幽门螺杆菌根除后早期胃癌的特点分析
[摘要] 目的 探讨幽门螺杆菌(Hp)根除后早期胃癌的临床、内镜表现及组织病理学特点,提高诊断准确率。
方法 回顾性分析2017年1月—2021年12月诊治,经内镜黏膜下剥离术(ESD)切除的Hp根除后早期胃癌病人50例临床资料,分析其临床特征、内镜下表现及组织病理学特点。
结果 50例病人共54处病灶,有临床症状42例(84.0%),服用质子泵抑制剂(PPI)超过1年43例(86.0%)。胃黏膜中重度萎缩29例(58.0%),既往有肠上皮化生40例(80.0%)。54处病灶位于胃体或胃窦42处(77.8%),病灶平均直径≤20 mm有44处(81.5%);巴黎分型0-Ⅱc 29处(53.7%),0-Ⅱa+Ⅱc 17处(31.5%),0-Ⅱa 7处(13.0%),0-Ⅱb 1处(1.8%)。典型内镜下表现为地图样发红43处(79.6%)。26例病人追踪到免疫组化结果,Ki67阳性表达率≤50%有17例(65.4%)。
结论 Hp根除后早期胃癌的内镜特点表现为背景黏膜多有中重度萎缩、地图样发红、病灶直径≤20 mm、浅表凹陷型,据此可提高Hp根除后早期胃癌的诊断准确率。
[关键词] 幽门螺杆菌;胃肿瘤;胃镜检查;回顾性研究
[中图分类号] R573.9;R735.2
[文献标志码] A
[文章编号] 2096-5532(2023)01-0055-05
doi:10.11712/jms.2096-5532.2023.59.002
[网络出版] https://kns.cnki.net/kcms/detail//37.1517.R.20230220.1440.001.html;2023-02-21 14:24:23
CHARACTERISTICS OF EARLY GASTRIC CANCER AFTER HELICOBACTER PYLORI ERADICATION
WANG Xinyu, LIU Xinyuan, MAO Tao, WEI Zhi, FU Jindong, LI Xiaoyu
(Department of Gastroenterology, The Affiliated Hospital of Qing-
dao University, Qingdao 266003, China)
; [ABSTRACT] "Objective "To investigate the clinical features, endoscopic findings, and histopathological features of early gastric cancer after Helicobacter pylori (Hp) eradication, and to improve diagnostic accuracy.
Methods "A retrospective analysis was performed for the clinical data of 50 patients with early gastric cancer after Hp eradication who underwent resection by endoscopic submucosal dissection (ESD) from January 2017 to December 2021, including clinical features, endoscopic findings, and histopathological features.
Results "The 50 patients had a total of 54 lesions, among whom 42(84.0%) had clinical symptoms and 43(86.0%) took proton pump inhibitors (PPI) for more than 1 year. Among these 50 patients, 29(58.0%) had moderate to severe gastric mucosal atrophy, and 40(80.0%) had a past history of intestinal metaplasia. Among the 54 lesions, 42(77.8%) were located in the gastric body or antrum and 44(81.5%) had a mean diameter of ≤20 mm; based on the Paris classification, there were 29(53.7%) 0-Ⅱc lesions, 17(31.5%) 0-Ⅱa+Ⅱc lesions, 7(13.0%) 0-Ⅱa lesions, and 1(1.8%) 0-Ⅱb lesion. The typical endosco-
pic finding was map-like redness in 43 lesions (79.6%). Immunohistochemical results were obtained for 26 patients, among whom 17(65.4%) had a positive Ki67 expression rate of ≤50%.
Conclusion "Endoscopic features of early gastric cancer after Hp eradication include moderate to severe atrophy and map-like redness of the background mucosa, a lesion diameter of ≤20 mm, and superficial depressed type, which can be used to improve the diagnostic accuracy of early gastric cancer after Hp eradication.
