CK7/SATB2/PAX8与肿瘤大小/侧向鉴别原发与下消化道转移性卵巢黏液癌
[摘要] 目的 探讨细胞角蛋白7(CK7)、特殊的含AT序列的结合蛋白2(SATB2)、配对盒基因8(PAX8)联合肿瘤大小、侧向性鉴别诊断原发性与下消化道转移性卵巢黏液癌的意义。
方法 选取原发性卵巢黏液性肿瘤(PMOC)标本74例,下消化道转移性卵巢黏液癌(MMOC)标本16例,统计肿瘤大小、侧向性,免疫组化方法检测标本CK7、SATB2、PAX8、细胞角蛋白20(CK20)及尾型同源盒转录因子2(CDX2)的表达,分析上述指标在二者鉴别诊断中的意义。
结果 CK7、PAX8在PMOC组织阳性表达率高于MMOC(P=0.000、0.000),SATB2、CK20、CDX2在MMOC组织阳性表达率高于PMOC(P=0.000,χ2=14.16~17.30,Plt;0.05);CK7、PAX8、SATB2联合肿瘤大小、侧向性诊断PMOC的准确度分别为82.2%、76.7%、74.4%,CK7/CDX2/CK20诊断PMOC的准确度为76.6%,CK7/PAX8/SATB2/大小/侧向性诊断PMOC准确度、灵敏度、特异度、约登指数分别为94.4%、93.2%、100.0%、93.2%。
结论 单个指标CK7、PAX8、SATB2可以鉴别PMOC与MMOC,联合肿瘤大小、侧向性诊断效能显著提高。
[关键词] 卵巢肿瘤;病理学,临床;角蛋白7;配对盒基因8;特殊的含AT序列的结合蛋白2;诊断,鉴别
[中图分类号] R737.31
[文献标志码] A
[文章编号] 2096-5532(2023)01-0122-05
doi:10.11712/jms.2096-5532.2023.59.035
[网络出版] https://kns.cnki.net/kcms/detail/37.1517.R.20230308.1058.017.html;2023-03-09 17:01:35
THE SIGNIFICANCE OF CK7, SATB2, AND PAX8 COMBINED WITH TUMOR SIZE AND LATERALITY IN DIFFERENTIAL DIAGNOSIS OF PRIMARY AND LOWER GASTROINTESTINAL METASTATIC OVARIAN MUCINOUS CARCINOMA
LI Hongxuan, XIA Nannan, SONG Kejuan, ZHANG Dan, ZHAO Han, YAO Qin
(Department of Gynecology, Qingdao Women and Children Hospital, Qingdao 266000, China)
; [ABSTRACT] "Objective "To explore the application of cytokeratin 7 (CK7), special AT-rich sequence-binding protein 2 (SATB2), and paired box gene 8 (PAX8) combined with tumor size and laterality in differential diagnosis of primary mucinous ovarian carcinoma (PMOC) and metastatic mucinous ovarian carcinoma (MMOC) of lower digestive tract.
Methods nbsp;Tumor size and laterality were measured using 74 specimens of PMOC and 16 specimens of MMOC. Immunohistochemical methods were used to detect the expression of CK7, SATB2, PAX8, cytokeratin 20 (CK20), and caudal type homeobox 2 (CDX2). The significance of the above indicators in the differential diagnosis of PMOC and MMOC was analyzed.
Results "The positive expression rate of CK7 and PAX8 was higher in PMOC than in MMOC (P=0.000,0.000), and the positive expression rate of SATB2, CK20, and CDX2 was higher in MMOC than in PMOC (P=0.000;χ2=14.16-17.30,Plt;0.05). The PMOC diagnostic accuracy of CK7, PAX8, and SATB2 combined with tumor size and laterality was 82.2%, 76.7%, and 74.4%, respectively. The PMOC diagnostic accuracy of CK7/CDX2/CK20 was 76.6%. The PMOC diagnostic accuracy, sensitivity, specificity, and Youden index of CK7/PAX8/SATB2/tumor size/laterality was 94.4%, 93.2%, 100.0%, and 93.2%, respectively.
Conclusion "CK7, PAX8, or SATB2 can be used to differentiate between PMOC and MMOC. The diagnostic efficiency is significantly improved in combination with tumor size and laterality.
