山东汉族人群ADD2基因rs3755351位点单核苷酸多态性与重度子痫前期遗传易感性的关系

[摘要] 目的 研究山东汉族人群内收蛋白2(ADD2)基因rs3755351位点单核苷酸多态性与重度子痫前期遗传易感性的关系。
方法 选取山东地区1 184例重度子痫前期病人(病例组)和1 421例健康对照者(对照组)作为研究对象,将病例组病人进一步分为早发型重度子痫前期(EOSP)和晚发型重度子痫前期(LOSP)两个亚组。提取外周血DNA,采用TaqMan探针PCR技术检测ADD2基因rs3755351位点基因型分布和等位基因频率。
结果
病例组ADD2基因rs3755351位点AA、AC、CC基因型的频率分别为10.81%、43.67%和45.52%,A、C等位基因的频率分别为32.64%和67.36%,与对照组相比,差异均无统计学意义(P>0.05)。EOSP组与对照组相比较,rs3755351位点的等位基因频率差异有显著性(χ2=6.394,P<0.05),但基因型频率差异无统计学意义(P>0.05)。EOSP组与LOSP组、LOSP组与对照组比较,rs3755351位点的基因型和等位基因频率差异均无统计学意义(P>0.05)。
结论 山东汉族人群ADD2基因rs3755351位点的单核苷酸多态性可能与子痫前期的严重程度有关,该基因是EOSP的易感基因之一。
[关键词] 先兆子痫;ADD2基因;多态性,单核苷酸;疾病遗传易感性
[中图分类号] R714.244;R363.25
[文献标志码] A
[文章编号] 2096-5532(2023)01-0097-04
doi:10.11712/jms.2096-5532.2023.59.024
[网络出版] https://kns.cnki.net/kcms/detail/37.1517.R.20230302.1736.003.html;2023-03-03 15:43:36
RELATIONSHIP BETWEEN SINGLE NUCLEOTIDE POLYMORPHISM AT RS3755351 IN ADD2 GENE AND GENETIC SUSCEPTIBILITY TO SEVERE PREECLAMPSIA IN HAN POPULATION OF SHANDONG PROVINCE, CHINA
YANG Zhencui, XU Longqiang, LIU Shuhui, ZONG Jinbao
(Department of Clinical Lab, The Affiliated Hospital of Qingdao University, Qingdao 266003, China)
; [ABSTRACT] "Objective "To study the relationship between single nucleotide polymorphism at rs3755351 in the beta-adducin gene (ADD2) and genetic susceptibility to severe preeclampsia in the Han population of Shandong province, China.
Methods
A total of 1 184 patients with severe preeclampsia (case group) and 1 421 healthy controls (control group) from Shandong province were included. The case group was further divided into early-onset severe preeclampsia (EOSP) and late-onset severe preeclampsia (LOSP) subgroups. DNA was extracted from peripheral blood. TaqMan PCR was used to analyze the genotype and allele frequencies at rs3755351 in the ADD2 gene.
Results "In the case group, the frequencies of AA, AC, and CC genotypes at rs3755351 in the ADD2 gene were 10.81%, 43.67%, and 45.52%, respectively, and the frequencies of alleles A and C were 32.64% and 67.36%, respectively, with no significant differences from those in the control group (Pgt;0.05). The EOSP group differed significantly from the control group in the allele frequency of rs3755351 (χ2=6.394,Plt;0.05), but not in the genotype frequency (Pgt;0.05). There were no significant differences in the genotype and allele frequencies of rs3755351 between the EOSP and LOSP groups, nor between the LOSP and control groups.
Conclusion "The single nucleotide polymorphism at rs3755351 in the ADD2 gene may be related to the severity of preeclampsia among the Shandong Han population. This gene is a susceptibility gene for EOSP.
