GO/HTPB composite liner for anti-migration of small molecules
Ho Li ,Jie Wei ,Y-nn Zhng ,* ,Yu-bing Hu ,Wei Jing ,Teng-yue Zhng
a College of Materials Science and Engineering,Nanjing Tech University,Nanjing,211816,China
b National Special Superfine Powder Engineering Research Center of China,Nanjing University of Science and Technology,Nanjing,210014,China
c Key Laboratory of Special Energy Materials,Nanjing University of Science and Technology,Ministry of Education,Nanjing,210094,China
d China Research and Development Academy of Machinery Equipment,Beijing,100089,China
Keywords:Graphene oxide Liner Structure-function Anti-migration Bonding
ABSTRACT Hydroxyl-terminated polybutadiene/toluene diisocyanate (HTPB/TDI) system is widely used in composite solid propellants.The migrations of plasticizers and water molecules from solid propellants and surrounding environment to the inhibitor have always been the important issues.This study focuses on the preparation,characterization and anti-migration behavior of graphene oxide (GO)/HTPB nanocomposite liner.The GO/HTPB (GH) composite liners affect the migration of small molecules through a tighter cross-linked structure and weakening function of small molecule adsorption.The anti-migration performance of the liner at different temperatures was analyzed,and the influence of the added amount of GO on the anti-migration performance and adhesion performance was also systematically studied.The overall performance of the liner is optimized when the amount of GO filler is 0.3 wt%.After adding 0.3 wt%GO,the concentration of dioctyl sebacate(DOS)migrated into the liner is decreased by 23.28%,and the concentration of water molecules is decreased by 51.89%,indicating that the introduction of GO can significantly improve the anti-migration performance of the liner.In addition,the bond strength is greatly increased from 0.25 MPa to 0.95 MPa,which meets the application requirements of the current propellant system.This research provides an important way for the preparation of structure-function synergistic anti-migration composite liners.
1.Introduction
As a kind of adhesive transition layer between propellant and insulation(or shell),liner is one of the decisive factors affecting the working state and service life of rocket motor [1].With the development of the solid propellant to high energy,higher-performance liner materials are urgently required.Most noteworthy,the good compatibility of liner material with propellant grain is one important prerequisite.HTPB is an important liquid rubber,which exhibits high strength,appropriate viscosity and good chemical resistance[2],and shows great compatibility with composite solid propellants [3].It is always cross-linked with polyisocyanate to form a cross-linked structure with higher bonding strength [4].Therefore,HTPB/TDI curing system with excellent processing performance has become an important part of the rocket propulsion[5].
For a long time,the bonding performance of propellant grain has been a serious problem during the development of solid rocket motor technology [6,7].The liner should be firmly bonded to the surface of the solid propellant to protect the inner wall of the combustion chamber[8].The poor bonding performance may lead to the propellant/inhibitor debonding and even cause accidents.During the long-term storage,there widely exists the migrations of small molecules in the propellant due to the external temperature,moisture,light and so on [9].Until now,many current researches have focused on the study of the anti-migration of plasticizers[10].A research shows that plasticizer migration from the rocket propellant grain to the inhibitor may cause serious damage to the interface,which is prone to accidents[11].Therefore,it is important to improve the anti-migration performance of the propellant liner,while maintaining the excellent mechanical properties and thermal stability [12,13].So far,a series of anti-migration methods have been conducted,such as applying materials with strong migration resistance as the matrix [14],setting a barrier liner layer between the coating layer and the propellant [15],and adding Inorganic fillers [16].Among them,adding a barrier liner shows remarkable anti-migration performance,while remaining the good adhesion performance.
Graphene has been considered a promising nano-barrier material [17,18],especially in polymer composites [19].Carbon ring’s high aspect ratio and high electron cloud density enable it can hinder the penetration of atoms and molecules.Graphene oxide(GO) is convenient for material modification because its surface contains a large number of oxygen-containing groups.In recent years,GO has been widely used to improve the mechanical properties,thermal decomposition efficiency and dimensional stability of the polymer matrix[20—23].Some studies have shown that GO also exhibits good barrier effect in polymer composites.Abraham[24]applied the hydrophobicity of few-layer graphene nanosheets to limit water intake.Lozada-Hidalgo [25]took advantages of the barrier properties of single crystal graphene films to construct heavy water separation functional devices.Zheng [26]introduced GO into styrene-butadiene rubber (SBR) to improve its gas barrier properties.Based on the above studies,GO in the HTPB propellant liner may exhibit a good anti-migration effect on small molecules through its unique structural properties.However,there are few reports on the application of GO to improve the anti-plasticizer migration of propellant.
In this study,we used GO as a filler of the liner material to enhance its anti-migration property.This new type of liner may meet the current anti-migration and adhesion requirements of high-performance propellants.Furthermore,an innovative structure-function synergy anti-migration mechanism was proposed(as shown in Fig.1).The effect of GO on the thermal stability,crystallinity and crosslinking degree of HTPB were evaluated through thermodynamics and Soxhlet extraction experiments.Furthermore,both the anti-migration and bonding performance of the GH composite liners were investigated systematically.
2.Materials and experiments
2.1.Materials
Hydroxyl-terminated polybutadiene (HTPB,99.9%,hydroxyl value ≥ 0.1 mol/100 g) was purchased from Tianjin Siense Biochemical Technology Co.,Ltd.,China.Toluene diisocyanate(TDI,≥98.0%),dibutyltin dilaurate (DBTL) and triethanolamine (TEA,≥99.0%) were purchased from Shanghai Aladdin Company,China.Dioctyl Sebacate (DOS,≥97.0%) was purchased from Shanghai Macleans Biochemical Technology Co.,Ltd.,China.Graphene oxide(GO) was prepared by a modified Hummers method.
2.2.Preparation of graphene oxide
Firstly,6 g graphite powder and 3 g NaNO3were added into a beaker,with 150 mL H2SO4under an ice bath.Then 18 g KMnO4was added in batches.The temperature was raised to 35°C in water bath.After 2 h,200 mL of deionized water was added,and kept stirring for another 2 h.The temperature was then raised to 98°C and the solution was diluted by adding 400 mL deionized water.Few 30% H2O2was added dropwise until the solution turned to bright yellow.The metal ions were washed away by diluted HCl.Centrifugated and washed until the solution was neutral.Lyophilized for later use.
