二氟/三氟甲基吡啶类化合物的合成研究进展
摘要:吡啶及其衍生物已广泛应用于农药化学、药物化学和材料化学等领域.其中,二氟/三氟甲基吡啶衍生物因其独特的生物活性,在近年来的农化和药化研究中备受关注.介绍以合成砌块构筑方法为主的二氟/三氟甲基吡啶化合物的合成反应研究,利于研究人员更好地了解氟代吡啶的合成背景,并设计出新的合成方法.
关键词:氟化试剂;合成砌块;吡啶;二氟甲基化;三氟甲基化
中图分类号:O621.3 文献标志码:A
文章编号:2095-6991(2023)01-0089-08
Abstract:Pyridines and its derivatives are a privileged class of nitrogen-containing heterocycles applied in fields of agrochemicals, pharmaceuticals and functional materials. Among its derivatives, di/trifluoromethylpyridines have attracted much attention in recent agrochemical and medicinal studies because of their unique biological activities. This review gives a brief summary on the synthesis of di/trifluoromethylpyridines by method of building blocks. The aim is to provide researchers with a convenient route to touch this topic and design new methods.
Key words:fluorination reagents; building blocks; pyridines; difluorination; trifluorination
0 引言
自Korner(1869年)和Dewar(1871年)确定了吡啶环的结构以来,吡啶及其衍生物作为一类独特的含氮杂环化合物,也是研究最多的杂环芳烃之一.同时,吡啶及其衍生物已被应用在不同的化学领域.在配位化学中,吡啶因其结构中的氮原子,可作为螯合金属离子的N-配体[1-4],也可作为高效的有机金属催化剂[5].吡啶类化合物在材料化学和超分子化学等领域同样也取得了不错的研究成果[6].其次,吡啶类化合物因其特殊的生物活性而吸引了科学家的研究兴趣.吡啶结构单元多存在于倍半萜、生物碱和多肽等复杂天然活性分子中.同时,含有吡啶结构单元的药物分子往往具有良好的药用价值和广泛的药理功效,且已有62种含吡啶结构单元的药物分子作为临床或上市药物[7-8].在农用化学品方面,含有吡啶结构单元的农药分子具有对环境友好且选择性高等特点,常用作除草剂、杀菌剂和杀虫剂等[9].
二氟甲基吡啶和三氟甲基吡啶衍生物作为含氟类化合物,体现出较强的生物活性,将含氟基团引入有机分子中,可显著地增强分子的代谢稳定性、亲油性、亲脂性和膜透性,被广泛应用于药物和农用化学品的合成中[10-13].本文对近年来二氟/三氟甲基吡啶类化合物合成反应进行总结,主要介绍含氟合成砌块在构筑二氟/三氟甲基吡啶类化合物的应用研究.
1 二氟甲基吡啶衍生物的合成研究
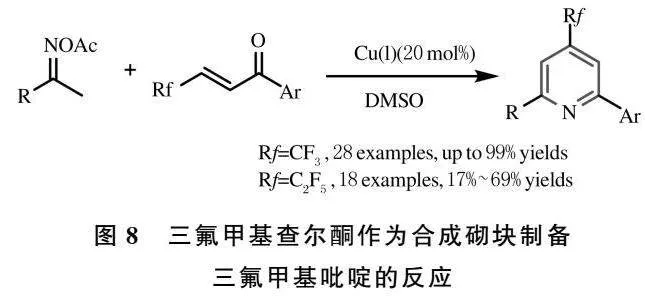
在众多的含氟基团中,二氟甲基(CF2H)基团已被证明在药物研发和设计中起到不可或缺的作用,其原因是二氟甲基基团可以看做甲氧基、巯基、酰胺和胺基等基团的等效体[14],也是生物体中良好的氢键供体[15-17].因此,引入二氟甲基的方法被不断地开发出来[18-20].同时,大多数二氟甲基吡啶衍生物已被用于农药及医药领域[21-23].例如,Thizopy和Dithiopy(图1)是一类市售的除草剂;二氟甲基胺基吡啶衍生物被用于皮肤病的治疗.
传统制备二氟甲基吡啶的方法,是由SF4或DAST及其衍生物对吡啶甲醛的脱氧氟化反应[24-26].然而,该方法会使用有毒、具有腐蚀性的氟化试剂,反应条件苛刻,底物范围受限,极大地限制了这些试剂在二氟甲基吡啶衍生物制备中的
应用.随着氟化试剂的发展,大多数二氟甲基试剂,如BrCHF2[27]、RMCHF2 (M=Sn,Zn,Ag)[28-31]、TMSCF2X (X=H,COOEt)[32-33]、CHF2COOH[34]、(CHF2SO2)2Zn[35]已被用于二氟甲基吡啶的合成中,这些合成方法使二氟甲基吡啶及其衍生物的制备取得了很大的进展.