[KEY WORDS] "pre-eclampsia; ADD2 gene; polymorphism, single nucleotide; genetic
子痫前期(PE)是一种以妊娠期高血压和蛋白尿为特征的妊娠特异性疾病,全球发病率为3%~5%,严重危害母婴健康[1]。在巴西,高血压疾病是导致孕产妇围生期死亡的主要原因,占所调查孕产妇死因的25%[2]。另外,高血压还与胎儿死亡、新生儿死亡、宫内生长受限和早产的风险增加有关[3]。研究表明,内皮细胞损伤、氧化应激、遗传因素和营养因素等与PE的发病有关[4]。内收蛋白(ADD)是
一种细胞膜骨架蛋白,由α、β、γ 等3个亚基组成异源二聚体。这3个亚基分别由基因ADD1、ADD2以及ADD3所编码[5]。有文献报道,ADD的遗传变异与高血压及相关疾病有关,且其基因多态性与高血压的易感性密切相关[6-8]。基于此,我们推测ADD2基因的遗传变异可能与PE有关。因此,本研究探讨了山东汉族人群ADD2基因rs3755351位点的单核苷酸多态性与重度PE遗传易感性的关系,旨在为阐明PE的发病机制提供新的思路。现将结果报告如下。
1 对象和方法
1.1 研究对象
选取2014年1月—2016年1月青岛大学附属医院、山东省立医院、滨州医学院附属医院的重度PE病人1 184例(病例组)和正常孕妇1 421例(对照组)作为研究对象。病例组只纳入孕周大于28周的重度PE病人,PE诊断均符合《妊娠期高血压疾病诊治指南(2020)》的标准[9]。根据发生重度PE的早晚,将病例组病人分为早发型重度PE(EOSP,指妊娠34周以前发生的重度PE)和晚发型重度PE(LOSP,≥34周发生的重度PE)[10]。本研究获青岛大学附属医院伦理委员会批准,所有研究对象均签署知情同意书。
1.2 研究方法
1.2.1 外周血DNA提取 采集所有研究对象外周肘静脉血2 mL,EDTA抗凝,冻存于-80 ℃冰箱。用天根血液基因组DNA提取试剂盒(离心柱式)提取400 μL外周血基因组DNA,-20 ℃保存备用。
1.2.2 ADD2基因分型分析 应用美国ABI公司合成的TaqMan探针,采用TaqMan探针PCR技术对ADD2基因的rs3755351位点进行单核苷酸多态性分析。rs3755351位点检测前引物序列为5′-TGTGTTCAGCGACAGTATCTCTTTA-3′,后引物序列为5′-GGACTAGTGACTTGGGAGCCAC-TTA-3′。实时荧光定量PCR反应体系共7 μL,由1.75 μL 2×MIX溶液、0.05 μL 20×SNP探针、1.00 μL模板DNA和4.20 μL的去离子水组成。将上述反应体系放入C1000TM热循环仪和CFX96TM实时系统(BIO-RAD,Hercules,CA)中进行扩增,条件如下:95 ℃变性10 min,95 ℃退火15 s,60 ℃延伸1 min,共45个循环。荧光信号在60 ℃延伸时进行捕捉。采用Bio-Rad CFX Manager 3.0软件进行基因型判别。为验证TaqMan探针法的准确性,随机抽取两组的20份DNA样本进行Sanger测序,结果显示两种方法的一致性为100%。
1.3 统计学分析
使用SPSS 24.0软件进行统计学分析。计量数据以±s表示,两组比较采用t检验;两组计数资料的比较采用卡方检验。以P<0.05为差异有统计学意义。
2 结" 果
2.1 临床资料比较
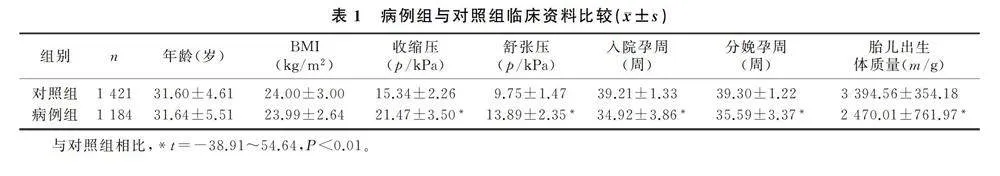
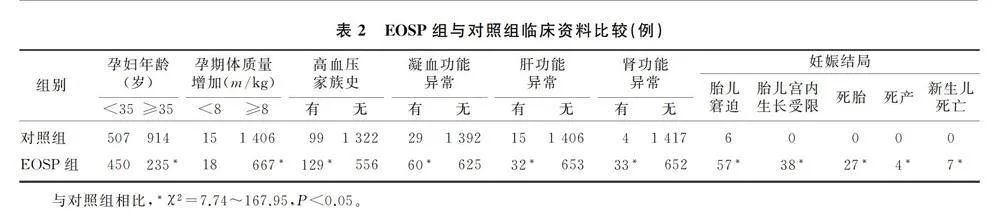
与对照组相比,病例组的收缩压和舒张压明显升高,入院孕周和分娩孕周明显提前,胎儿出生体质量明显降低(t=-38.91~54.64,P<0.01)。见表1。EOSP组与对照组相比,年龄、孕期体质量增加、高血压家族史、凝血功能、肝肾功能等指标差异均有统计学意义(χ2=7.74~167.95,P<0.05);对妊娠结局进行分析,EOSP组胎儿窘迫、胎儿宫内生长受限、死胎、死产、新生儿死亡的发生率均显著高于对照组,差异具有统计学意义(χ2=8.31~99.37,P<0.05)。见表2。