[KEY WORDS] "Helicobacter pylori; stomach neoplasms; gastroscopy; retrospective studies
幽门螺杆菌(Hp)感染可引起胃黏膜慢性炎症,逐渐进展成癌前病变,导致萎缩性胃炎和肠上皮化生[1-3],胃癌发生风险明显增加[4]。Hp为胃癌的Ⅰ类致癌因子,尽早发现Hp感染并治疗是胃癌的预防措施之一[5-6]。多项研究表明,根除 Hp可有效降低胃癌发生风险,但根除Hp后仍可发生胃癌 [7-8]。近年来Hp根除后早期胃癌越来越多地受到关注。Hp根除后胃癌是除菌1年后发现的胃癌,包括除菌后发生的胃癌以及除菌前发生、但在除菌后被发现的胃癌 [9]。本文回顾分析确诊的Hp根除后早期胃癌病人的临床资料,总结其临床特征、内镜表现及组织病理学特点,以提高其诊断的准确率。
1 资料和方法
1.1 研究对象
收集青岛大学附属医院、山东省第二人民医院、日照市人民医院、青岛市中心医院2017年1月—2021年12月诊治,经内镜黏膜下剥离术(ESD)切除后早期胃癌病人4 620例,通过查阅病历资料以及电话随访,从中筛选出确诊为Hp根除后早期胃癌病人共50例。纳入标准:①既往胃镜病理或碳13呼气试验示有Hp感染,已行根除Hp治疗;②除菌治疗1年后发现早期胃癌病变,并行ESD治疗,ESD术后病理诊断为腺癌、印戒细胞癌、高级别上皮内瘤变;③病理检查示Hp阴性或未检出或碳13呼气试验提示Hp阴性。同时满足以上3项即可诊断为Hp根除后早期胃癌。本研究通过我院伦理委员会审查批准。
1.2 研究方法
收集病人临床资料,包括年龄、性别、吸烟及饮酒史、家族史、既往史、临床表现、是否长期使用质子泵抑制剂(PPI)、内镜表现(包括背景黏膜、病变的内镜下分型、病灶部位、大小等)以及组织学类型等。背景黏膜包括是否有地图样发红及萎缩程度,地图样发红指内镜下表现为多发的平坦状或伴有轻微凹陷的红斑样病灶,成功根除Hp后弥漫性发红消失,非萎缩黏膜和萎缩黏膜之间色差对比增强,即相关研究提出的“色调逆转”[9];根据木村-竹本分类法将胃黏膜萎缩分为轻度(C-1、C-2)、中度(C-3、O-1)、重度(O-2、O-3)[10]。病变的内镜下分型根据2005年巴黎标准[11]。病灶部位分为贲门、胃底、胃体、胃角、胃窦、幽门前庭。组织学类型根据WHO分类标准分为乳头状腺癌、管状腺癌、印戒细胞癌、未分化癌和高级别上皮内瘤变等,根据胃癌组织细胞的分化程度分为高分化癌、中分化癌、低分化癌和未分化癌。
2 结" 果
2.1 Hp根除后早期胃癌病人临床特点
