微粒体甘油三酯转运蛋白基因rs1800591变异与老年人群非酒精性脂肪性肝病发生风险的关系
赵锦涵 张晶 张洋 徐潇艺 勾钰淞 徐航飞 万妍 吴剑
摘要:目的 探討老年人群微粒体甘油三酯转运蛋白(MTTP)基因rs1800591多态性与非酒精性脂肪性肝病(NAFLD)发病风险的关系。方法 本研究的临床队列建立在北京京煤集团总医院门矿医院,2020年1月11日—2021年9月30日在北京门头沟社区共招募参加健康体检1098例老年志愿者,其中NAFLD患者614例,非NAFLD患者484例,采用基因芯片法检测MTTP rs1800591基因型,收集人口学资料并检测受试者的血液生化指标。
符合正态分布的计量资料两组间比较采用独立样本t检验;对非正态分布的计量资料两组间比较采用Mann-Whitney U检验;计数资料两组间比较采用χ2检验。应用χ2检验分析基因型频率的分布是否符合Hardy-Weinberg (H-W) 平衡检验以确认样本的群体代表性。以非条件Logistic回归模型计算比值比(OR)及其95%CI以评估基因多态性与NAFLD发生风险及其他合并症的关系。结果 两组间性别、年龄差异均有统计学意义(P值均<0.05)。相比于非NAFLD组,NAFLD组的BMI、腰臀比、TG、ALT、AST、CAP、LSM水平均显著提高,而HDL明显降低(P值均<0.05)。NAFLD组中高血压、糖尿病、肥胖及代谢综合征患者的比例也均高于非NAFLD组(P值均< 0.05)。MTTP rs1800591多态性在对照组基因型频率分布符合Hardy-Weinberg平衡(χ2=1.097,P=0.29)。MTTP rs1800591不同基因型及等位基因分布在NAFLD患者与对照组中均有显著性差异(P值均<0.001)。总人群中T等位基因(GT+TT,n=351)携带率在男性中比例偏低,而BMI和CAP值显著高于非携带者(GG,n=747)(P值均<0.001)。相比于非携带者,T等位基因携带者(GT+TT,n=232)中肥胖患者比例明显提高,但NFS评分却显著降低(P值均<0.05)。在NAFLD受试者中,T等位基因携带者男性比例和腰臀比显著降低, T等位基因携带者HDL高于非携带者(GG,n=382),T等位基因携带者NFS评分仍明显低于非携带者(P值均<0.05)。非条件Logistic回归分析表明,在校正性别、年龄、BMI混杂因素后,MTTP rs1800591 GT+TT型仍显著增加了NAFLD的发生风险(OR=1.643, 95%CI:1.226~2.203, P=0.001),而T等位基因携带则增加了总人群中肥胖的发生风险(OR=1.371, 95%CI:1.051~1.788, P=0.02)。结论 老年人群中MTTP rs1800591多态性与NAFLD的发生有关,T等位基因携带者可能促进了NAFLD肝脏脂肪变性,增加肥胖症发生风险,但可能抑制了肝纤维化进展。
关键词:非酒精性脂肪性肝病; 基因; 老年人
基金项目:北京市百千万人才工程资助项目(2019A15); 北京市属医学科研院所公益发展改革试点项目(京医研2021-10)
Association between the rs1800591 variation of the microsomal triglyceride transfer protein gene and the risk of nonalcoholic fatty liver disease in the elderly population
ZHAO Jinhan1, ZHANG Jing1, ZHANG Yang2, XU Xiaoyi1, GOU Yusong1, XU Hangfei1, WAN Yan3, WU Jian3. (1. Third Department of Liver Disease Center, Beijing YouAn Hospital, Capital Medical University, Beijing 100069, China; 2. Beijing Institute of Hepatology, Beijing 100069, China; 3. Capital University of Physical Education and Sports, Beijing 100191, China)
Corresponding author:
WU Jian, wujiancupes@126.com (ORCID:0000-0001-6690-4561)
Abstract:
Objective To investigate the association between the polymorphism of the microsomal triglyceride transport protein (MTTP) gene at rs1800591 locus and the risk of nonalcoholic fatty liver disease (NAFLD) in the elderly population. Methods The clinical cohort of this study was established in Menkuang Hospital, Beijing Jingmei Group General Hospital. A total of 1098 healthy elderly volunteers were recruited for physical examination in communities in Mentougou District of Beijing, China, from January 11, 2020 to September 30, 2021, among whom there were 614 patients with NAFLD and 484 individuals without NAFLD. Gene microarray was used to determine the genotypes of MTTP rs1800591; demographic data were collected, and blood biochemical parameters were measured. The independent samples t-test was used for comparison of normally distributed continuous data between groups, and the Mann-Whitney U test was used for comparison of non-normally distributed continuous data between groups; the chi-square test was used for comparison of categorical data between groups. The chi-square test was used