2.3.Preparation of GO/HTPB composite liner
The preparation of GO/HTPB (GH) composite consists of the following five steps (GH slurry mixing was shown in Fig.2): (1) A quantitative GO(0、0.1、0.3、0.5 wt%)was placed in acetone,and a well-dispersed GO suspension was obtained by sonication.(2)HTPB and DBTL (1:0.001) were mixed with GO suspension by mechanical stirring,acetone was removed by heat preservation,and TDI was introduced according to curing coefficient R=1.3.(3)The mixture was placed in an oil bath at 80°C and stirred for 2 h.(4)Added TEA,stirred evenly,and poured the mixture into the mold(150×150×2 mm3).(5)The mold was put into the oven,and then cured at 80°C for 36 h to obtain the composite.
2.4.Characterization
2.4.1.TEM and SEM test
The microstructure and morphology of GO were observed by transmission electron microscope (TEM,Hitachi HT7700).The fracture morphology of GH liners was characterized using scanning electron microscopy(SEM,Hitachi S-4800).A thin layer of gold was deposited on the surface by sputtering prior to SEM analysis.
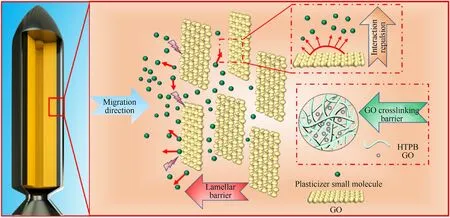
Fig.1.Schematic diagram of graphene oxide anti-migration.
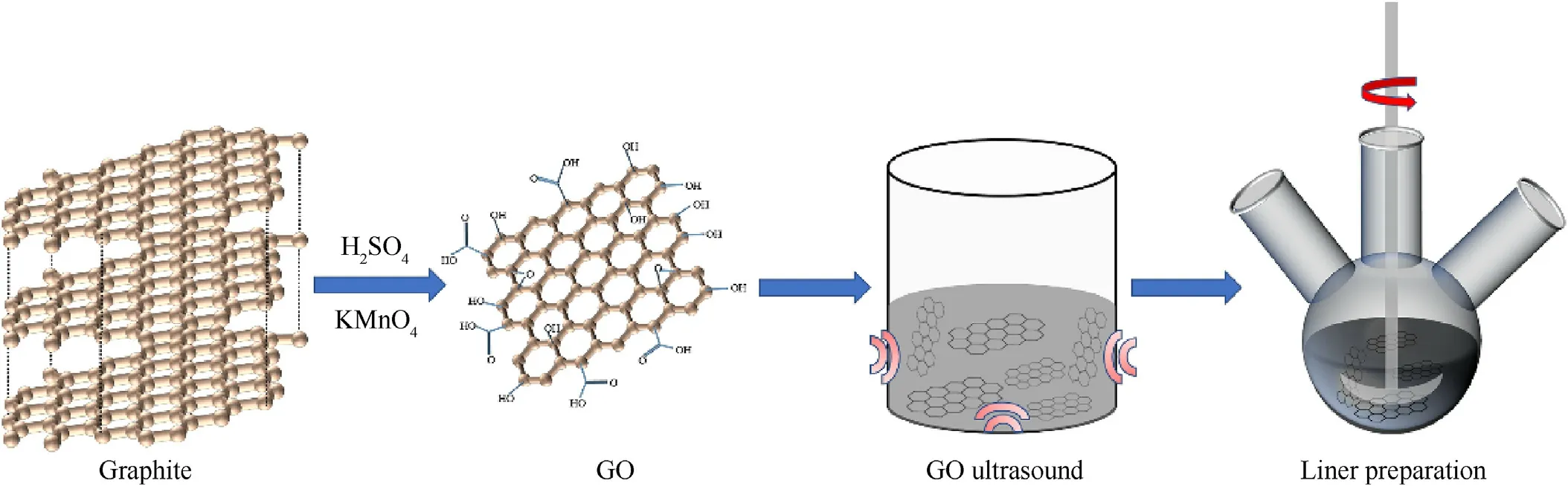
Fig.2.Flow chart of material preparation.
2.4.2.Thermodynamic test
The glass transition temperature (Tgs),melting temperature(Tmh) and crystallization enthalpy (ΔHcc) were obtained by processing the data measured by differential scanning calorimetry(DSC).The samples were shifted from room temperature to~80°C,and then heated to 250°C,the heating rate was 10°C/min,N2protection.The initial decomposition temperature (T5%) and the maximum weight loss rate temperature (Trmax) were directly obtained by thermogravimetric analysis (TG Mettler-Toledo thermogravimetric analyzer).The samples with a typical weight of 10 mg were heated from room temperature to 500°C,at a heating rate of 10°C/min under N2atmosphere.The crystallinity was calculated according to the following equation:
where |ΔHcc| represents absolute value of the measured crystallization enthalpy,ΔH100%represents the enthalpy corresponding to complete crystallization of HTPB(1.9 J/g),andXHTPBrepresents the weight fraction of HTPB in the GH composite.
2.4.3.Cross-link density test
Soxhlet extraction was used to determine the density of crosslink of liners.The soluble component in the resin was extracted with acetone close to the boiling temperature,and the insoluble component was considered to be the cured resin.The cross-link density test process is shown in Fig.3.
(1) Weighed the blank filter paper before loading the sample and recorded it asm1.The difference between the weighing results was the mass of the sample before extractionm2.(2)Installed condenser,extractor and flask.Injected 150 mL of acetone into the flask.Continuously extracted for 6 h under a constant temperature water bath of 80±2°C.(3)Took out of the extracted filter paper bag,drained it for a few minutes,put it in an oven under 105 ± 2°C for 2 h,then the sample was dried and weighed.After extraction,The total mass of the filter paper and the sample after extraction wasm3.(4)A blank test was repeated under the same conditions as above,and use it to calibrate the sample filter paper bag during calculation.The mass of the filter paper after extraction was recorded asm4.
The cross-link density result was calculated according to the following formula:
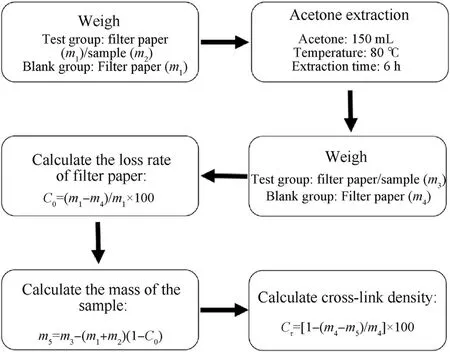
Fig.3.Flow chart of cross-link density test.
Quality loss rate of blank filter paper:
wherem1represents the mass of blank filter paper before extraction,andm4represents the mass after extraction of blank filter paper.
The remaining quality of the sample after extraction:
wherem2represents the quality of the sample before extraction,m3represents the total mass of the filter paper and the sample after extraction.
The degree of cross-link density:
wherem5represents the mass of the sample before extraction,andm4represents the mass of the sample after extraction.