本文共纳入50例病人,其中男36例,女14例;年龄42~75岁,平均(60.98±7.24)岁,其中≥60岁28例(56.0%)。有临床症状42例(84.0%),其中腹痛13例(31.0%),腹胀5例(11.9%),同时有腹痛、腹胀8例(19.0%),有反酸及烧心5例(11.9%),仅有烧心症状1例(2.4%),嗳气1例(2.4%),同时存在2种以上症状者9例(21.4%);无临床症状8例(16.0%)。有吸烟及饮酒史者19例(38.0%),仅有吸烟史3例(6.0%),仅有饮酒史4例(8.0%),无吸烟饮酒史24例(48.0%)。有癌症家族史者15例(30.0%),既往有高血压史19例(38.0%),糖尿病等其他病史14例(28.0%),既往无病史者17例(34.0%)。服用PPI超过1年43例(86.0%)。
2.2 内镜下表现及病理学特点
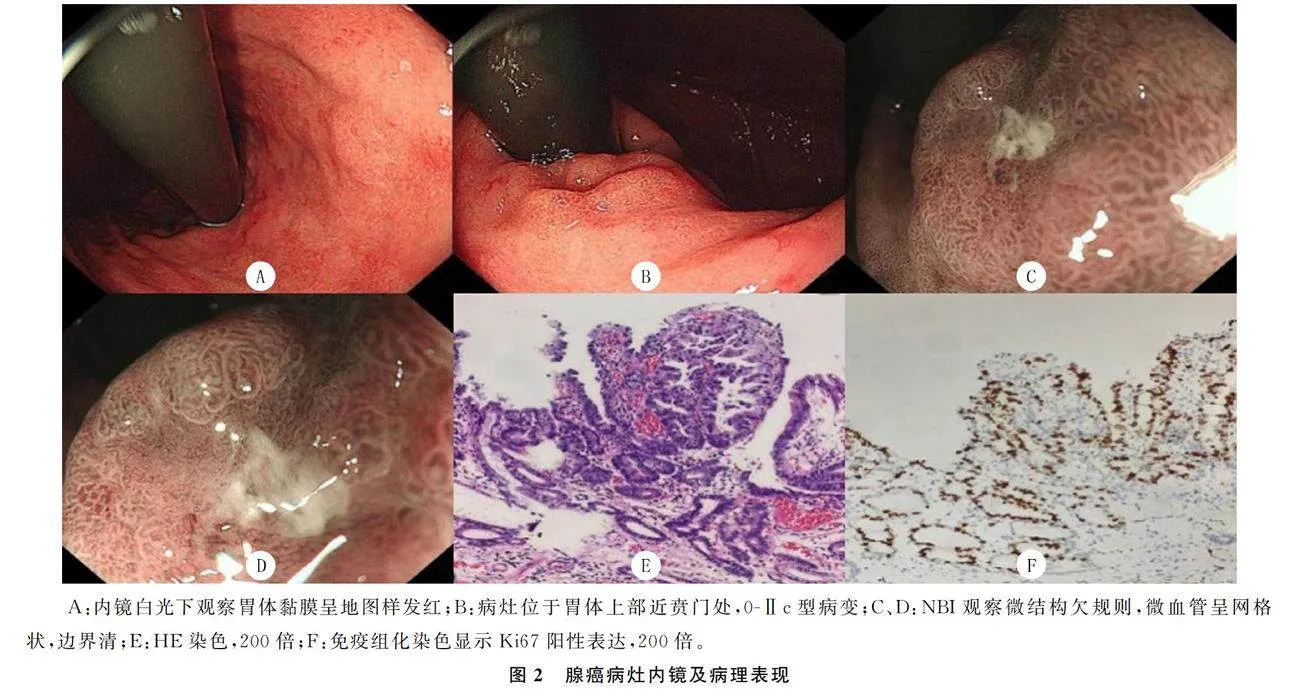
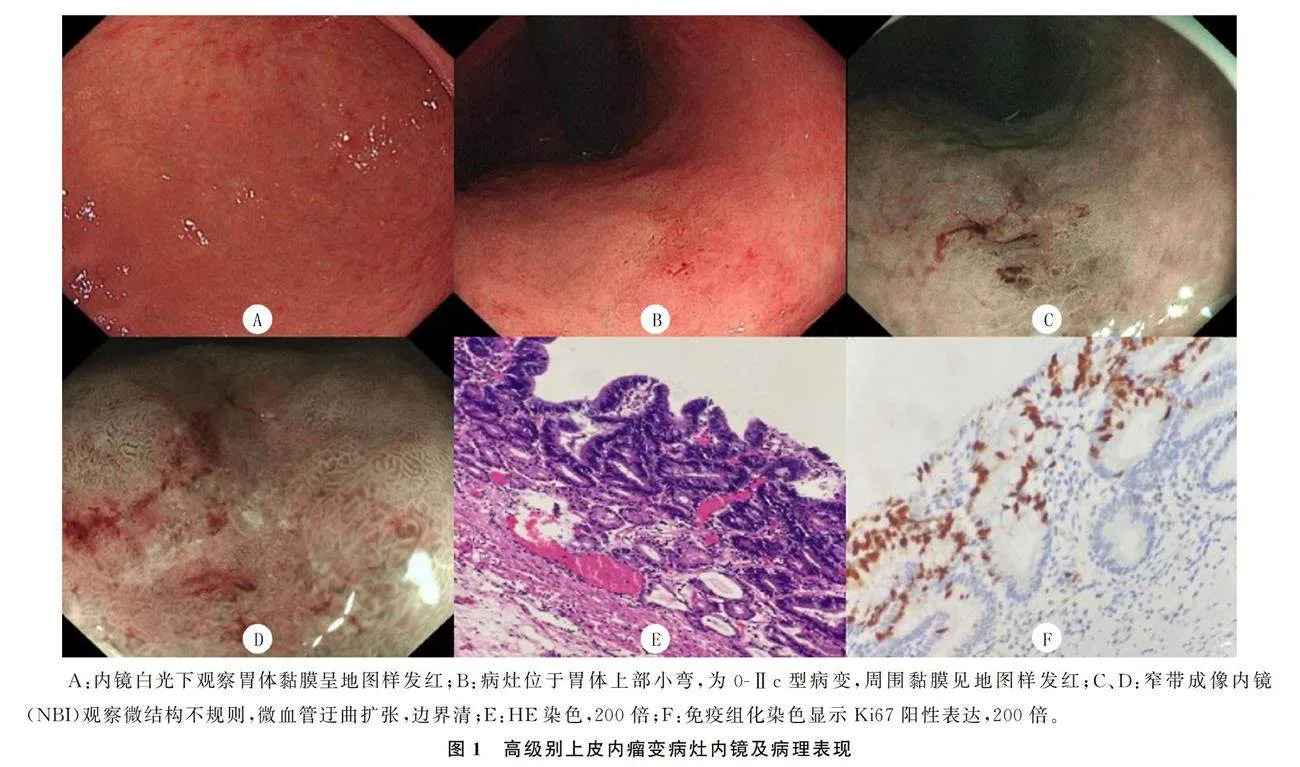
本文纳入的50例病人共有54处病灶,胃黏膜萎缩为轻度萎缩者21例(42.0%),其中C-1者8例(16.0%),C-2者13例(26.0%);中重度萎缩者29例(58.0%),该29例中C-3者11例(22.0%),O-1者9例(18.0%),O-2者3例(6.0%),O-3者6例(12.0%)。肠上皮化生者40例(80.0%)。54处病灶中背景黏膜为地图样发红有43处(79.6%)。有25处病灶位于胃体(46.3%),17处病灶位于胃窦(31.5%),8处位于胃角(14.8%),1处位于胃窦胃体交界部(1.8%),1处位于贲门(1.8%),2处位于幽门(3.7%)。病灶直径为1.5~90.0 mm,平均(18.01±12.29)mm,≤20 mm有44处(81.5%),gt;20 mm有10处(18.5%)。巴黎分型为0-Ⅱc有29处(53.7%),0-Ⅱa+Ⅱc有17处(31.5%),0-Ⅱa 7处(13.0%),0-Ⅱb 1处(1.8%)。病理诊断为高级别上皮内瘤变有38处(70.4%)(图1),腺癌16处(29.6%)(图2)。腺癌病灶中,镜下表现为0-Ⅱc 9处(56.2%),0-Ⅱa+Ⅱc 4处(25.0%),0-Ⅱa 2处(12.5%),0-Ⅱb 1处(6.2%)。26例病人追踪到免疫组化结果,其中Ki67阳性表达率≤50%有17例(65.4%)。
3 讨" 论