to investigate whether the distribution of genotype frequency was in accordance with Hardy-Weinberg equilibrium. The unconditional logistic regression model was used to calculate odds ratio (OR) and its 95% confidence interval (CI) to investigate the association of gene polymorphism with the risk of NAFLD and other comorbidities. Results There were significant differences in sex and age between the two groups (P<0.05). Compared with the non-NAFLD group, the NAFLD group had significantly higher levels of body mass index (BMI), waist-hip ratio, triglyceride, alanine aminotransferase, aspartate aminotransferase, controlled attenuation parameter (CAP), and liver stiffness measurement and a significantly lower level of high-density lipoprotein (HDL) (all P<0.05). Compared with the non-NAFLD group, the NAFLD group had a significantly higher proportion of patients with hypertension, diabetes, obesity, and metabolic syndrome (all P<0.05). The distribution of genotype frequency at MTTP rs1800591 locus was in accordance with Hardy-Weinberg equilibrium in the control group (χ2=1.097, P=0.29). There were a significant differences in the genotype and the distribution of alleles at MTTP rs1800591 locus between the patients with NAFLD and the control group (all P<0.001). In the total population, there was a significantly lower carrying rate of T allele (GT+TT, n=351) in male individuals, and the individuals carrying T allele had significantly higher BMI and CAP than those carrying GG allele (n=747) (P<0.001). Compared with the individuals who did not carry T allele, the individuals carrying T allele (GT+TT, n=232) had a significantly higher proportion of patients with obesity and a significantly lower NFS score (P<0.05). As for the individuals with NAFLD, the individuals carrying T allele had a significantly lower proportion of male individuals, a significantly lower waist-hip ratio, and a significantly higher level of HDL compared with those who did not carry T allele (GG, n=382), and the GT+TT group had a significantly lower NFS score than the GG group (all P<0.05). The non-conditional logistic regression analysis showed that after adjustment for the confounding factors of sex, age, and BMI, the GT+TT genotype at MTTP rs1800591 locus significantly increased the risk of NAFLD (OR=1.643, 95%CI: 1.226-2.203, P=0.001), and carrying T allele also increased the risk of obesity in the total population (OR=1.371, 95%CI: 1.051-1.788, P=0.02). ConclusionMTTP rs1800591 polymorphism is associated with the development of NAFLD in the elderly population, and carrying T allele may promote hepatic steatosis and increase the risk of obesity in NAFLD, while it may inhibit the progression of liver fibrosis.
Key words:
Non-alcoholic Fatty Liver Disease; Genes; Aged
Research funding:
Beijing Hundred Thousand Talents Project (2019A15); Beijing Municipal Institute of Public Medical Research Development and Reform Pilot Project (2021-10)