2.4.4.Anti-migration test
Anti-migration test was conducted by immersion method.The prepared liners were cut into small pieces of 10×10×2 mm3,the initial massm0was recorded.Then the small pieces were put into a sealed sample bottle with DOS (the sample need to be completely immersed)at 30°C,40°C,50°C,60°C and 70°C.The samples were taken out regularly,and the DOS on the surface of the samples was wiped with filter paper.After being weighed,it was put back into the sample bottle until the weight of the sample was stable.During the experiment,the concentration of DOS(Ct)in the sample at timetcould be calculated by the following formula:
wheremtandm0respectively represents the quality of the liner at timetand the initial quality of the liner.
2.4.5.Bonding test
Titanium alloy sheets(100×25×1.5 mm3)were all sandblasted and then washed with acetone to remove the surface contaminants.Tiled GH liner slurry flat on the joint between two titanium plates(length:20 mm,width:25 mm).The samples were cured at 80°C for 3 h under vacuum.The instrument used for the adhesion performance test is the CMT4254 microcomputer-controlled electronic universal testing machine from Shenzhen Xinsansi Material Testing Co.,Ltd.,Shenzhen,China.The samples were tested at a speed of 10 mm/min under 25°C.The bonding strength was calculated according to the following equation:
whereFrepresents the maximum load when the sample bonding surface breaks,andArepresents the sample bonding area.
3.Results and discussion
3.1.Morphology and characteristics
From Fig.4(a),the prepared GO sheet is similar to tulle,and exhibits a lot of wrinkles.Fig.4(b)—(c) is the blank HTPB sample without GO.Fig.4(d)—(f)shows the SEM images of GH with 0.1 wt%,0.3 wt% and 0.5 wt% GO contents.It can be observed that the surface of GH-0 is smooth and there is no obvious gap between the interfaces.As shown in Fig.4(d)—(f) by increasing of GO content,the surface of GH gradually becomes rougher and GO keeps relatively good dispersion.When the amount of GO filling increases to 0.5 wt% clear particle aggregations are appeared in Fig.4(f).The agglomeration of particles will induce micro-cracks at the interface,which will have an adverse effect on the mechanical properties[27].Therefore,the GO dispersion of GH-0.3 is the best.
3.2.Thermal stability analysis
Currently,most of the liner materials are high molecular polymers.However,the thermal decomposition temperature is always under 200°C.Therefore,the study of liner material with better thermal stability becomes very important.
Fig.5 is the TG-DTG and DSC curves of GH-0、GH-0.1、GH-0.3 and GH-0.5.Table 1 lists the initial decomposition temperature(T5%),maximum weight loss rate temperature (Trmax),glass transition temperature (Tgs),melting temperature (Tmh),enthalpy of crystallization (ΔHcc) and crystallinity (Xc) of GH liners.As a longchain polymer,HTPB is chemically inert [28].The decomposition of GH liner undergoes two stages.The first one is the endothermic stage of the soft segment phase decomposition at 250—400°C,and 10—15%mass weight loss occurs during this stage.The other one is the exothermic stage at 400—480°C and the remaining hard segment phase is further degraded.It can be seen thatT5%of the liner with GO is higher than that of the blank sample.The pyrolysis temperature of GH-0.5 is increased by 71.6°C,hence its thermal stability is the best.However,as shown in the DTG curves in Fig.4(a),theTrmaxof GH were nearly the same.The change of the peak position between 400 and 450°C may be due to the different hard phase composition of the gasket samples prepared with different GO filler contents.Tgvalue is almost unaffected by filler doping and filler content[29].From Fig.5(b)and Table 1,Tgsof the soft segment of each sample are basically the same,indicating that there are few hard segments doped in the soft segment,and the degree of microphase separation is very high.The crystallinity of GH liners are calculated from the crystallization enthalpy measured by the DSC curve.After loading GO,the crystallinity of GH liner increases significantly.Especially the crystallinity of GH-0.3 has reached 25.21%,indicating that the introduction of GO promotes the formation of an ordered structure of the hard segment phase of HTPB.In addition,the influence of different GO loadings on crystallinity can be attributed to the hindrance of GO to the polymer chain binding.
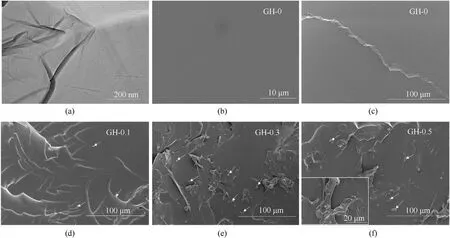
Fig.4.(a)TEM image of GO;(b)—(f)SEM images of the fracture surface of GH composite liners with different GO loadings.The inset in(f)shows the dispersion between filler and matrix at high magnification.Arrows indicate GO sheets.
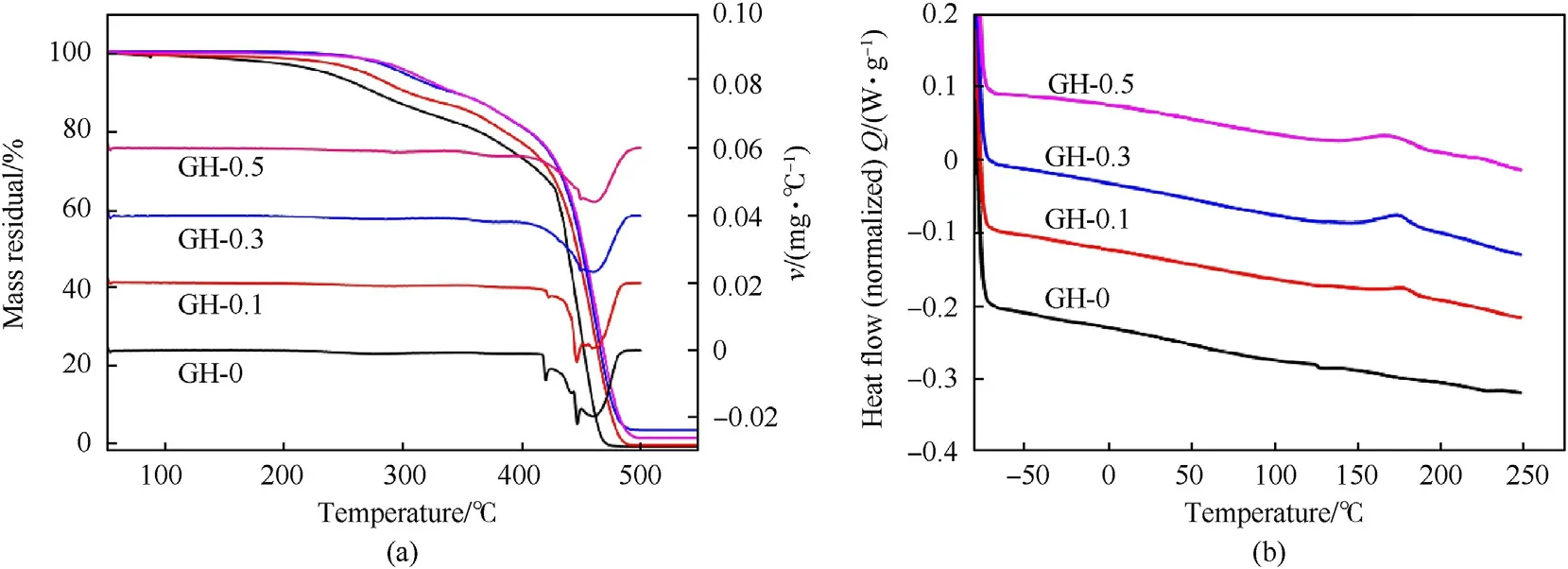
Fig.5.(a) TG-DTG curves of GH liners;(b) DSC curves of GH liners.
