytotoxicity of Tx7.29 on ND7/23 cells
3 Discussion
According to the statistics of ConoServer, the O-superfamily which has 953 nucleic acid sequences,is the most abundant superfamily in conotoxins[6].Because of the same cysteine framework but different signal peptide sequences, O-superfamily was divided into three superfamilies: O1- , O2- , and O3-superfamily[18]. Although there are nearly 200 conotoxins in O2 superfamily, the function of most is unknown[19]. In this study, we obtained a novel O2-conotoxin Tx7.29 from the cDNA library ofConus textile. In the process of synthesis of Tx7.29,we replaced the original amino acid residues (6P, 12E and 20E) by the modified residues (O and γ). Tx7.29 could significantly inhibit the calcium currents and showed analgesic effects in the hotplate assay.
Several O2 superfamily conotoxins with known functions have a unique structural motif -E(γ)CCS-[12].Initially, this motif was considered to be related to the effects of γ-PnVIIA and γ-TxVIIA on the pacemaker related channels. However, PiVIIA, which also has this motif, increased the magnitude of the calcium currents in DRG neurons. The biological activities of As7a and De7a are still unknown[20-21]. In this work,Tx7.29 with that motif, inhibited the calcium currents.In the mature peptides of TxVIIA and Tx7.29, almost only the cysteines and -E(γ)CCS- motif are conserved(Figure 1b). That -E(γ) CCS- motif could be important for the three-dimensional structure and stability of these O2-conotoxins. Biochemical parameters resulting from the comparison of several O2-conotoxins showed that they have differential pI and net charges, that might be related to the differences found in the biological activity and the molecular target specificity (Table 2). Further experiments should be performed to unravel the structural-activity relationship of Tx7.29.

Table 2 Biochemical parameters for comparison of several O2-conotoxins reported
A previous study suggested that the conotoxin γ-PnVIIA might have actions on cationic channels permeable to Ca2+and Na+[10]. Thus, we analyzed the effect of Tx7.29 over Na+, K+and Ca2+currents in rat DRG neurons. Perfusion of Tx7.29 in the µmol/L range produces a significant decrease in the Ca2+currents, without significantly modifying the Na+and K+currents. The results indicated that the activity of Tx7.29 is similar to that of ω-conotoxins. Most ω-conotoxins characterized to date selectively block the N-type CaVchannels, leading to their development as intrathecal analgesics for severe pain[22]. The main analgesic conotoxin is the ω-conotoxin MVIIA, which was approved by FDA for the management of intractable chronic pain[5]. In the mice hot plate assay,Tx7.29 also showed obvious analgesic activities.Almost all ω-conotoxins blocks the CaV2.2 channels,and some of them also blocks the CaV1.2 channels[23].The effects of Tx7.29 in different calcium channel subtypes will be carefully studied in the future work.
4 Conclusion
The synthesis, identification, and physiological functions of a O2-superfamily conotoxin Tx7.29 was performed in this study. Tx7.29 could significantly inhibit calcium currents and increase the hot plate latency. Tx7.29 may be a useful tool for analgesic drug development and could expand our visions of the molecular targets of O2-conotoxins.