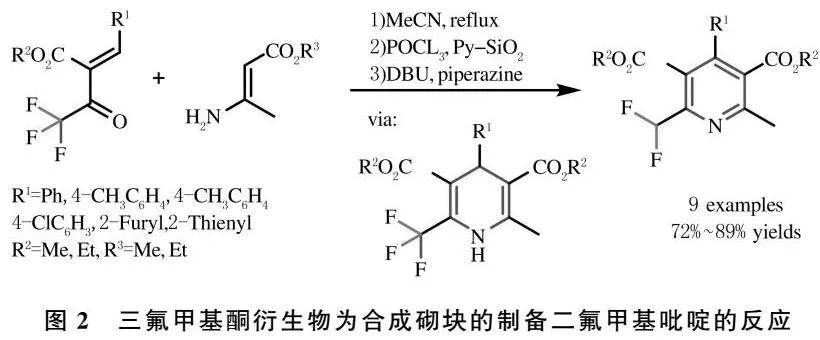
此外,利用含氟甲基合成砌块是制备二氟甲基吡啶的另一种重要的方法.1997年Shibata K.课题组以三氟甲基酮衍生物为合成砌块,与β-胺基酯在DBU/哌嗪的作用下,通过脱氟反应以高收率制备了多取代的二氟甲基吡啶衍生物[36](图2).
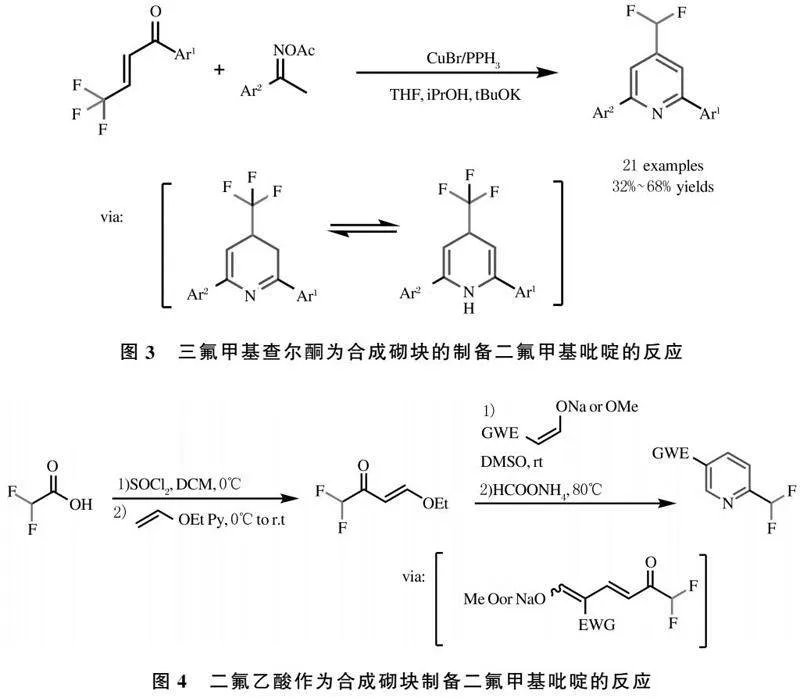
2018年李兴伟课题组实现了4-二氟甲基吡啶衍生物的多样性合成.该反应选取三氟甲基查尔酮为含氟合成砌块,在亚铜盐的用下与酮肟,形成4-三氟甲基二氢吡啶衍生物,并在叔丁醇钾的作用下发生脱氟反应得到目标产物[37](图3).
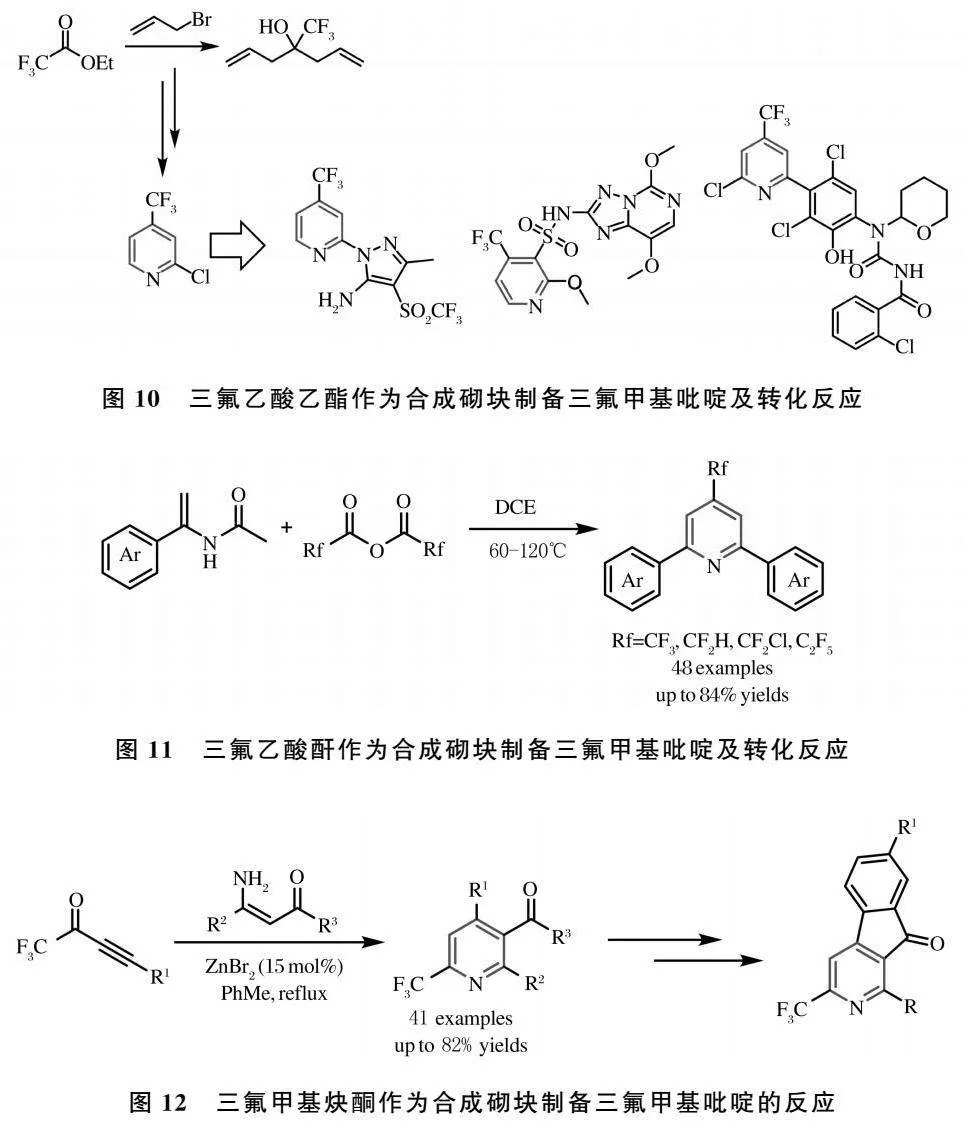
前面论述的制备方法均是利用三氟甲基合成砌块得到三氟甲基二氢吡啶衍生物,随后在碱的作用下,发生脱氟反应,制备得到相应的二氟甲基吡啶化合物.然而,使用二氟甲基合成砌块来构筑二氟甲基吡啶化合物的研究开展较晚.2014年,Desrosiers课题组选择二氟乙酸为合成砌块,通过多步的de novo反应合成了2-二氟甲基吡啶酯类化合物[38](图4).