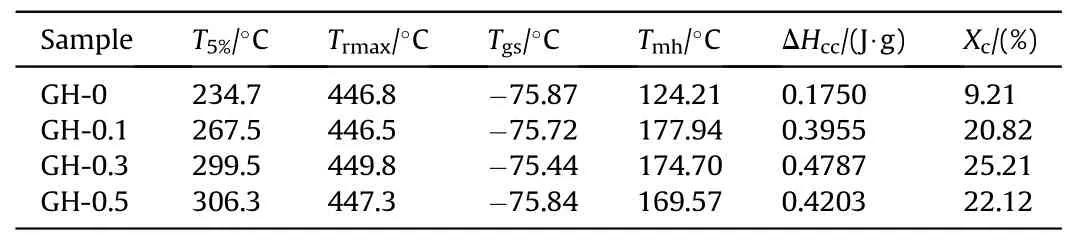
Table 1 Thermodynamic parameters and absolute crystallinity of GH liners.
Above all,the introduction of GO can significantly improve the overall thermal stability of HTPB composites.This is because GO has a stable structure.The addition of GO filler in HTPB can increase the crystallinity of the liner,and the destruction of the ordered crystal structure requires higher conditions.In addition,GO filler changes the segment composition of the composite liner,thereby increasing the pyrolysis temperature of the liner.
3.3.Cross-link density analysis
According to the migration theory,the difficulty for small molecules such as plasticizers to migrate into the liner is related to the crosslink density of the liner material [30].The increase of crosslink density results from the compact microstructure and mesh size and results in the improvement of anti-migration performance.Therefore,the crosslink density of the GH composite liners were investigated.
Fig.6 shows the change trend of the cross-link density of the GH composite liner.The average values of the GH crosslink density are summarized in Table 2.Compared with the blank sample,the crosslink density of GH composites are significantly improved.In this GH liner composites,GO is not only the inorganic nanofiller,but also the crosslink agent during the curing reaction.A large number of oxygen-containing groups exposed on the surface of GO can react with the active hydrogen of HTPB to extend the molecular chain,leading to the strong covalent bonding and interfacial interaction between GO and HTPB,which may promote the formation of polymer networks.Whereas the crosslink density of GH-0.5 is slightly lower than the others.This is due to the large specific surface areas as well as high surface energy of GO that makes GO easy to agglomerate.The agglomerated GO may block the active sites of the cross-link reaction,destroy the cross-link structure to a certain extent,and then reduce the cross-link density of the composite.Therefore,the dispersion of GO fillers in HTPB shows an important impact on the composite cross-link.More than that,the reaction between the Hydroxyl groups from GO and the polyisocyanate groups of TDI,the reaction between polyisocyanate groups and HTPB is avoided,which will lead to the decrease of eater groups.These above impacts may have a positive impact on the anti-migration properties of the liner composite.Hence,the antimigration of the composite liners were further investigated.
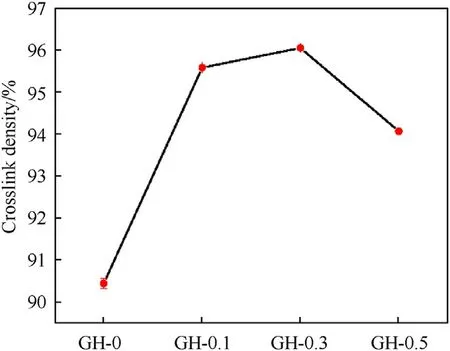
Fig.6.Cross-link density change of GH with different GO contents.
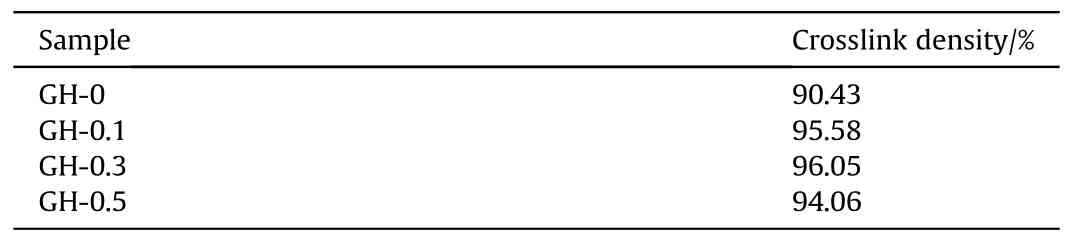
Table 2 The calculation results of the crosslinking density of GH liner.
3.4.Anti-migration by immersion method
Fig.7 shows the DOS concentration change in GH liner obtained by the immersion method.It can be found that the migration equilibrium concentrations of the liner doped with GO are significantly lower than that of the liner without GO,and the time required to reach the migration equilibrium is much longer.GH-0.3 achieves the lowest migration equilibrium concentration.On the one hand,GO can be the cross-link agent of GH system,so that the cross-link density is increased,then the mobility of molecular segments is weakened,and the probability of constructing pore through which plasticizer molecules can pass is reduced;On the other hand,with the introduction of GO,the content of soft segments in the GH system decreases,resulting in a reduction of the produced ester groups,which ultimately leads to a decrease of the migration.Above all,we initially realized the structure-function synergy liner to resist the migration of small molecules.In addition,the dispersion of GO in HTPB is severely affected by the amount of GO filler.If the amount of GO is too small,GO cannot be fully dispersed in the matrix,which will limit its lamellar barrier effect and reduce the anti-migration performance.On the contrary,too much agglomerated GO will block the cross-link sites in the cross-link reaction,and thus have a negative impact on the antimigration performance.