Hp感染可导致慢性胃炎,经过萎缩性胃炎、肠上皮化生、上皮内瘤变逐渐发展为胃癌[12-13]。有研究表明,根除Hp可降低胃癌特别是肠型胃癌的发生[14]。一项Meta分析表明,内镜下切除早期原发胃癌病灶后根除Hp可降低异时胃癌的发生率[15]。
然而有些研究发现,即使成功根除Hp后胃癌也会发生,但根除Hp后胃癌的发生率明显降低[16-19]。还有研究认为,Hp感染、Hp未感染以及除菌后胃黏膜状态不同:未感染Hp黏膜存在集合细静脉的规则排列,Hp感染胃黏膜萎缩、肿胀,可见弥漫性、点状发红,除菌后胃黏膜弥漫性发红消失,胃体或胃窦地图样发红较显著;Hp根除后胃癌内镜下表现为胃炎样改变,普通内镜下白光观察癌灶区域不明确,病理切片可见癌表层部分被非癌上皮所覆盖,呈现“马赛克样改变”[9]。 Hp根除后早期胃癌内镜下诊断较困难。本研究通过回顾性分析Hp根除后早期胃癌病人的临床资料,总结其临床及内镜特点,以期提高Hp根除后早期胃癌的诊断率。
本研究50例病人中以老年病人居多(56.0%),平均年龄为(60.98±7.24)岁(42~75岁),男性病人居多(72.0%)。胃癌的流行病学特点为发病率随年龄增加而增加,且男性发病率比女性高,本文研究结果符合其发病趋势。本文研究中84.0%病人有临床症状,然而仅凭临床症状无法判断是否为Hp根除后早期胃癌,需进一步行内镜等检查。有研究对根除Hp后胃癌组与根除Hp后未发现胃癌组比较发现,年龄大的病人(根除Hp时gt;54岁)比年轻病人更易发生除菌后胃癌,而两组间性别没有明显差异[20]。目前,Hp根除后早期胃癌的发生与病人吸烟史、饮酒史、既往患病史及癌症家族史等是否有关尚无明确文献报道。本研究病人既往有其他病史(66.0%)、无癌症家族史者(70.0%)居多,而有吸烟或饮酒史病人(52.0%)与无吸烟饮酒史(48.0%)病人的占比基本相当。本研究50例病人中服用PPI超过1年者有43例,占86.0%,与相关研究结果相符[21]。长期使用PPI可增加Hp除菌后发生胃癌风险,PPI使用时间越长,风险越高[21]。
这可能与长期使用PPI后,胃酸分泌受到抑制,胃内酸碱环境发生变化,微生物群过度生长有关。
有研究结果表明,长期使用PPI增加胃癌风险与是否根除Hp无关[7]。目前长期使用PPI对Hp根除后早期胃癌的发生是否有明确影响尚未达成共识。