[KEY WORDS] "ovarian neoplasms; pathology, clinical; keratin-7; paired box gene 8; special AT-rich sequence-binding protein 2; diagnosis, differential
卵巢黏液癌是上皮性卵巢癌比较少见的一种类型,约占上皮性卵巢肿瘤的1%~3%[1]。卵巢是其他部位恶性肿瘤常见的转移位点[2],初步诊断为原发性卵巢黏液癌(PMOC)的病人中有50%~70%来自于其他部位的转移,并以下消化道来源的黏液癌转移最为常见[3]。PMOC和转移性卵巢黏液癌(MMOC)组织学及生物学行为相似,鉴别诊断较困难[4],并且二者的治疗与预后差异较大[5],有效鉴别PMOC与MMOC具有重要意义。目前临床上无有效区分PMOC和MMOC的方法。相关研究结果显示,可以根据肿瘤大小及侧向性区分原发性与转移性肿瘤[6-7]。细胞角蛋白7(CK7)、细胞角蛋白20(CK20)、尾型同源盒转录因子2(CDX2)是区分原发瘤与转移瘤的经典指标,下消化道来源肿瘤通常CK20、CDX2阳性表达;PMOC常为CK7阳性表达,CK20、CDX2阴性表达,但由于CK20、CDX2也可以在PMOC中表达,使得其诊断灵敏度、准确度降低[8]。近年来,特殊的含AT序列的结合蛋白2(SATB2)被证实为结直肠上皮和阑尾上皮特异和敏感的标志物[9]。配对盒基因8(PAX8)是卵巢黏液性恶性肿瘤特异性较强,但表达率较低的一个指标[10]。本研究探讨CK7、SATB2、PAX8联合肿瘤大小、侧向性在鉴别PMOC和MMOC中的应用,为二者临床鉴别诊断提供新思路。 现将研究结果报告如下。
1 资料与方法
1.1 标本及其来源
选取青岛大学附属医院2014年10月—2019年2月手术切除的PMOC和MMOC标本90例,均经40 g/L甲醛溶液固定、石蜡包埋。其中原发性卵巢黏液腺癌27例,原发性卵巢交界性黏液性肿瘤47例;阑尾MMOC 9例,结直肠MMOC 6例,小肠MMOC 1例。所有标本均由两名经验丰富的病理科医生复诊。收集所有病人的年龄、肿瘤大小等临床及病理特征资料。所选病人术前均未接受放疗和化疗,未合并其他恶性肿瘤及影响效应指标观测、判断的其他疾病。
1.2 检测指标及方法
使用免疫组织化学方法对标本进行SATB2、CK7、PAX8、CK20和CDX2染色。以PBS替代一抗作为阴性对照,乳腺浸润性导管癌标本作为CK7的阳性对照,结直肠癌标本作为CK20、CDX2以及SATB2的阳性对照,卵巢低分化浆液癌作为PAX8的阳性对照。所用免疫组织化学染色试剂均购自abcam公司。
1.3 结果判定