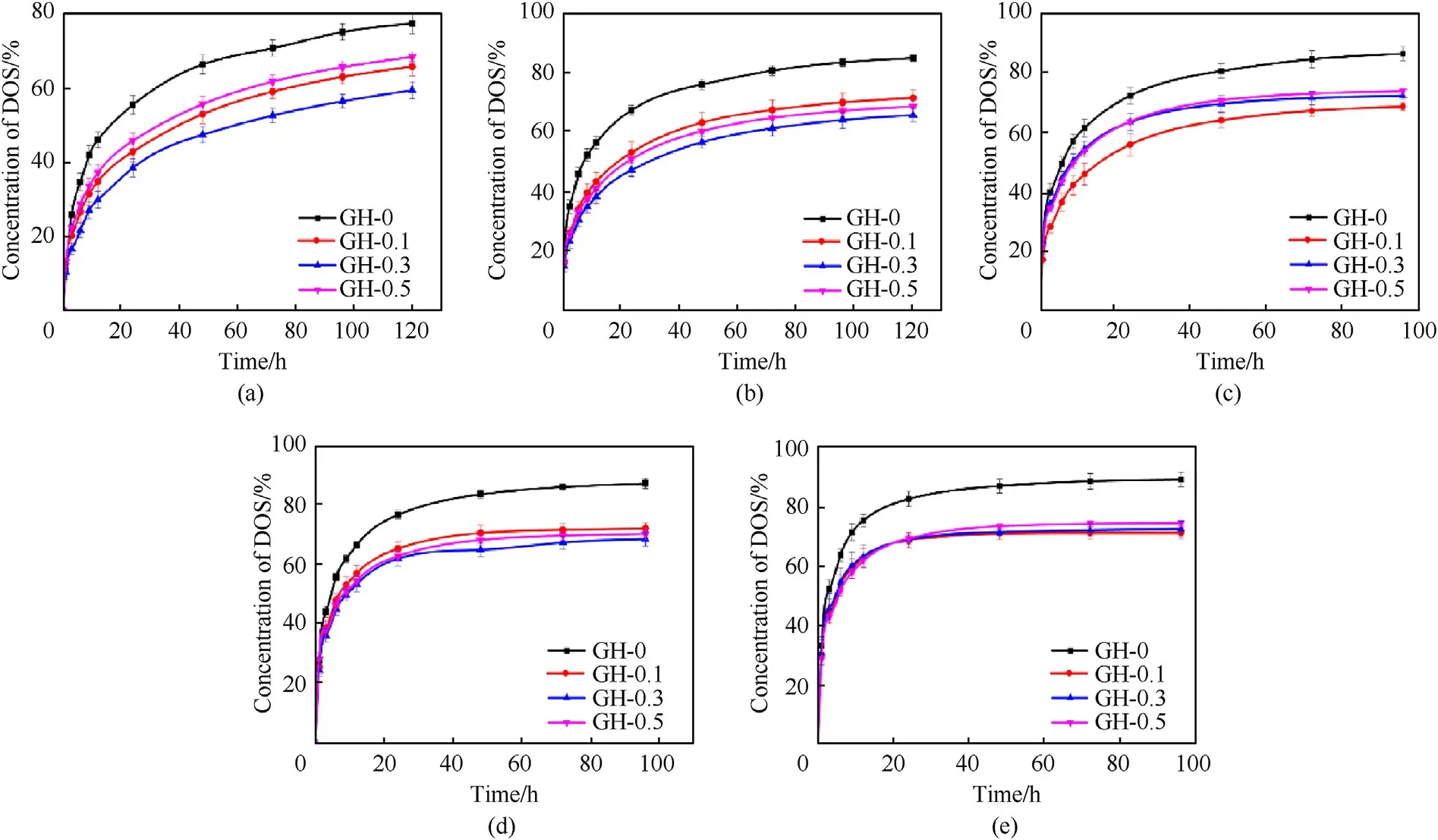
Fig.7.Time-varying DOS concentration in the liners with different temperatures: (a) 30 °C;(b) 40 °C;(c) 50 °C;(d) 60 °C;(e) 70 °C.
We also explored the anti-migration performance under different temperatures.Fig.8 shows a comparative picture with the anti-migration performance under different temperature for a specific sample.As the temperature increases,the migration equilibrium concentration of DOS increases,and the time required to reach equilibrium decreases.When temperature goes up,the kinetic capacities of both HTPB segment and the DOS molecule increase,and their activities become much stronger,which promote the migration of plasticizers.In addition,the accelerated movement of polymer molecular chains can lead to the structural disturbances and will cause some structural damage.Then more ester groups of the cross-link molecular segments will be released,which is conducive to the adsorption of more DOS molecules.
In addition to the migration concentration and time,the migration rate can also reflect the anti-migration performance of the sample.As an important migration kinetic parameter,the migration coefficient can vividly present the speed of the migration process.According to Fick’s second law [1]:
whereCrepresents the volume concentration of the migrating substance,trepresents the expansion migration time,xrepresents the distance,andDrepresents the diffusion coefficient.
Assuming that the migration is unidirectional under non-steady state conditions,with a constant diffusion coefficient,the amount of migration through the sample:
whereMtrepresents the amount of migration at timet,andM∞represents the amount of migration at equilibrium.