本研究结果显示,Hp根除后早期胃癌病人胃黏膜中、重度萎缩者占58.0%,有肠上皮化生者占80.0%。严重的胃黏膜萎缩、胃体黏膜肠化生被认为是Hp根除后早期胃癌发生的危险因素[22-23]。本研究病人内镜下背景黏膜呈地图样发红者占大多数(79.6%),黏膜地图样发红提示黏膜为除菌后状态,结合NBI观察病灶微结构及微血管表现,可判断病灶是否为Hp根除后早期胃癌。黏膜地图样发红有可能成为预测Hp根除后胃癌的一项指标[24]。对于萎缩性胃炎病人,建议根除Hp后1年行内镜随访,避免Hp根除后早期胃癌漏诊[25]。
本研究结果显示,Hp根除后早期胃癌的病灶直径≤20 mm者占81.5%,77.8%的病灶位于胃体及胃窦,多表现为浅表凹陷型。有研究发现,Hp根除后早期胃癌病灶较Hp阳性胃癌病灶更小(≤20 mm),多数为凹陷型,病灶多位于非贲门位置,大多为肠型胃癌[26-28]。本文结果与既往研究发现的病灶大小、位置、镜下分型基本相符[27-28]。本文研究病理分型根据WHO分类标准进行,既往研究采用的是Lauren分型标准,因此病理分型方面无法与既往研究相比较。除菌后胃癌“胃炎样”外观更多见,根除Hp后胃癌表面出现低度异型性上皮细胞,病灶边界不清、非肿瘤上皮的覆盖会影响内镜下对Hp根除后胃癌的判断[29-30],使得诊断更加困难;同时提示内镜医师在行ESD切除病灶时需注意切除范围,避免病灶切除不完全。由于本文研究所收集病人的部分内镜图片不清及病理资料缺失,对胃炎样外观病灶占比未做统计。
Ki67可作为细胞增殖程度的一项指标,在胃癌组织中Ki67的表达率高于癌旁组织,可用于胃癌肿瘤成分增殖程度的评估[31-32]。
本文研究有26例病人进行了免疫组化检查,结果显示Ki67阳性表达率≤50%者占65.4%。相较于未除菌胃癌,Hp根除后早期胃癌中Ki67的表达水平更低[33]。内镜下胃黏膜萎缩严重、除菌前有肠上皮化生及长期使用PPI是Hp根除后早期胃癌发展的危险因素,对具有危险因素的人群进行定期内镜检查并随访,有助于及时发现Hp根除后早期胃癌[33]。
综上所述,Hp根除后早期胃癌的镜下特征表现为病灶位于非贲门位置,凹陷型,背景黏膜呈地图样发红。对除菌前胃黏膜中重度萎缩、有肠上皮化生、长期服用PPI人群进行内镜随访,可做到Hp根除后早期胃癌的早发现、早治疗。
[参考文献]
[1]VALENZUELA M A, CANALES J, CORVALN A H, et al. Helicobacter pylori-induced inflammation and epigenetic changes during gastric carcinogenesis[J]. World Journal of Gastroenterology, 2015, 21(45):12742-12756.