CK7及CK20在细胞质表达,CDX2、SATB2及PAX8在细胞核表达。由经验丰富的病理学专家使用双盲、二级计分法判定结果。阳性细胞比例计分标准:lt;1%为0分,1%~25%为1分,26%~50%为2分,51%~75%为3分,gt;75%为4分。染色强度评分:未着色为0分,淡黄色为1分,黄色或深黄色且背景未着色为2分,深褐色并浅棕色背景为2分,黄褐色或棕褐色为3分。以上述两项分数的乘积作为最终评分,评分lt;3分为阴性表达,评分≥3分为阳性表达。
1.4 统计学分析
应用SPSS 22.0软件进行统计分析。数据资料比较采用t检验、χ2检验、Fisher精确检验或Mann-Whitney U检验。Plt;0.05表示差异有显著性。
2 结" 果
2.1 PMOC与MMOC病人临床及免疫组化特征比较
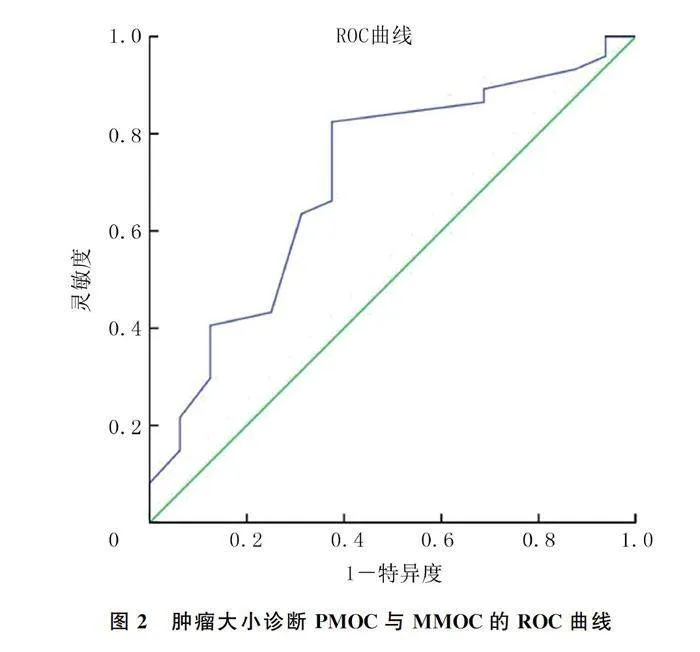
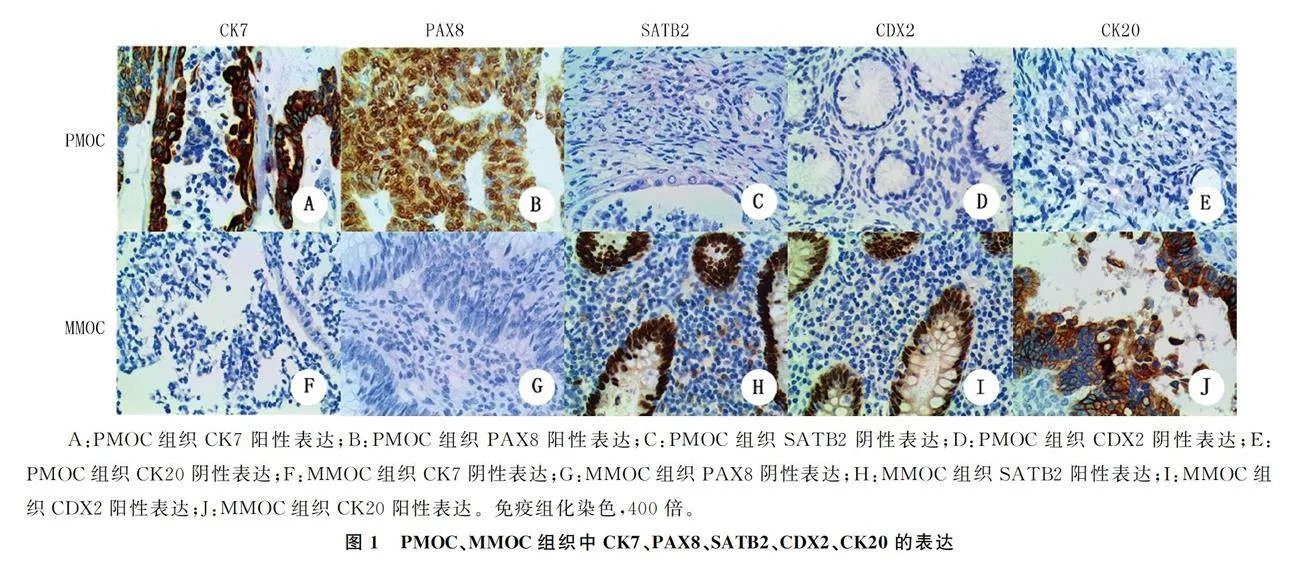
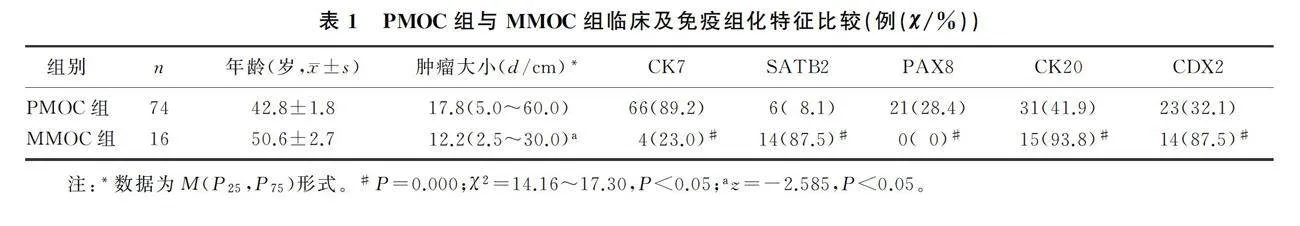
PMOC与MMOC病人年龄差异无统计学意义(Pgt;0.05)。PMOC组CK7、PAX8的阳性表达率高于MMOC组,SATB2、CK20、CDX2的阳性表达率低于MMOC组,差异有统计学意义(P=0.000;χ2=14.16~17.30,Plt;0.05),PAX8在MMOC组织中不表达(图1)。PMOC组肿瘤直径显著大于MMOC组(Z=-2.585,Plt;0.05)。见表1。ROC曲线分析显示,根据肿瘤大小鉴别诊断PMOC与MMOC的曲线下面积为0.706(95%CI=0.563~0.849,P=0.010)。选取肿瘤直径10 cm作为最佳界值,PMOC组有61例(82.4%)肿瘤直径gt;10 cm,MMOC组仅有6例(37.5%)肿瘤直径gt;10 cm(图2),两组比较差异有显著性(P=0.001)。PMOC组肿瘤单侧者68例(91.9%),MMOC组肿瘤双侧者11例(68.8%),两组比较差异有统计学意义(P=0.000)。
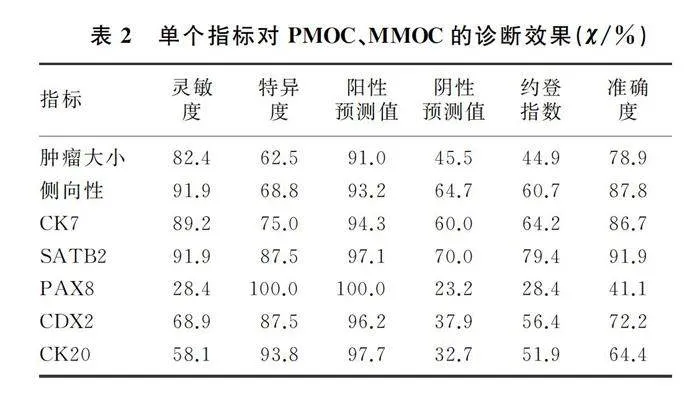
2.2 单个指标对PMOC、MMOC的诊断效果
CDX2、CK20对PMOC与MMOC进行鉴别诊断的准确度分别为72.2%、64.4%,灵敏度则分别为68.9%、58.1%,是所有指标中除PAX8外较低的两个。虽然PAX8诊断PMOC与MMOC的准确度仅为41.1%,但其在MMOC中不表达,诊断特异度为100.0%。CK7、SATB2、肿瘤大小及侧向性诊断PMOC与MMOC的准确度较高,其中SATB2的准确度最高,为91.9%。见表2。
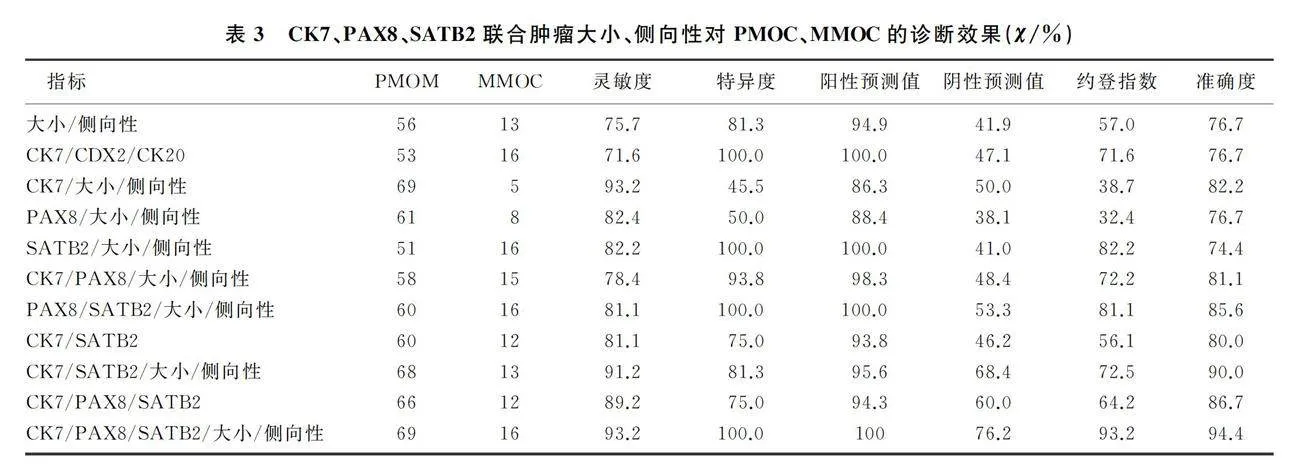
2.3 CK7、PAX8、SATB2联合肿瘤大小、侧向性对PMOC、MMOC的诊断效果