The expression formula of the required data and convert it into the existing parameter form Eq.(9):
Derivative of the differential to obtain the calculation formula of the migration coefficient:
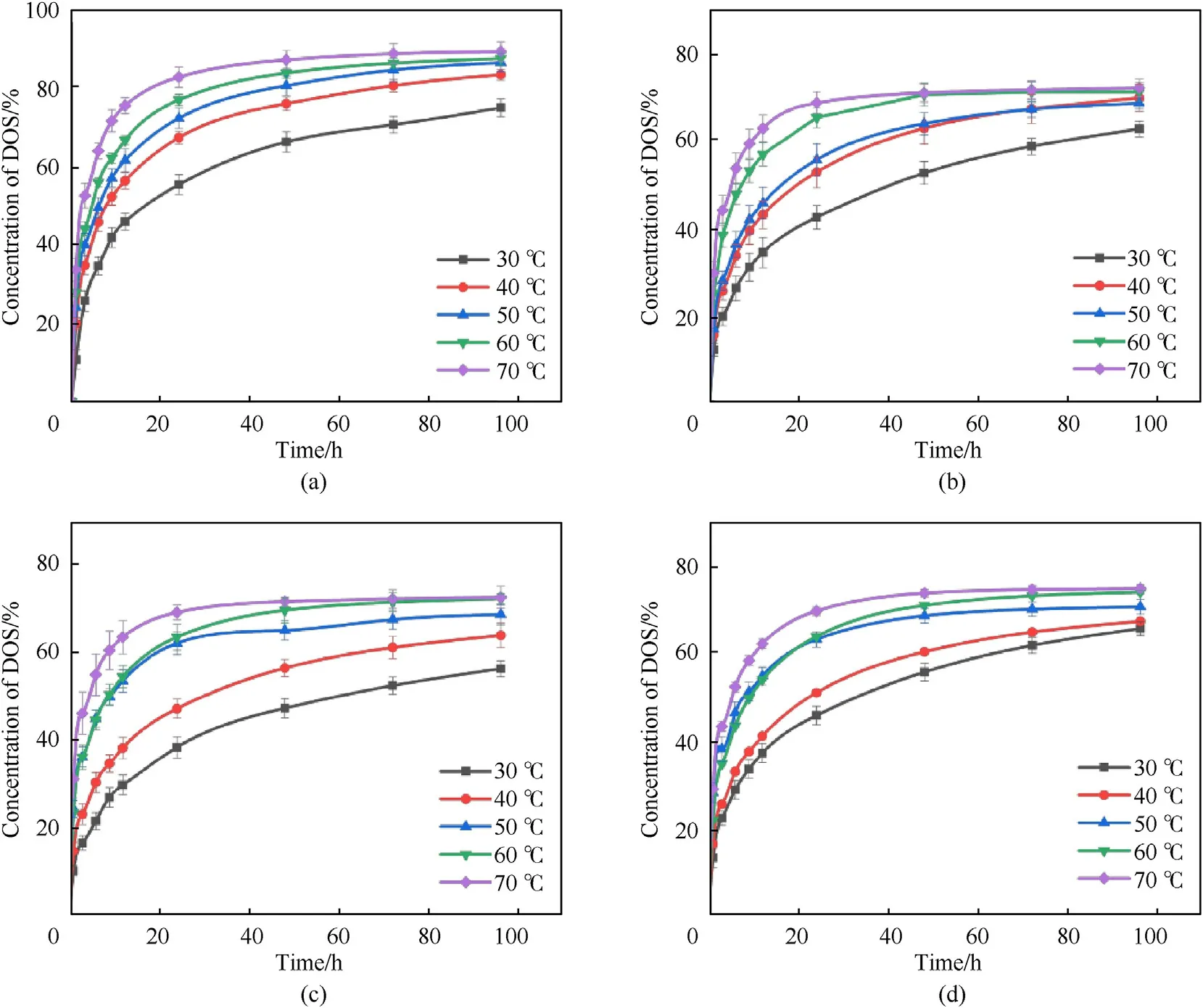
Fig.8.Time-varying DOS concentration in the specific liners with different temperatures: (a) GH-0;(b) GH-0.1;(c) GH-0.3;(d) GH-0.5.
where Δmrepresents the amount of DOS migrated into the liner at timet,m0represents the initial mass of the liner,ρ represents the density of DOS as 0.918 g/cm3,ρirepresents the liner density,anddirepresents the thickness of the liner.Uset1/2and Δm/m0as the abscissa and ordinate respectively to draw the point diagram.The linear slope after linear fitting is substituted into Eq.(12) to calculate the migration coefficient.The linear fitting result is shown in Fig.9:
Both the linear correlation coefficient (R2) of the fitting results and the diffusion coefficient (D) calculated from the slope were collected in Table 3.It can be seen from the table that the linear correlation of the fitting results at a temperature of 30°C is close to 1,indicating that the linear correlation is good.However,when the temperature rises to 70°C,the linear correlation becomes worse,indicating that the data is more volatile.At the same temperature,the migration coefficient of GH liner is one order of magnitude lower than the liner without GO.The migration coefficient of GH-0.3 at 30°C shows the greatest reduction of 1.30E-8.At the same temperature,the change trend of the migration coefficient is opposite to the cross-link density,which also shows that the crosslink density is an important factor affecting the migration of DOS.The change of migration coefficient is also related to the dispersion of GO in HTPB.With the same GO loading,the temperature will significantly affect the migration coefficient of liners.The migration coefficient of GH-0 at 70°C is 8.07E-7,which is much higher than the migration coefficient at 30°C.This is due to the fact that the structure of GH will change and some of the chain segments will be broken with the increase of temperature,so the speed of molecule and chain segment movement will accelerate,leading to an increase in the migration coefficient.
In addition,the relationship between the migration coefficient and temperature can be expressed in the form of the Arrhenius equation:
whereDrepresents the mobility coefficient,Rrepresents the molar constant,Trepresents the thermodynamic temperature,Earepresents the activation energy,andArepresents the frequency factor.
A dot plot is made with 1000/Tand lnDas the abscissa and ordinate respectively.Obviously linear relationship is observed after linear fitting.The linear fitting result is shown in Fig.10.Based on this,the migration activation energy of DOS in the GH liner can be calculated by Eq.(13).
The linear correlation coefficients (r2) and the apparent migration activation energy of DOS (Ea) are summarized in Table 4.
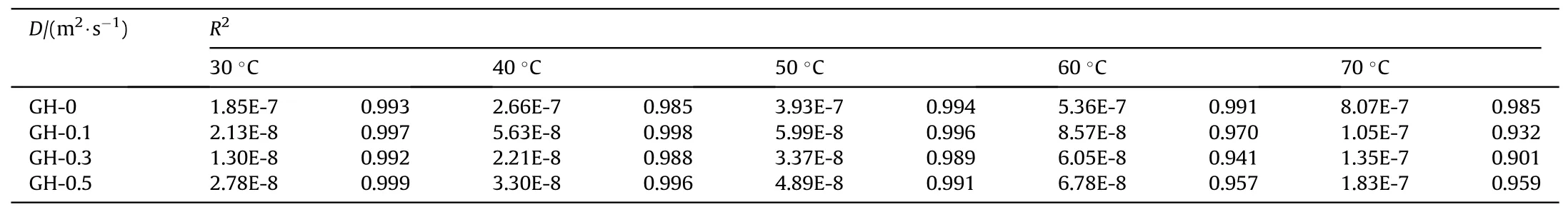
Table 3 Migration coefficient of DOS in GH liners and linear correlation coefficient.
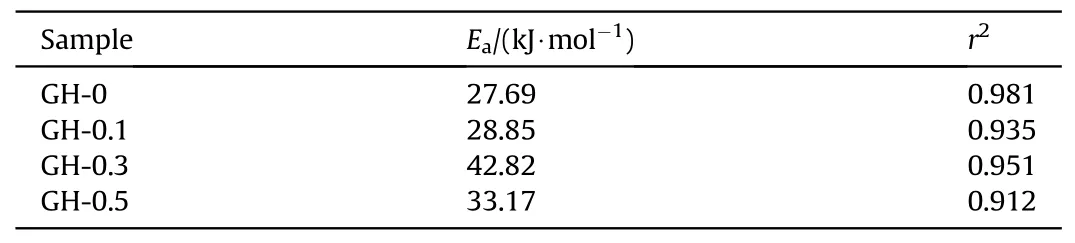
Table 4 Activation energy of migration of DOS in GH liners and Linear correlation coefficient.