[2]EGI Y, ITO M, TANAKA S, et al. Role of Helicobacter pylori infection and chronic inflammation in gastric cancer in the cardia[J]. Japanese Journal of Clinical Oncology, 2007,37(5):365-369.
[3]WATARI J, CHEN N, AMENTA P S, et al. Helicobacter pylori associated chronic gastritis, clinical syndromes, precancerous lesions, and pathogenesis of gastric cancer development[J]. World Journal of Gastroenterology, 2014, 20(18):5461-5473.
[4]DE FALCO M, LUCARIELLO A, IAQUINTO S, et al. Molecular mechanisms of Helicobacter pylori pathogenesis[J]. Journal of Cellular Physiology, 2015, 230(8):1702-1707.
[5]王姝,于亚男,丁雪丽,等. 岩藻多糖联合标准四联疗法根除幽门螺杆菌的临床疗效观察[J].精准医学杂志,2021,36(2):181-184.
[6]徐珊,张雪琳,田字彬. COL5A2在胃癌组织中的表达及其与患者预后的关系[J].精准医学杂志,2021,36(1):6-13.
[7]FUKASE K, KATO M, KIKUCHI S, et al. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric can-
cer: an open-label, randomised controlled trial[J]. Lancet (London, England), 2008,372(9636):392-397.
[8]CHEY W D, WONG B C Y, PRACTICE PARAMETERS COMMITTEE OF THE AMERICAN COLLEGE OF GASTROENTEROLOGY. American College of Gastroenterology guideline on the management of Helicobacter pylori infection[J]. The American Journal of Gastroenterology, 2007,102(8):1808-1825.
[9]八木一芳,味冈洋一编著;宫健,刘石译. H.pylori除菌后发现胃癌的内镜诊断[M]. 沈阳:辽宁科学技术出版社, 2017.
[10]KIMURA K, TAKEMOTO T. An endoscopic recognition of the atrophic border and its significance in chronic gastritis[J].
Endoscopy, 1969,1(3):87-97.
[11]ENDOSCOPIC CLASSIFICATION REVIEW GROUP. Update on the Paris classification of superficial neoplastic lesions in the digestive tract[J]. Endoscopy, 2005,37(6):570-578.
[12]CHO S J, CHOI I J, KOOK M C, et al. Staging of intestinal- and diffuse-type gastric cancers with the OLGA and OLGIM staging systems[J]. Alimentary Pharmacology amp; Therapeutics, 2013,38(10):1292-1302.
[13]CORREA P. Human gastric carcinogenesis: a multistep and multifactorial process: first American cancer society award lecture on cancer epidemiology and prevention[J]. Cancer Research, 1992,52(24):6735-6740.
[14]TAKENAKA R, OKADA H, KATO J, et al. Helicobacter pylori eradication reduced the incidence of gastric cancer, especially of the intestinal type[J]. Alimentary Pharmacology amp; Therapeutics, 2007, 25(7):805-812.
[15]SEO J Y, LEE D H, CHO Y, et al. Eradication of Helicobac-
ter pylori reduces metachronous gastric cancer after endoscopic resection of early gastric cancer[J]. Hepato-gastroenterology, 2013,60(124):776-780.
[16]CHOI J, KIM S G, YOON H, et al. Eradication of Helicobacter pylori after endoscopic resection of gastric tumors does not reduce incidence of metachronous gastric carcinoma[J]. Clinical Gastroenterology and Hepatology, 2014,12(5):793-800.
[17]BAE S E, JUNG H Y, KANG J E, et al. Effect of Helicobacter pylori eradication on metachronous recurrence after endoscopic resection of gastric neoplasm[J]. The American Journal of Gastroenterology, 2014,109(1):60-67.