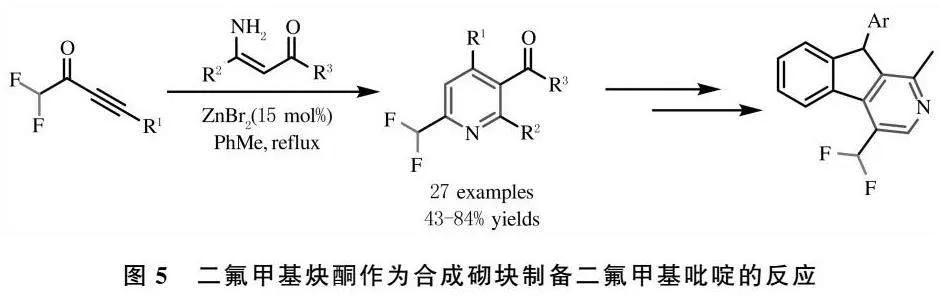
在有机合成中,α,β-炔酮类化合物常用于构筑杂环化合物的合成砌块[38].2021年,胡雨来课题组选择二氟甲基-α,β-炔酮为二氟甲基合成砌块,通过Bohlmann-Rahtz反应实现了多取代的6-二氟甲基吡啶衍生物,并且吡啶衍生物通过后官能团化制备得到具有潜在生物活性的二氟甲基芴类衍生物[39],图5为二氟甲基炔酮作为合成砌块制备二氟甲基吡啶的反应.
2 三氟甲基吡啶衍生物的合成研究
在有机合成化学和药物研发中,具有广泛生物活性的药物分子的设计、合成和评价是研究人员不断努力的目标.活性片段作为化学药物结构的关键组成部分,已被广泛应用于药物衍生化、农药产品创新、靶标化合物设计和结构-生物活性关系表征等领域,而且生物活性片段的识别和利用对于探索新药结构、优化药物疗效、降低研发成本具有重要意义[40].