CK7/大小/侧向性联合诊断PMOC与MMOC的准确度高于CK7/CDX2/CK20联合(82.2% vs 76.7%)。PAX8联合肿瘤大小、侧向性使得诊断的灵敏度、准确度较PAX8升高。PAX8分别与CK7、SATB2联合无鉴别诊断意义(P=1.000、0.553),但再联合肿瘤大小、侧向性诊断的特异度、准确度均升高。CK7/PAX8/SATB2联合诊断PMOC与MMOC的准确度高于CK7/CDX2/CK20联合(86.7% vs 76.7%)。CK7/PAX8/SATB2与大小、侧向性联合诊断PMOC与MMOC具有较高的灵敏度、特异度、准确度及约登指数,为所有指标单独或联合诊断中最高者。见表3。
3 讨" 论
PMOC与MMOC的鉴别诊断一直是临床工作中的挑战。卵巢黏液癌可与其他组织学类型的肿瘤同时存在,表现为持续的恶性进展过程,与结直肠癌的进展过程相似[11],MMOC的生长模式与PMOC类似[12]。原发性与转移性卵巢癌治疗方案与预后截然不同[13],因此有效区分原发与转移性肿瘤对病人的临床管理十分重要。本文研究结果显示,单个指标CK7、PAX8、SATB2可以用于鉴别PMOC与MMOC,联合肿瘤大小、侧向性诊断效能显著提高。
继发性卵巢肿瘤通常双侧受累[14],肿瘤体积大于胃肠道原发部位,有时转移症状先于原发症状出现,导致病人最初的临床表现可能为卵巢或盆腔肿物[7]。本文研究结果显示,应用侧向性可正确诊断87.8%病人,PMOC病人中有91.1%表现为单侧,与既往文献结果相一致[15]。本文研究有68.8%的MMOC病人肿瘤为双侧,较相关研究46.3%结果稍高[16],但低于LEE等[17]结果(75%),原因可能与国内外人群差异或标本量不同有关。应用肿瘤侧向性诊断PMOC与MMOC的特异度较低,有误诊的可能。转移瘤体积通常小于原发性卵巢肿瘤[14-15]。本文研究结果显示,与MMOC组相比,PMOC组肿瘤直径显著增大,82.4%的PMOC病人肿瘤最大直径gt;10 cm。肿瘤大小与侧向性的联合应用可提高PMOC诊断的特异度(81.3%),当卵巢或盆腔包块较小且为双侧时,应高度考虑恶性转移可能[18]。虽然肿瘤大小和侧向性可以区分原发与转移癌,但约登指数仅为57.0%,准确度仅为76.7%,仍需更准确的方法鉴别PMOC与MMOC。
CK7、CK20、CDX2是区分卵巢原发性与下消化道转移癌最常用、最典型的标志物。但它们在肿瘤组织免疫组化中的重叠表达降低了诊断的准确性[19]。既往研究显示,CK7、CK20是鉴别PMOC与MMOC的有效指标[20],但本研究中CK7、CK20联合鉴别诊断PMOC与MMOC诊断效能差异无统计学意义,这可能与CK20灵敏度低[8]或者样本量较小有关。CDX2是结直肠癌特异性的标记物,本研究中CDX2在87.5%的MMOC中表达,诊断PMOC与MMOC的特异度达87.5%,与既往文献结果相一致[21]。但CDX2诊断的灵敏度较低[22],本研究中CDX2诊断PMOC与MMOC的灵敏度为68.9%,阴性预测值仅为37.9%,可能会导致误诊。在SATB2、CDX2、CK20等指标中,SATB2区分原发与转移癌的灵敏度、准确度最高。PAX8是苗勒管来源肿瘤敏感特异的标志物,在卵巢黏液性