2.2 病例组与对照组基因型和等位基因频率比较
病例组和对照组ADD2基因rs3755351位点的基因型分布符合Hardy-Weinberg平衡定律(P>0.05)。两组基因型和等位基因频率比较差异均无统计学意义(χ2=3.345、3.308,P>0.05)。见表3。
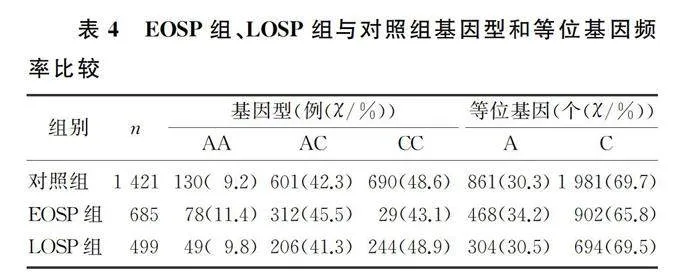
2.3 EOSP组、LOSP组与对照组基因型和等位基因频率比较
EOSP组与对照组比较,rs3755351位点的等位基因频率差异有显著性(χ2=6.394,P<0.05),但基因型频率差异无统计学意义(χ2=1.065,P>0.05)。EOSP组与LOSP组比较、LOSP组与对照组比较,rs3755351位点的基因型和等位基因频率差异均无统计学意义(χ2=0.010~4.018,P>0.05)。见表4。
3 讨" 论
PE是一种妊娠特异性疾病,全球的发病率为3.2%~12.0%,我国的发病率为4.2%[11-12]。有研究表明,妊娠早期胎盘发育受损和螺旋动脉重构受损通常与EOSP有关,而LOSP可能与母体内皮功能障碍有关[13]。一项全面的前瞻性研究表明,PE与心血管疾病死亡独立相关,并且,与LOSP相比,EOSP胎儿生长受限更严重,孕产妇围生期的发病率和死亡率更高[14],远期发生心血管疾病死亡的风险也更高[15]。
ADD2基因位于染色体2p13-p14上,有13个外显子,大小为100 kb。ADD2在大脑、胚胎和肾中表达最高,在维持细胞形态、参与物质运输、传递细胞形态信号、影响和控制细胞增殖方面发挥重要作用[16-18]。例如,敲除ADD2基因的小鼠会表现出以轻度贫血和代偿性溶血为特征的表型[19-20]。在小鼠大脑中,ADD2被认为是突触结构的组成部分,在海马的突触可塑性中协调运动和学习记忆过程[21]。在肾脏组织中,ADD2蛋白的突变改变了肾脏Na+-K+-ATP酶内吞作用的动态调节,还通过调节特定足细胞的表达,参与肾小球病变的发展[18,22]。流行病学研究还发现,ADD2可能是盐敏感型原发性高血压的易感基因,通过增加肾小管基底膜Na+-K+-
ATP酶活性,增加肾小管上皮细胞对Na+的重吸
收,导致高血压[5,23]。例如,KATO等[24]在对日本高血压人群进行大量病例-对照关联研究中发现,ADD2基因rs3755351位点多态性与高血压显著相关。耿启彬等[25]研究发现,在福建人群中,ADD2基因的rs3755351位点与高血压遗传易感性相关,与AC或CC基因型相比,携带AA基因型能显著降低罹患高血压的风险,但在广东人群中未发现该位点与高血压的遗传易感性相关。在中国藏族人群中,ADD2基因rs3755351位点多态性与藏族高原高血压的发生有关,是世居藏族人群高原高血压发生的易感基因之一[26]。
本研究对1 184例重度PE孕妇和1 421例健康孕妇的一般临床资料分析显示,与对照组相比,重度PE孕妇收缩压和舒张压都升高,入院孕周和分娩孕周均提前,胎儿出生体质量较低,这进一步佐证了孕产妇发生不良结局的风险与PE的严重程度相关。基因多态性分析结果显示,两组孕妇ADD2基因rs3755351位点的基因型和等位基因频率差异无显著意义。LOSP组与EOSP组、LOSP组与对照组比较,rs3755351位点的基因型和等位基因频率差异均无统计学意义;但EOSP组与对照组相比,rs3755351位点的等位基因频率差异有统计学意义,而基因型频率差异无统计学意义。提示山东汉族人群ADD2基因rs3755351位点的单核苷酸多态性可能与PE严重程度有关,该基因是EOSP的易感基因之一。由于本研究仅局限于山东地区人群,而基因位点的多态性在不同种族和地域间往往有较大的差异,因此,ADD2基因rs3755351位点与PE易感性的相关性尚需在中国其他地区人群中加以验证。