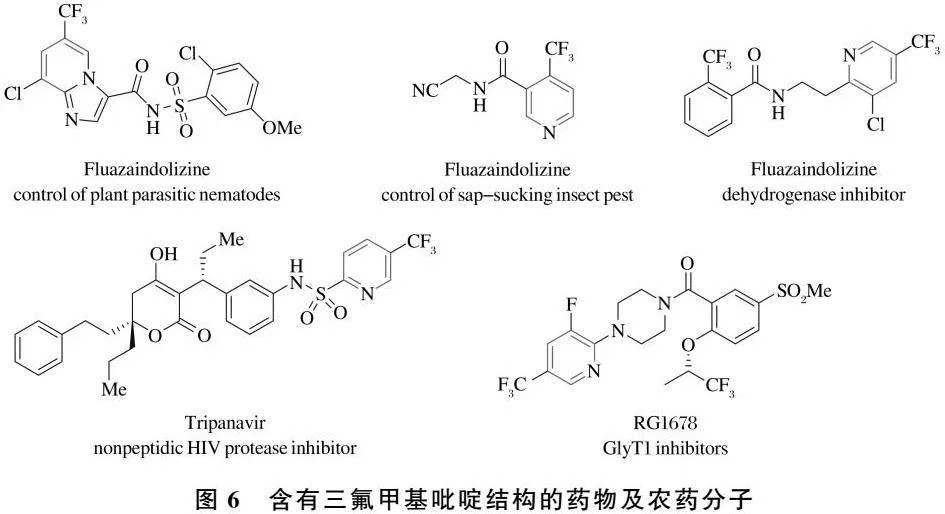
近年来的研究发现,具有特殊结构的三氟甲基吡啶衍生物体现出良好的除草、抗菌和杀虫等广泛的生物活性,常被作为创新农药合成的活性片段[41].同时,部分三氟甲基吡啶衍生物作为药物已用于临床研究[42-45].Tripanavir,NVP-BKM120和Bitopertin已在临床二期使用(图6).
基于三氟甲基吡啶衍生物在农药化学中的重要性,开发简便高效的构筑三氟甲基衍生物的方法是很有必要的.早期,三氟甲基吡啶的合成方法是利用氟氯交换[46].该方法是第一例实现三氟甲基吡啶合成方法,需要使用有毒的氯气和腐蚀性强的氟化氢,反应条件苛刻且对反应设备要求较高.利用三氟甲基试剂对吡啶环的直接三氟甲基化是行之有效的.其中常见的三氟甲基化试剂主要有TMSCF3[47-48]、CuCF3[49]、CF3SO2Cl[50]、Tongi试剂[51]、Langlois试剂[52]和CF3COOH[53].
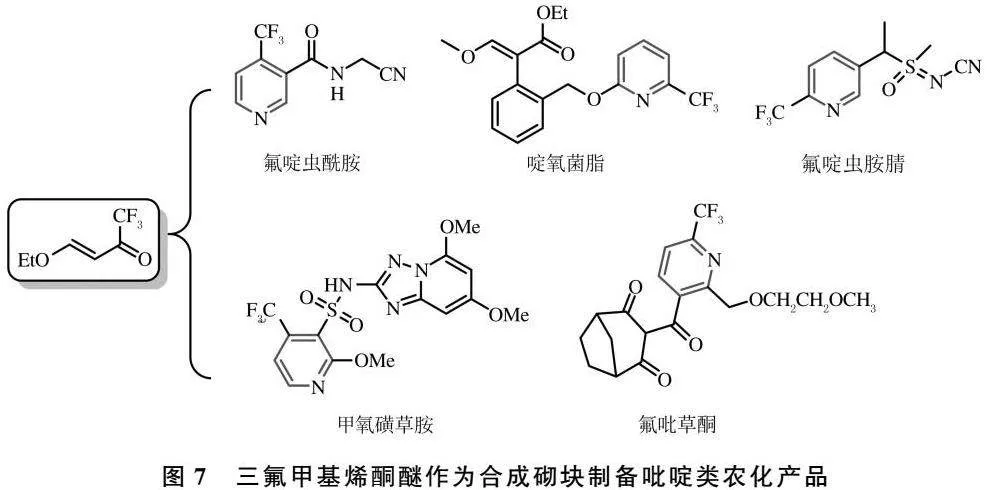
三氟甲基吡啶衍生物的构筑也可通过三氟甲基合成砌块实现.其中,三氟甲基烯酮醚因其制备简便、反应活性高等特点,已被用于多类含氟吡啶类农化产品的生产中[46](图7).
查尔酮类衍生物本身具有良好的药物活性,可用于癌症及肿瘤的治疗[54].同时,其在有机合成中也受到了极大关注.三氟甲基查尔酮是一类经典构建三氟甲基化合物的合成砌块.李兴伟课题组和白大昌课题组利用铜催化下肟酯的[3+3]环加成反应,分别制备了4-三氟甲基取代和4-五氟乙基取代的吡啶类化合物,反应具有较好的底物适用性以及优异的区域选择性[37,55](图8).