肿瘤中表达不稳定,表达率为0~70%,在下消化道
上皮组织中基本不表达[23]。本文结果显示,PAX8在PMOC组织表达阳性率为28.4%,在MMOC组织中不表达。HU等[24]研究显示,PAX8在53.2%的PMOC组织中表达。本文结果与其不一致,可能与检测所用抗体或样本量不同有关。PAX8表达阳性应高度怀疑为PMOC而非MMOC。
本文研究首次联合CK7、PAX8、SATB2与肿瘤大小、肿瘤侧向性鉴别诊断PMOC与MMOC,结果显示,CK7、PAX8与肿瘤大小、侧向性联合诊断PMOC与MMOC的灵敏度、准确度均较单个指标升高,SATB2联合肿瘤大小、侧向性诊断PMOC与MMOC具有100%的特异度,CK7/PAX8/SATB2三者联合诊断两种疾病的灵敏度、准确度高于CK7/CDX2/CK20联合;CK7/PAX8/SATB2与肿瘤大小、侧向性联合诊断具有最高的灵敏度(93.2%)、特异度(100.0%)、准确度及约登指数(93.2%),诊断效果最好。因此,可以考虑在不增加成本的基础上,用PAX8、SATB2替代CDX2、CK20,联合肿瘤大小、侧向性提高PMOC与MMOC鉴别诊断的效率。
综上所述,CK7、PAX8、SATB2表达检测可以鉴别PMOC与MMOC,联合肿瘤大小、侧向性诊断效能显著提高,联合诊断效能优于单个或多个免疫组化指标。PAX8阳性表达应高度怀疑为PMOC,而非MMOC。但本文研究样本量较小,且为单中心样本,为提高鉴别诊断的效率,需要扩大样本量、纳入多地区、多种族人群继续研究。卵巢黏液癌的确诊仍需要详尽的影像学检查、彻底的术中探查及准确的组织病理学检查。
[参考文献]
[1]SHIMADA M, KIGAWA J, OHISHI Y, et al. Clinicopatho-
logical characteristics of mucinous adenocarcinoma of the ovary[J]. Gynecol Oncol, 2009,113(3):331-334.
[2] JANG J Y A, YANAIHARA N, PUJADE-LAURAINE E, et al. Update on rare epithelial ovarian cancers: based on the Rare Ovarian Tumors Young Investigator Conference[J]. Journal of Gynecologic Oncology, 2017,28(4):e54.
[3] PERREN T J. Mucinous epithelial ovarian carcinoma[J]. Annals of Oncology, 2016,27: i53-i57.