[参考文献]
[1]ARMALY Z, JADAON J E, JABBOUR A, et al. Preeclampsia: novel mechanisms and potential therapeutic approaches[J]. Frontiers in Physiology, 2018,9:973.
[2]COSTA P R F, ASSIS A M O, CUNHA C M, et al. Hypertriglyceridemic waist phenotype and changes in the fasting gly-
cemia and blood pressure in children and adolescents over oneyear follow-up period[J]. Arquivos Brasileiros De Cardiologia, 2017,109(1):47-53.
[3]PAYNE B A, HUTCHEON J A, ANSERMINO J M, et al. A risk prediction model for the assessment and triage of women with hypertensive disorders of pregnancy in low-resourced settings: the miniPIERS (Pre-eclampsia Integrated Estimate of RiSk) multi-country prospective cohort study[J]. PLoS Medicine, 2014,11(1):e1001589.
[4]YOUNG B C, LEVINE R J, KARUMANCHI S A. Pathoge-
nesis of preeclampsia[J]. Annual Review of Pathology: Me-
chanisms of Disease, 2010,5:173-192.
[5]LI X C, HAI J J, TAN Y J, et al. miR-218 suppresses metastasis and invasion of endometrial cancer via negatively regulating ADD2[J]. European Review for Medical and Pharmacological Sciences, 2019,23(4):1408-1417.
[6]NEWTON-CHEH C, JOHNSON T, GATEVA V, et al. Genome-wide association study identifies eight loci associated with blood pressure[J]. Nature Genetics, 2009,41(6):666-676.
[7]LEVY D, EHRET G B, RICE K, et al. Genome-wide association study of blood pressure and hypertension[J]. Nature Genetics, 2009,41(6):677-687.
[8]CHO Y S, GO M J, KIM Y J, et al. A large-scale genome-wide association study of Asian populations uncovers genetic factors influencing eight quantitative traits[J]. Nature Gene-
tics, 2009,41(5):527-534.
[9]杨孜,张为远. 《妊娠期高血压疾病诊治指南(2020)》解读[J]. 中华妇产科杂志, 2020,55(6):425-432.