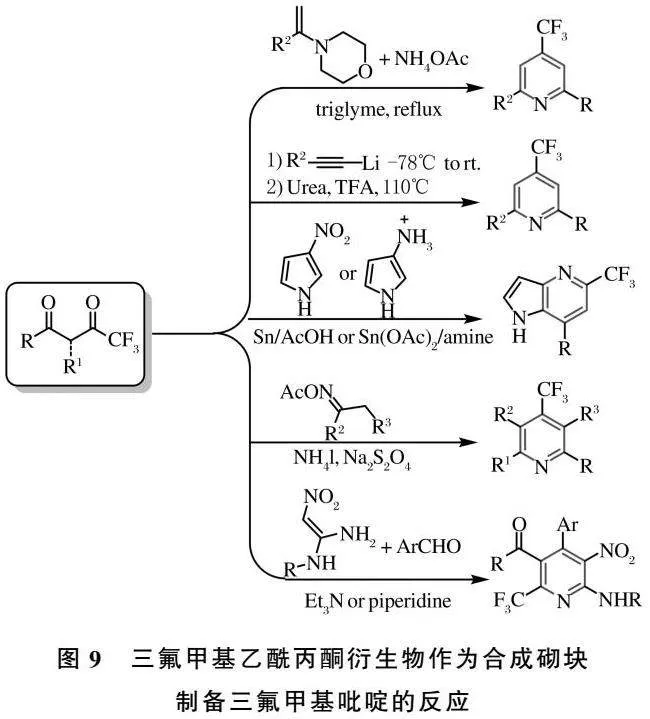
三氟乙酰丙酮是重要的有机合成中间体,由于其在结构和生物学上独特的性质,使其在有机化学、分析化学、配位化学、材料化学和生物化学中有着重要作用[56].同时,三氟乙酰丙酮类衍生物也可用于三氟甲基吡啶类化合物的构建.反应过程通过不同的氮源,可实现4-三氟甲基或6-三氟甲基吡啶的合成[57-61](图9).
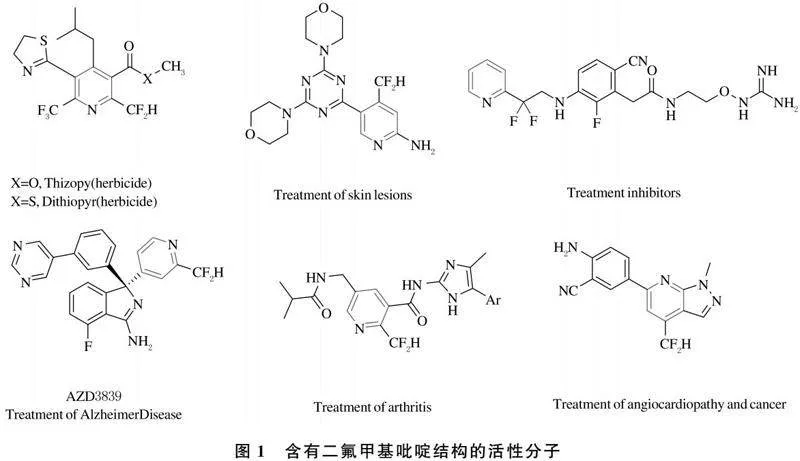
三氟乙酸在有机合成中常作为基团的保护/脱保护的试剂、反应的脱水剂及环化反应的催化剂等[62].早期,姜标课题组[63]选用三氟乙酸乙酯作为含氟砌块,通过多步反应实现了4-三氟甲基吡啶的合成,且该化合物也是转换为其他类型三氟甲基吡啶的精细化产品的起始原料(图10).
最近,翁志强课题组[64-66]利用三氟乙酸酐及衍生物作为含氟合成砌块,实现了多类含氟以及三氟甲基化合物的构建.在这一系列的研究中,该课题组利用商业易得氟代乙酸酐作为含氟砌块,实现了4-氟代吡啶衍生物构筑.该制备反应条件温和,底物的官能团耐受性高等特点[67](图11).
α,β-炔酮也是构建吡啶类衍生物的重要合成砌块[68-69].三氟甲基炔酮也常被应用于吡唑、喹啉和嘧啶等杂环化合物的构建[70].胡雨来课题组[71]扩展了三氟甲基炔酮的应用范围,通过Bohlmann-Rahtz环化反应实现了三氟甲基吡啶衍生物的构建,且相应的三氟甲基吡啶化合物通过后续的官能团化,可转化为三氟甲基芴酮衍生物(图12).
3 结语
随着二氟/三氟吡啶衍生物的生物活性的研究不断推进,高效且简便制备含氟吡啶类化合物的方法也将不断地推陈出新.但二氟/三氟吡啶衍生物的制备方法中仍面临着挑战:①更加温和、绿色且高效的制备反应的开发;②具有多官能团化的二氟/三氟吡啶衍生物的制备,以及手性含氟吡啶的制备反应研究;③实现含氟砌块在氟代吡啶类精细化工品生产中的应用.相信在不远的将来,会有更多的二氟/三氟吡啶衍生物将走进我们的生活中,这将加速有机氟化学及氟化工业的发展.
参考文献:
[1] BORA D,DEB B,AMY L F,et al.Dicarbonyliridium(I) complexes of pyridine ester ligands and their reactivity towards various electrophiles[J].Inorg Chim Acta,2010,363(7):1539-1546.
[2] ZHOU M D,JAIN K R,GUNYAR A,et al.Bidentate lewis base adducts of methyltrioxidorhenium(VII):ligand influence on catalytic performance and stability[J].Eur J Inorg Chem,2009,2009(20):2907-2914.
[3] CHAN Y T,MOOREFIELD C N,SOLER M,et al.Unexpected isolation of a pentameric metallomacrocycle from the FeII-mediated complexation of 120° juxtaposed 2,2′:6′,2″-terpyridine ligands[J].Chem Eur J,2010,16(6):1768-1771.