[4] MILLS A M, SHANES E D. Mucinous ovarian tumors[J]. Surgical Pathology Clinics, 2019,12(2):565-585.
[5] KAJIYAMA H, SUZUKI S, UTSUMI F, et al. Comparison of long-term oncologic outcomes between metastatic ovarian carcinoma originating from gastrointestinal organs and advanced mucinous ovarian carcinoma[J]. International Journal of Clinical Oncology, 2019, 24(8):950-956.
[6] SEIDMAN J D, KURMAN R J, RONNETT B M. Primary and metastatic mucinous adenocarcinomas in the ovaries: incidence in routine practice with a new approach to improve intraoperative diagnosis[J]. The American Journal of Surgical Pathology, 2003, 27(7):985-993.
[7] KHUNAMORNPONG S, SUPRASERT P, POJCHAMARNWIPUTH S, et al. Primary and metastatic mucinous adenocarcinomas of the ovary: Evaluation of the diagnostic approach using tumor size and laterality[J]. Gynecologic Oncology, 2006,101(1):152-157.
[8] MEAGHER N S, WANG L Y, RAMBAU P F, et al. A combination of the immunohistochemical markers CK7 and SATB2 is highly sensitive and specific for distinguishing primary ova-
rian mucinous tumors from colorectal and appendiceal metastases[J]. Modern Pathology: an Official Journal of the United States and Canadian Academy of Pathology, Inc, 2019,32(12):1834-1846.