[10]REN Z L, GAO Y F, GAO Y, et al. Distinct placental mole-
cular processes associated with early-onset and late-onset preeclampsia[J]. Theranostics, 2021,11(10):5028-5044.
[11]SUN N X, QIN S T, ZHANG L, et al. Roles of noncoding RNAs in preeclampsia[J]. Reproductive Biology and Endocrinology: RBamp;E, 2021,19(1):100.
[12]STAFF A C. The two-stage placental model of preeclampsia: an update[J]. Journal of Reproductive Immunology, 2019,134-135:1-10.
[13]OGGE G, CHAIWORAPONGSA T, ROMERO R, et al. Placental lesions associated with maternal underperfusion are more frequent in early-onset than in late-onset preeclampsia[J]. Journal of Perinatal Medicine, 2011,39(6):641-652.
[14]PARA R, ROMERO R, GOMEZ-LOPEZ N, et al. Maternal circulating concentrations of soluble Fas and Elabela in early- and late-onset preeclampsia[J]. The Journal of Maternal-Fetal amp; Neonatal Medicine, 2022,35(2):316-329.
[15]MONGRAW-CHAFFIN M L, CIRILLO P M, COHN B A. Preeclampsia and cardiovascular disease death: prospective evidence from the child health and development studies cohort[J]. Hypertension (Dallas, Tex: 1979), 2010,56(1):166-171.
[16]ZHANG J R, HU W N, LI C Y. A review of the epidemiolo-
gical evidence for adducin family gene polymorphisms and hypertension[J]. Cardiology Research and Practice, 2019,2019:7135604.
[17]KIANG K M Y, LEUNG G K K. A review on adducin from functional to pathological mechanisms: future direction in cancer[J]. BioMed Research International, 2018,2018:3465929.
[18]FERRANDI M, CUSI D, MOLINARI I, et al. alpha- and beta-Adducin polymorphisms affect podocyte proteins and proteinuria in rodents and decline of renal function in human IgA nephropathy[J]. Journal of Molecular Medicine (Berlin, Germany), 2010,88(2):203-217.
[19]PORRO F, COSTESSI L, MARRO M L, et al. The erythrocyte skeletons of beta-adducin deficient mice have altered levels of tropomyosin, tropomodulin and EcapZ[J]. FEBS Letters, 2004,576(1-2):36-40.
[20]MURO A F, MARRO M L, GAJOVI S, et al. Mild spherocytic hereditary elliptocytosis and altered levels of alpha- and gamma-adducins in beta-adducin-deficient mice[J]. Blood, 2000,95(12):3978-3985.
[21]PORRO F, ROSATO-SIRI M, LEONE E, et al. Beta-adducin (Add2) KO mice show synaptic plasticity, motor coordination and behavioral deficits accompanied by changes in the expression and phosphorylation levels of the alpha- and gamma-adducin subunits[J]. Genes, Brain, and Behavior, 2010,9(1):84-96.
[22]EFENDIEV R, KRMAR R T, OGIMOTO G, et al. Hypertension-linked mutation in the adducin alpha-subunit leads to higher AP2-mu2 phosphorylation and impaired Na+, K+-ATPase trafficking in response to GPCR signals and intracellular sodium[J]. Circulation Research, 2004,95(11):1100-1108.
[23]LIU C M, HSU W H, LIN W Y, et al. Adducin family proteins possess different nuclear export potentials[J]. Journal of Biomedical Science, 2017,24(1):30.
[24]KATO N, MIYATA T, TABARA Y, et al. High-density association study and nomination of susceptibility genes for hypertension in the Japanese National Project[J]. Human Mole-
cular Genetics, 2008,17(4):617-627.
[25]耿启彬,李媛媛,李红,等. Beta-adducin(Add2)基因rs3755351多态性与高血压遗传易感的相关性研究[J]. 分子诊断与治疗杂志, 2013,5(5):301-305.
[26]汪晓洲,刘玉林,姚京豫,等. ADD2基因rs3755351位点多态性与藏、汉民族高原高血压发生的相关性[J]. 中国高原医学与生物学杂志, 2020,41(4):233-237.
(本文编辑 马伟平)