[4] HUSSON J,KNORR M.2,2′:6′,2″-terpyridines functionalized with thienyl substituents:synthesis and applications[J].J Heterocycl Chem,2012,49(3):453-478.
[5] JARUSIEWICZ J,YOO K,JUNG K.Highly regioselective heck coupling reactions of aryl halides and dihydropyran in the presence of an NHC-pyridine ligand[J].Synlett,2009,3:482-486.
[6] DE RUITER G,LAHAV M,VAN DER BOOM M E.Pyridine coordination chemistry for molecular assemblies on surfaces[J].Acc Chem Res,2014,47(12):3407-3416.
[7] VITAKU E,SMITH D T,NJARDARSON J T.Analysis of the structural diversity,substitution patterns,and frequency of nitrogen heterocycles among U.S.FDA approved pharmaceuticals[J].J Med Chem,2014,57(24):10257-10274.
[8] BAUMANN M,BAXEENDALE I R.An overview of the synthetic routes to the best selling drugs containing 6-membered heterocycles[J].Beilstein J Org Chem,2013,9:2265.
[9] REN M,NIU J,HU B,et al.Block of kir channels by flonicamid disrupts salivary and renal excretion of insect pests[J].Insect Biochem Mol Biol,2018,99:17-26.
[10] MEI H,HAN J,FUSTERO S,et al.Fluorine-containing drugs approved by the FDA in 2018[J].Chem Eur J,2019,25(51):11797-11819.
[11] MEANWELL N A.Fluorine and fluorinated motifs in the design and application of bioisosteres for drug design[J].J Med Chem,2018,61(14):5822-5880.
[12] FUJIWARA T,O’HAGAN D.Successful fluorine-containing herbicide agrochemicals[J].J Fluorine Chem,2014,167:16-29.
[13] JESCHKE P.The unique role of halogen substituents in the design of modern agrochemicals[J].Pest Manage Sci,2010,66(1):10-27.
[14] 张寅生.生物电子等哌替二氟甲基在药物化学中的应用及其化学合成方法进展[J].药物学报,2021,56(8):2182-2196.
[15] ZAFRANI Y,YEFFET D,SOD-MORIAH G,et al.Difluoromethyl bioisostere:examining the “lipophilic hydrogen bond donor” concept[J].J Med Chem,2017,60(2):797-804.
[16] SESSLER C D,RAHM M,BECKER S,et al.CF2H,a hydrogen bond donor[J].J Am Chem Soc,2017,139(27):9325-9332.
[17] ZAFRANI Y,SAPHIER S,GERSHONOV E.Utilizing the CF2H moiety as a H-bond-donating group in drug discovery[J].Future Med Chem,2020,12(5):361-365.
[18] FENG Z,XIAO Y L,ZHANG X.Transition-metal (Cu,Pd,Ni)-catalyzed difluoroalkylation via cross-coupling with difluoroalkyl halides[J].Acc Chem Res,2018,51(9):2264-2278.
[19] RONG J,NI C,HU J.Metal-catalyzed direct difluoromethylation reactions[J].Asian J Org Chem,2017,6(2):139-152.
[20] YERIEN D,BARATA-VALLEJO S,POSTIGO A.Difluoromethylation reactions of organic compounds[J].Chem Eur J,2017,23(59):14676-14701.
[21] GOURE W F,LESCHINSKY K L,WRATTEN S J,et al.Synthesis and herbicidal activity of N-substituted 2,6-bis(polyfluoromethyl)dihydropyridine-3,5-dicarboxylates[J].J Agric Food Chem,1991,39(5):981-986.
[22] LEE F L,STIKES G L.Synthesis of a new class of pyridine herbicide[J].Pestic Sci,1991,31(4):555-568.
[23] RAGEOT D,THOMAS B,BORSARI C,et al.Scalable,economical,and practical synthesis of 4 (difluoromethyl)pyridin-2-amine,a key intermediate for lipid kinase inhibitors[J].Org Process Res Dev,2019,23(11):2416-2424.
[24] HASS A,SPITZER M,LIEB M.Synthese seitenkettenfluorierter aromatischer verbindungen und deren chemische reaktivitat[J].Chem Ber,1988,121(7):1329-1340.
[25] SUBOTA A I,RYABUKHIN S V,GORLOVA A O,et al.An approach to the synthesis of 3-substituted piperidines bearing partially fluorinated alkyl groups[J].J Fluorine Chem,2019,224:61-66.
[26] MELVIN P R,FERGUSON D M,SCHIMLER,S D,et al.Room temperature deoxyfluorination of benzaldehydes and α ketoesters with sulfuryl fluoride and tetramethylammonium fluoride[J].Org Lett,2019,21(5):1350-1353.
[27] BACAUANU V,CARDINAL S,YAMAUCHI M,et al.Metallaphotoredox difluoromethylation of aryl bromides [J].Angew Chem Int Ed,2018,57(38):12543-12548.