The DOS migration activation energy (27.69—42.82 kJ/mol) obtained in this article is higher than that of NG (17.4—20.9 kJ/mol)[31]because of the larger molecular weight of DOS.The data shows that the migration activation energy of the liner introduced with GO has been increased,and it changes with the increase of GO loading.The migration activation energy of DOS in GH-0.3(42.82 kJ/mol) is significantly higher than other samples,which indicates that the migration and diffusion of DOS in GH liners is much more difficult.This is mainly because the good dispersion of GO leads to the reduction of polar groups,thereby reducing the migration ability of the plasticizer in the liner.
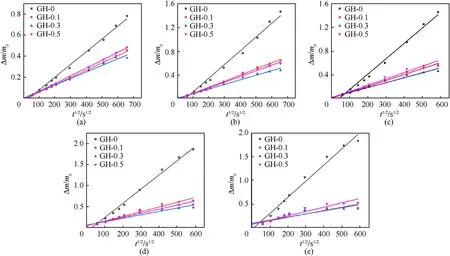
Fig.9.Linear fitting of the DOS migration coefficient in GH liners at different temperatures: (a) 30 °C;(b) 40 °C;(c) 50 °C;(d) 60 °C;(e) 70 °C.
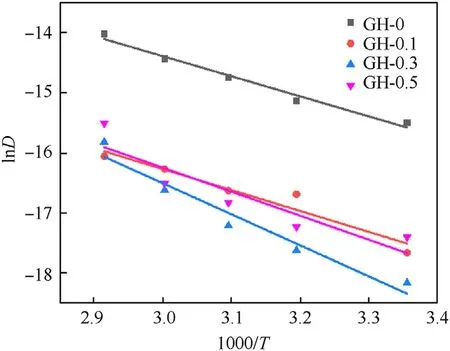
Fig.10.Fitting results of DOS migration activation energy in GH liners.
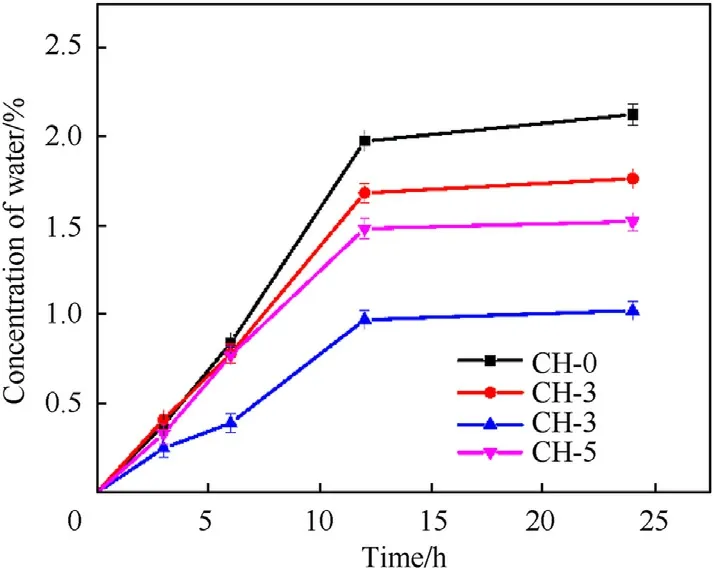
Fig.11.Time-varying water concentration in GH liners.
During the storage of the propellant,except for the migration of plasticizers,there also widely exists the migration of water molecules from the surrounding environment.Therefore,we conducted the water resistance performance of the liners.It can be seen from Fig.11 that GH-0 already has good water resistance.While maintaining its own water resistance,GO filler improves the water migration resistance of HTPB to a certain extent.The concentration of water molecules that migrated into the GH-0.3 liner decreased from 2.12%to 1.02%(a decrease of 51.89%).GO is dispersed in HTPB in the form of sheets,which not only delays the process of solvent adsorption and diffusion,but also improves the strength of the HTPB matrix,making the matrix less likely to deform during the swelling process,and increasing the swelling resistance.Therefore,the prepared GH liner has good resistance to water molecule migration.
3.5.Adhesion performance of GH composite materials
Improving the interfacial bonding performance of the propellant is critical for solid rocket motors.The migration of small molecules from propellant to inhibitor can cause terrible interface debonding.Therefore,we also conducted uni-axial tensile experiments to test the bonding performance of GH liners.
Fig.12 shows the typical load-displacement curve of the samples.The maximum load of the GH liner is significantly increased by adding GO.The failure of the bonding performance of the GH liner is an instantaneous and irreversible process.When the load reaches its maximum value,the interface of the specimen will break and the load will dissipate immediately.The maximum load of HTPB liner(GH-0)is much lower than other GH liners.When GH-0 reaches the maximum load,the sample interface is not instantaneously broken,and the downward trend of the load curve is similar to the upward trend.As an elastomer after curing,GH-0 has low hardness and excellent toughness,so the maximum load during the bonding and stretching process is low.GH liner loaded with GO has a higher crosslinking density after curing,which increases the hardness of the GH liner,so the cohesion of the liner itself is improved,and the maximum load of the GH liner bonding and stretching is significantly increased.As the hardness of the GH liner increases,its toughness decreases.Therefore,when the maximum load is reached,the interface of GH liners are instantaneously broken,and the load curve drops instantaneously.
The critical strength value required for solid rocket propellant is between 0.15 and 4.0 MPa[32].In Table 5,the adhesive strength of the gasket sample without GO is only 0.25 MPa,which can barely meet the requirements of propellant.After loading GO,the bond strength of GH liner increases significantly.Especially the bond strength of GH-0.5 has reached 1.1 MPa,which is 4.4 times that of GH-0.Because of the large specific surface area,high tensile strength and high elastic modulus of GO,when GO is uniformlydistributed in HTPB,the interface bonding between GO and HTPB polymer chains will be prominently promoted,along with an obvious increasing of the total bonding strength.In addition,during the vacuum curing process,under the action of pressure,HTPB and GO lamellas are firmly binding to form an effective load transfer.