[9]STRICKLAND S, PARRA-HERRAN C. Immunohistochemical characterization of appendiceal mucinous neoplasms and the value of special AT-rich sequence-binding protein 2 in their distinction from primary ovarian mucinous tumours[J]. Histopathology, 2016,68(7):977-987.
[10]LI Z B, ROTH R, ROCK J B, et al. Dual immunostain with SATB2 and CK20 differentiates appendiceal mucinous neoplasms from ovarian mucinous neoplasms[J]. American Journal of Clinical Pathology, 2017,147(5):484-491.
[11]MORICE P, GOUY S, LEARY A. Mucinous ovarian carcinoma[J]. The New England Journal of Medicine, 2019,380(13):1256-1266.
[12]PARK C K, KIM H S. Clinicopathological characteristics of ovarian metastasis from colorectal and pancreatobiliary carcinomas mimicking primary ovarian mucinous tumor[J]. Anticancer Research, 2018,38(9):5465-5473.
[13]BABAIER A, GHATAGE P. Mucinous cancer of the ovary: overview and current status[J]. Diagnostics, 2020,10(1):52.
[14]ZHANG W, TAN C, XU M D, et al. Appendiceal mucinous neoplasm mimics ovarian tumors: Challenges for preoperative and intraoperative diagnosis and clinical implication[J]. European Journal of Surgical Oncology, 2019,45(11):2120-2125.
[15]SIMONS M, BOLHUIS T, HAAN A F, et al. A novel algorithm for better distinction of primary mucinous ovarian carcinomas and mucinous carcinomas metastatic to the ovary[J]. Virchows Archiv, 2019,474(3):289-296.
[16]BRULS J, SIMONS M, OVERBEEK L I, et al. A national population-based study provides insight in the origin of malignancies metastatic to the ovary[J]. Virchows Archiv, 2015,467(1):79-86.
[17]LEE K R, YOUNG R H. The distinction between primary and metastatic mucinous carcinomas of the ovary: gross and histologic findings in 50 cases[J]. The American Journal of Surgical Pathology, 2003, 27(3):281-292.
[18]ACKROYD S A, GOETSCH L, BROWN J, et al. Pancreaticobiliary metastasis presenting as primary mucinous ovarian neoplasm: a systematic literature review[J]. Gynecologic Oncology Reports, 2019, 28:109-115.
[19]VANG R, GOWN A M, BARRY T S, et al. Cytokeratins 7 and 20 in primary and secondary mucinous tumors of the ovary: analysis of coordinate immunohistochemical expression profiles and staining distribution in 179 cases[J]. The American Journal of Surgical Pathology, 2006,30(9):1130-1139.
[20]MISSAOUI N, SALHI S, BDIOUI A, et al. Immunohistochemical characterization improves the reproducibility of the histological diagnosis of ovarian carcinoma[J]. Asian Pacific Journal of Cancer Prevention: APJCP, 2018,19(9):2545-2551.
[21]CECCHINI M J, WALSH J C, PARFITT J, et al. CDX2 and Muc2 immunohistochemistry as prognostic markers in stage Ⅱ colon cancer[J]. Human Pathology, 2019,90:70-79.
[22]VANG R, GOWN A M, WU L S, et al. Immunohistochemical expression of CDX2 in primary ovarian mucinous tumors and metastatic mucinous carcinomas involving the ovary: comparison with CK20 and correlation with coordinate expression of CK7[J]. Modern Pathology: an Official Journal of the Uni-
ted States and Canadian Academy of Pathology, Inc, 2006,19(11):1421-1428.
[23]ATES OZDEMIR D, USUBUTUN A. PAX2, PAX8 and CDX2 expression in metastatic mucinous, primary ovarian mucinous and seromucinous tumors and review of the literature[J]. Pathology Oncology Research: POR, 2016,22(3):593-599.
[24]HU A, LI H W, ZHANG L T, et al. Differentiating primary and extragenital metastatic mucinous ovarian tumours: an algorithm combining PAX8 with tumour size and laterality[J]. Journal of Clinical Pathology, 2015,68(7):522-528.
(本文编辑 黄建乡)