[28] PRAKASH G KS,GANESH S K,JONES J P,et al.Copper-mediated difluoromethylation of (hetero)aryl iodides and styryl halides with tributyl(difluoromethyl)stannane[J].Angew Chem Int Ed,2012,51(48):12090-12094.
[29] XU L,VICIC D.Direct difluoromethylation of aryl halides via base metal catalysis at room temperature [J].J Am Chem Soc,2017,139(10):3917.
[30] LU C,GU Y,WU J,et al.Palladium-catalyzed difluoromethylation of heteroaryl chlorides,bromides and iodides[J].Chem Sci,2017,8:4848-4852.
[31] ZHAO H,HERBERT S,KINZEL T,et al.Two ligands transfer from Ag to Pd:en route to (SIPr)Pd(CF2H)(X) and its application in one-pot C-H borylation/difluoromethylation[J].J Org Chem,2020,85(5):3596-3604.
[32] JIANG X L,CHEN Z H,XU X H,et al.Copper-mediated difluoromethylation of electron-poor aryl iodides at room temperature[J].Org Chem Front,2014,1(7):774-776.
[33] FUJIKAWA K,KOBAYASHI A,AMLI H.An efficient route to difluoromethylated pyridines[J].Synthesis,2012,44(19):3015-3018.
[34] TUNG T T,CHRUSTENSEN S R,NIELSEN J.Difluoroacetic acid as a new reagent for direct C-H difluoromethylation of heteroaromatic compounds[J].Chem Eur J,2017,23(72):18125-18128.
[35] FUJIWARA Y,DIXON J A,O’HARA F,et al.Practical and innate carbon-hydrogen functionalization of heterocycles[J].Nature,2012,492:95-99.
[36] KATSUYAMA I,FUNABIKI K,MATSUI M,et al.A convenient synthesis of difluormethyl-substituted pyridines[J].Synlett,1997,5:591-592.
[37] BAI D,WANG X,ZHANG G,et al.Redox-divergent synthesis of fluoroalkylated pyridines and 2-pyridones through Cu-catalyzed N-O cleavage of oxime acetates[J].Angew Chem Int Ed ,2018,57(22):6633-6637.
[38] DESROSIER J N,KELLY C B,FANDRICK D R,et al.A Scalable and regioselective synthesis of 2 difluoromethyl pyridines from commodity chemicals[J].Org Lett,2014,16(6):1724-1727.
[39] YANG T,DENG Z,WANG K H,et al.Access to 6-difluoromethylpyridines by ZnBr2-catalyzed cascade michael addition/ annulation[J].Tetrahedron,2021,79:131833-131840.
[40] ZDRAZIL B,GUHA R.The rise and fall of a scaffold:a trend analysis of scaffolds in the medicinal chemistry literature[J].J Med Chem,2018,61(11):4688-4703.
[41] ZHENG Z,DAI A,JIN Z,et al.Trifluoromethylpyridine:an important active fragment for the discovery of new pesticides[J].J Agric Food Chem,2022.10.1021/acs.jafc.1c08383.
[42] LAHM G P,DESAGER J,SMITH B K,et al.The discovery of fluazaindolizine:a new product for the control of plant parasitic nematodes[J].Bioorg Med Chem Lett,2017,27(7):1572-1575.
[43] WEI P,LIU Y,LI W,et al.Metabolic and dynamic profiling for risk assessment of fluopyram,a typical phenylamide fungicide widely applied in vegetable ecosystem[J].Sci Rep,2017,7:41347.
[44] MAIRA S-M,PECCHI S,HUANG A,et al.Identification and characterization of NVP-BKM120,an orally available pan-class I PI3-kinase inhibitor[J].Mol Cancer Ther,2012,11(2):317-328.
[45] BURGER M T,PECCHI S,WAGMAN A,et al.Identification of NVP-BKM120 as a potent,selective,orally bioavailable class I PI3 kinase inhibitor for treating cancer[J].ACS Med Chem Lett,2011,2(10):774-779.
[46] 曹晓峰,张瑞峰.三氟甲基吡啶在作物保护中的重要性[J].世界农药,2018,40(4):7-14.
[47] GONDA Z,KOVACS S,WEBER C,et al.Efficient copper-catalyzed trifluoromethylation of aromatic and heteroaromatic iodides:the beneficial anchoring effect of borates[J].Org Lett,2014,16(16):4268-4271.
[48] KREMLEV M M,MUSHTA A I,TYRRA W,et al.Me3SiCF3/AgF/Cu:a new reagents combination for selective trifluoromethylation of various organic halides by trifluoromethylcopper,CuCF3[J].J Fluorine Chem,2012,133,67-71.
[49] LIN X,LI Z,HAN X,et al.Trifluoromethylation of (Hetero)aryl iodides and bromides with copper(I) chlorodifluoroacetate complexes[J].RSC Adv,2016,6(79):75465-75469.
[50] NAGIB D A,MAXMILLAN S W C.Trifluoromethylation of arenes and heteroarenes by means of photoredox catalysis[J].Nature,2011,480: 224-228.
[51] MEJIA E,TOGNI A.Rhenium-catalyzed trifluoromethylation of arenes and heteroarenes by hypervalent iodine reagents[J].ACS Catal,2012,2(4):521-527.