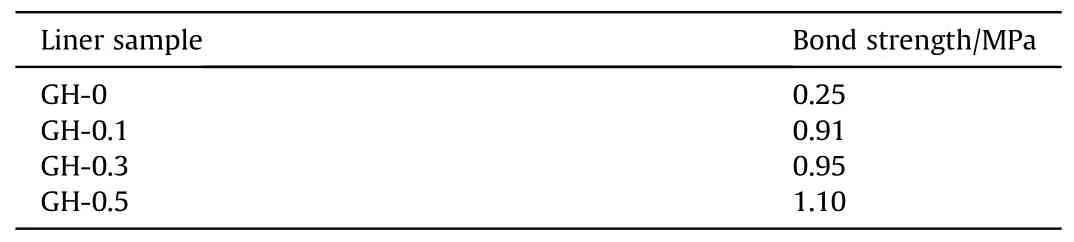
Table 5 Average bond strength of different GH composite liners.
4.Conclusions
In this article,we prepared an innovative GO/HTPB composite liner,and studied its anti-small molecule migration performance and interface bonding performance.The following conclusions were obtained:
(1) By introducing GO,the liner composition was improved with ordered lattice structures and higher degree of cross-link network.In addition,the thermal stability of the GH liner was significantly enhanced.For GH-0.5,T5%was increased by 71.6°C.GH-0.3 was the most significant,of which the crystallinity was increased from 9.21% to 25.21%,and the crosslink density was increased by 6.21%.(2) GH-0.3 showed the most notable anti-migration effect,including the migration concentration of DOS was decreased by 23.28%at 30°C,the migration coefficient was an order of magnitude lower,and the migration activation energy of DOS was increased by 15.13 kJ/mol.(3) The introduction of GO can structurally enhance the interfacial bonding between GO and HTPB polymer chains,and increase cohesion,thereby improved the bond strength.With the increase of GO content,both the maximum load and bonding strength at the breaking point of GH showed an increase.The bonding strength ofGH-0.5 can reach 1.10 MPa,which was 4.4 times that of GH-0.
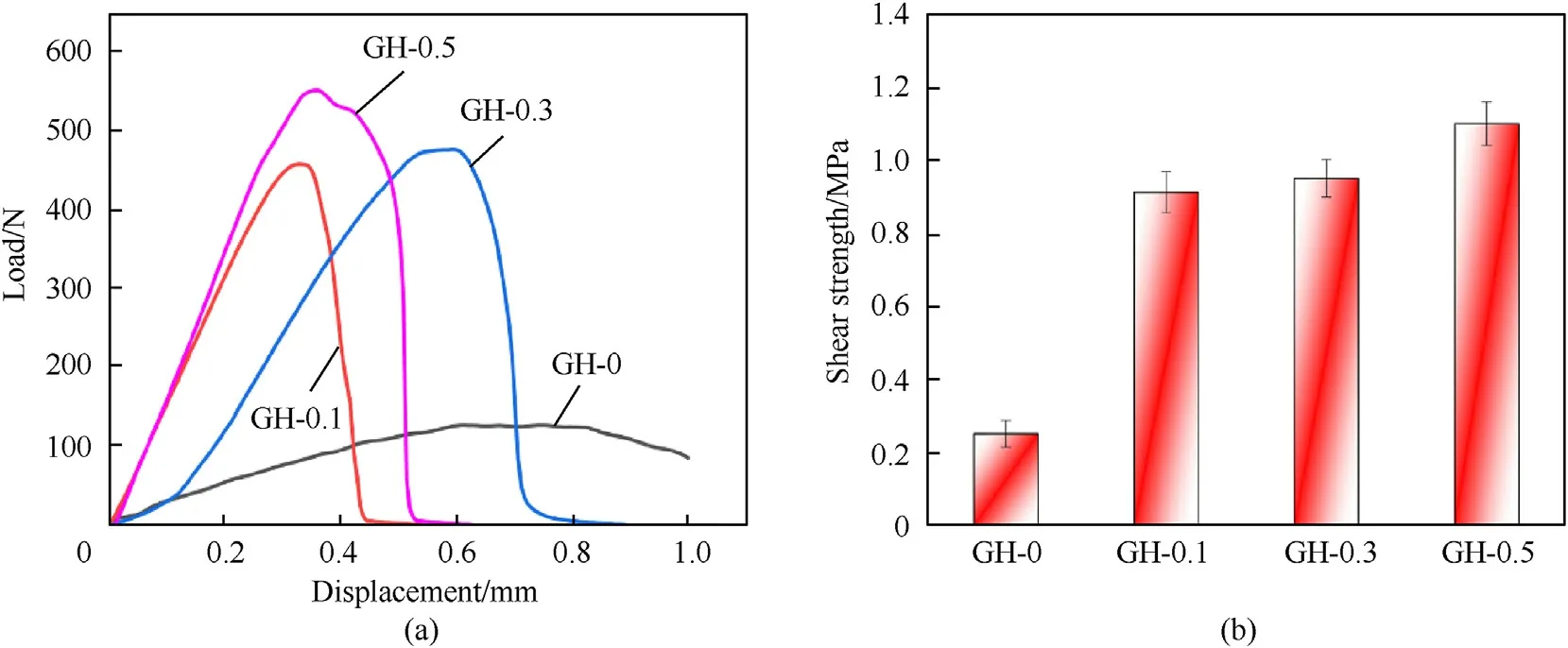
Fig.12.(a)Load-displacement curve of the bonded sample;(b) Bond strength of bonded samples.
In a word,a structure-function synergistic anti-migration composite liner was initially realized.Structurally,with the increase of crystallinity and cross-link density,the pores through which small molecules pass are reduced;functionally,GO can reduce the generation of ester groups,then the adsorption capacity between small molecules is weakened.This new type of liner meets the current anti-migration and adhesion requirements of highperformance propellants.In addition,it is possible to further realize functional anti-migration by modifying Go with groups that repel plasticizers.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors gratefully acknowledge the financial support of the National Natural Science Foundation of China (grant number 22005145)and the Natural Science Foundation of Jiangsu Province(grant number BK20180495,BK20180698),the Opening Project of Key Laboratory of Special Energy Materials (Nanjing University of Science and Technology),and the Fundamental Research Funds for the Priority Academic Program Development of Jiangsu Higher Education Institutions (grant number 30919011404).
- Defence Technology的其它文章
- Dual Attribute Adversarial Camouflage toward camouflaged object detection
- Electrophoretic deposition of hybrid organic-inorganic PTFE/Al/CuO energetic film
- A bi-population immune algorithm for weapon transportation support scheduling problem with pickup and delivery on aircraft carrier deck
- One-step green method to prepare progressive burning gun propellant through gradient denitration strategy
- Benchmark calculations and error cancelations for bond dissociation enthalpies of X—NO2
- Resilient tightly coupled INS/UWB integration method for indoor UAV navigation under challenging scenarios