[18]KWON Y H, HEO J, LEE H S, et al. Failure of Helicobacter pylori eradication and age are independent risk factors for recurrent neoplasia after endoscopic resection of early gastric cancer in 283 patients[J]. Alimentary Pharmacology amp; Therapeutics, 2014,39(6):609-618.
[19]YOON S B, PARK J M, LIM C H, et al. Effect of Helicobacter pylori eradication on metachronous gastric cancer after endoscopic resection of gastric tumors: a meta-analysis[J]. Helicobacter, 2014,19(4):243-248.
[20]TAKATA S, ITO M, YOSHIHARA M, et al. Host factors contributing to the discovery of gastric cancer after successful eradication therapy of Helicobacter pylori: preliminary report[J]. Journal of Gastroenterology and Hepatology, 2007, 22(4):571-576.
[21]HAGIWARA T, MUKAISHO K, NAKAYAMA T, et al. Proton pump inhibitors and Helicobacter pylori-associated pathogenesis[J]. Asian Pacific Journal of Cancer Prevention: APJCP, 2015,16(4):1315-1319.
[22]KODAMA M, MURAKAMI K, OKIMOTO T, et al. Histological characteristics of gastric mucosa prior to Helicobacter pylori eradication may predict gastric cancer[J]. Scandinavian Journal of Gastroenterology, 2013,48(11):1249-1256.
[23]ASONUMA S, IMATANI A, ASANO N, et al. Helicobacter pylori induces gastric mucosal intestinal metaplasia through the inhibition of interleukin-4-mediated HMG box protein Sox2 expression[J]. American Journal of Physiology Gastrointestinal and Liver Physiology, 2009, 297(2):312-322.
[24]NAGATA N, SHIMBO T, AKIYAMA J, et al. Predictability of gastric intestinal metaplasia by mottled patchy erythema seen on endoscopy[J]. Gastroenterology Research, 2011,4(5):203-209.
[25]ASAKA M, MABE K, MATSUSHIMA R, et al. Helicobac-
ter pylori eradication to eliminate gastric cancer: the Japanese strategy[J]. Gastroenterology Clinics of North America, 2015,44(3):639-648.
[26]MAEHATA Y, NAKAMURA S, ESAKI M, et al. Characteristics of primary and metachronous gastric cancers disco-
vered after Helicobacter pylori eradication: a multicenter propensity score-matched study[J]. Gut and Liver, 2017,11(5):628-634.
[27]YAMAMOTO K, KATO M, TAKAHASHI M, et al. Clinicopathological analysis of early-stage gastric cancers detected after successful eradication of Helicobacter pylori[J]. Helicobacter, 2011,16(3):210-216.
[28]KAMADA T, KUSUNOKI H, HATA J, et al. Helicobacter pylori infection and development of gastric cancer[J]. Kawasaki Med J, 2005,102(6):673-680.
[29]TABATA H, FUCHIGAMI T, KOBAYASHI H, et al. Helicobacter pylori and mucosal atrophy in patients with gastric cancer: a special study regarding the methods for detecting Helicobacter pylori[J]. Digestive Diseases and Sciences, 1999,44(10):2027-2034.
[30]KITAMURA Y, ITO M, MATSUO T, et al. Characteristic epithelium with low-grade atypia appears on the surface of gastric cancer after successful Helicobacter pylori eradication the-
rapy[J]. Helicobacter, 2014,19(4):289-295.
[31]ZHENG Y, WANG L, ZHANG J P, et al. Expression of p53, c-erbB-2 and Ki67 in intestinal metaplasia and gastric carcinoma[J]. World Journal of Gastroenterology, 2010,16(3):339-344.
[32]AL-MOUNDHRI M S, NIRMALA V, AL-HADABI I, et al. The prognostic significance of p53, p27kip1, p21waf1, HER-2/neu, and Ki67 proteins expression in gastric cancer: a clinicopathological and immunohistochemical study of 121 Arab patients[J]. Journal of Surgical Oncology, 2005,91(4):243-252.
[33]徐勤伟,胥明. 幽门螺杆菌根除后早期胃癌的临床特点[J]. 胃肠病学和肝病学杂志, 2019,28(1):4-9.
(本文编辑 黄建乡)