[52] JI Y,BRUECKL T,BAXTER R D,et al.Innate C-H trifluoromethylation of heterocycles.[J] Proc Natl Acad Sci U S A,2011,108(35):14411-14415.
[53] YANG X,SUN R,LI S,et al.Regioselective direct C-H trifluoromethylation of pyridine[J].Org Lett,2020,22(18):7108-7112.
[54] YANG H M,SHIN H R,CHO S H,et al.Structural requirement of chalcones for the inhibitory activity of interleukin[J].Bioorg Med Chem,2007,15(1):104-111.
[55] 杨思琪,李鑫,彭卓金,等.铜催化肟酯参与的[3+3]环加成反应合成 4-五氟乙基取代的吡啶类化合物[J].有机化学,2019,39:1623-1629.
[56] 戴佳亮,徐卫国,李华,等.三氟乙酰丙酮的合成与应用[J].有机氟工业,2015(1):38-44.
[57] FUNABIKI K,ISOMURA A,YAMAGUCHI Y,et al.Efficient and convenient entry to β-hydroxy-β-trifluoromethyl-β-substituted ketones and 2,6-disubstituted 4-trifluoromethylpyridines based on the reaction of trifluoromethyl ketones with enamines or imines[J].J Chem Soc Perkin Trans 1,2001,20:2578-2582.
[58] IAROSHENKO V O,OSTROVSKYI D,AYUB K,et al.Synthesis of 4-Trifluoromethylpyridines by [5+1] cyclization of 3-hydroxy-pent-4-yn-1-ones with urea[J].Adv Synth Catal,2005,355(2-3):576-588.
[59] DE ROSA M,ARNOLD D,HARTLINE D,et al.Effect of bronsted acids and bases,and LEWIS acid (Sn2+) on the regiochemistry of the reaction of amines with trifluoromethyl-β-diketones:reaction of 3-aminopyrrole to selectively produce regioisomeric 1H-pyrrolo[3,2-b]-pyridines[J].J Org Chem,2015,80(24):12288-12299.
[60] HUANG H,CAI J,XIE H,et al.Transition-metal-free N-O reduction of oximes:a modular synthesis of fluorinated pyridines[J].Org Lett,2017,19(14):3743-3746.
[61] DU X-X,ZI Q X,WU Y M,et al.An Environmentally benign multi-component reaction:regioselective synthesis of fluorinated 2-aminopyridines using diverse properties of the nitro group[J].Green Chem,2019,21(6):1505-1516.
[62] NORRIS M D.Trifluoroacetic acid (TFA) [J].Synlett,2015,26(3):418-419.
[63] JIANG B,XIONG W,ZHANG X,et al.Convenient approaches to 4-trifluoromethylpyridine[J].Org Process Res Dev,2001,5(5):531-534.
[64] LIN B,WU W,WENG Z.Synthesis of 3-perfluoroalkyl-substituted 1,2,4-triazinones through copper(I)-catalyzed interrupted click reaction[J].Tetrahedron,2019,75(19):2843-2847.
[65] CHEN T,WU W,WENG Z.Visible-light photoredox catalyzed synthesis of polysubstituted furfuryl trifluoroacetamide derivatives[J].Tetrahedron,2019,75(51):130751.
[66] YUAN Z,CHEN S,WENG Z,et al.Copper-catalyzed synthesis of trifluoromethylated bis(indolyl)arylmethanes from 2-arylindoles and 2,2,2-trifluoroacetohydrazide[J].Org Chem Front,2020,7(3):482-486.
[67] WANG Z,YOU C,WANG C,et al.Perfluorocarboxylic anhydrides triggered cyclization:access to 4-perfluoroalkylpyridines[J].J Org Chem,2019,84(22):14926-14935.
[68] AULAKH V S,CIUFOLINI M,A.Total synthesis and complete structural assignment of thiocillin I[J].J Am Chem Soc,2011,133(15):5900-5904.
[69] AULAKH V S,CIUFOLINI M A.An improved synthesis of pyridine-thiazole cores of thiopeptide antibiotics[J].J Org Chem,2009,7(15):5750-5753.
[70] ZHANG C.Synthesis of trifluoromethyl or trifluoroacetyl substituted heterocyclic compounds from trifluoromethyl-α,β-ynones[J].J Chin Chem Soc,2022,69(4):594-603.
[71] YANG T,DENG Z,WANG K H,et al.Synthesis of polysubstituted trifluoromethylpyridines from trifluoromethyl-α,β-ynones[J].J Org Chem,2020,85(2):924-933.
[责任编辑:纪彩虹]
基金项目:甘肃省高等学校创新基金项目(2020B-253);甘肃省自然科学基金项目(20JR10RA144);国家大学生创新项目(202111562004);甘肃省大学生创新项目(S202111562012, S202111562011)
作者简介:杨天宇(1990-),男,辽宁沈阳人,讲师,博士,研究方向:有机氟化学、稀土发光材料.E-mail:maxli101@sina.com.