非酒精性脂肪性肝病(NAFLD) 是一种代谢应激性肝损伤,其全球患病率约为25%,我国目前有超过2.4亿的患者[1]。NAFLD的病理进程可由单纯脂肪变性进展为非酒精性脂肪性肝炎 (NASH)、纤维化、肝硬化和肝细胞癌,目前尚无有效的治疗药物[2]。此外,我国也面临着迅速发展的老龄化问题,数据显示[3]我国60岁及以上人口超2.64亿,占全人口总数的18.7%,预计2050年将达到4.8亿。在老龄化人群中,NAFLD将增加肝脏并发症和肝外疾病发生风险,严重影响老年人群的生活质量,老年NAFLD患者亟需得到早期关注[4]。
NAFLD是遗传因素和环境因素共同作用的结果,遗传背景在其发生发展中的作用约占50%[5]。多个关键基因的单核苷酸多态性 (single nucleotide polymorphism, SNP)已被证明与NAFLD的遗传易感性密切相关。近年来,基于微粒体甘油三酯转运蛋白 (microsomal triglyceride transfer protein, MTTP)多态性 (rs1800591)与脂质代谢的密切关系,陆续开展了几项MTTP多态性与NAFLD关系的研究,但多集中于欧美及非洲人群,對亚洲队列的研究极少,目前尚无中国老年NAFLD人群与MTTP多态性的研究报道。
MTTP是一种异二聚体伴侣,主要在肝细胞和肠细胞中表达[6]。它是组装和分泌极低密度脂蛋白 (very low density lipoprotein, VLDL)及乳糜微粒的关键酶[7],通过与载脂蛋白B (apolipoprotein B, apoB) 的特异性结合在脂蛋白生物合成中发挥重要作用[8]。研究表明,MTTP rs1800591多态性 (G>T) 可能与NAFLD的易感性相关。非洲学者Gouda等[9]在包括174例平均年龄40岁的NAFLD队列中发现,MTTP rs1800591 TT基因型相比G等位基因携带者血清TG和VLDL明显降低,欧洲Musso等[10]研究结果与此一致,表明其可引发肝脏脂代谢的异常,是NAFLD发病的重要致病因素。Namikawa等[11]对63例活检证实的NASH日本患者进行了MTTP rs1800591多态性研究发现,G等位基因增加了NASH及肝脏脂肪变性的发生风险。与上述研究不同,在意大利[12](114例NASH) 和巴西人群[13](129例NASH) 的病例对照研究则显示,rs1800591多态性与NAFLD、其临床或组织学特征无显著相关性。我国仅有一项相关研究[14]在平均年龄44岁的580例NAFLD患者中进行,结果也显示rs1800591与NAFLD无显著相关性。尽管大量研究提示MTTP rs1800591与NAFLD的发生发展密切相关,但仍有少数研究发现二者并无关联,其原因可能是MTTP功能更易受到种族、地域、饮食、年龄、性别等因素的影响。为此,需要在不同种族不同年龄段的更多人群中进一步明确其易感性和风险等位基因,以及与NAFLD脂肪变性和纤维化进展的关系。
基于上述研究背景和目前我国老龄化现状,我们以65岁以上人群为目标,建立了一个社区老年脂肪肝病的队列,并开展了MTTP rs1800591多态性的检测工作。探究MTTP rs1800591多态性与NAFLD之间的临床相关性,为老年人群NAFLD综合诊治提供研究基础。
1 资料与方法
1.1 研究对象 本研究的临床队列建立在北京京煤集团总医院门矿医院,2020年1月11日—2021年9月30日在北京门头沟社区共招募参加健康体检1098例老年志愿者,分为NAFLD组(n=614)和非NAFLD组(n=484)。NAFLD诊断标准符合中华医学会肝病学分会NAFLD标准[2]。纳入标准:年龄≥65岁[15];所有NAFLD患者均经B超诊断。排除标准: 有过量饮酒史和其他可以导致脂肪肝的特定疾病;患有严重的肝、肾、心脏和脑部疾病以及恶性肿瘤者[2]。
1.2 临床及化验资料 2名训练有素的研究人员负责招募体检患者,从社区健康记录中提取人口统计学指标和病史。对受试者的身高、体质量、腰围、臀围进行标准测量,并计算BMI和腰臀比。使用FibroScan (法国Echosens公司,502 型,M探针) 对患者进行肝脂肪的定量测定,结果以受控衰减参数CAP表示,并测量肝脏硬度值(LSM)。研究对象于12 h空腹后在上午取静脉血进行生化分析,检测受试者的肝功能、脂质谱、空腹血浆葡萄糖(FPG)、糖化血红蛋白等指标。
1.3 纤维化及合并症定义 使用NAFLD纤维化评分(NAFLD fibrosis score, NFS) 来评估肝纤维化的严重程度。NFS=-1.675+0.037×年龄 (岁) +0.094×BMI (kg/m2)+1.13×空腹血糖受损/糖尿病 (是=1,否=0)+0.99×(AST/ALT)-0.013×PLT(×109/L)-0.66×白蛋白 (g/dL)[16]。合并症的诊断标准如下:收缩压≥130 mmHg或舒张压≥85 mmHg或服用降压药时,诊断为高血压[17]。Ⅱ型糖尿病诊断为:FPG≥7.0 mmol/L或口服葡萄糖耐量试验餐后2 h血糖≥11.1 mmol/L或既往有确切糖尿病病史[18]。肥胖症被定义为BMI≥25 kg/m2[19]。代谢综合征 (metabolic syndrome, MS) 的标准定义[20],需至少存在以下3项或3项以上:(1)肥胖,即BMI≥25 kg/m2和/或男性腰围>90 cm(女性腰围>80 cm);(2)高三酰甘油血症,即三酰甘油≥1.7 mmol/L,或患者因脂质异常而接受治疗;(3)低HDL-C血症,即男性HDL-C<1.03 mmol/L,女性HDL-C<1.29 mmol/L;(4)高血压,即收缩压≥130 mmHg和/或舒张压≥85 mmHg,或患者被诊断出患有高血压并曾接受过药物治疗;(5)高血糖,即空腹血糖升高(≥5. 6 mmol/L),或患者被诊断出患有Ⅱ型糖尿病并曾接受过药物治疗。
1.4 基因组DNA提取和基因分型 从患者全血标本中提取基因组DNA。DNA通过分光光度法 (Nanodrop 2000, Thermo Scientific, Wilmington, DE) 测定DNA的浓度和质量,并在基因分型前标准化至约50 ng/mL。MTTP rs1800591的G和T等位基因探针由美国富鲁达公司设计和合成 (Fluidigm, South San Francisco, CA, USA), 使用96.96微液流动态芯片(IFC)和JunoTM系统(Fluidigm, South San Francisco, CA, USA)对队列样品进行SNP分型分析,采用Fluidigm SNP基因分型分析软件(4.5.1版) 对MTTP rs1800591不同等位基因的分布进行分析。
1.5 统计学方法 数据的统计分析采用SPSS 26.0统计软件。符合正态分布的计量资料以x±s表示,两组间比较采用独立样本t检验;对非正态分布的计量资料,以M(P25~P75)表示,两组间比较采用Mann-Whitney U检验;计数资料两组间比较采用χ2检验。应用χ2检验分析基因型频率的分布是否符合Hardy-Weinberg (H-W) 平衡检验以确认样本的群体代表性。以非条件Logistic回归模型计算比值比(OR)及其95%CI以评估基因多态性与NAFLD发生风险及其他合并症的关系。P<0.05为差异有统计学意义。
2 结果
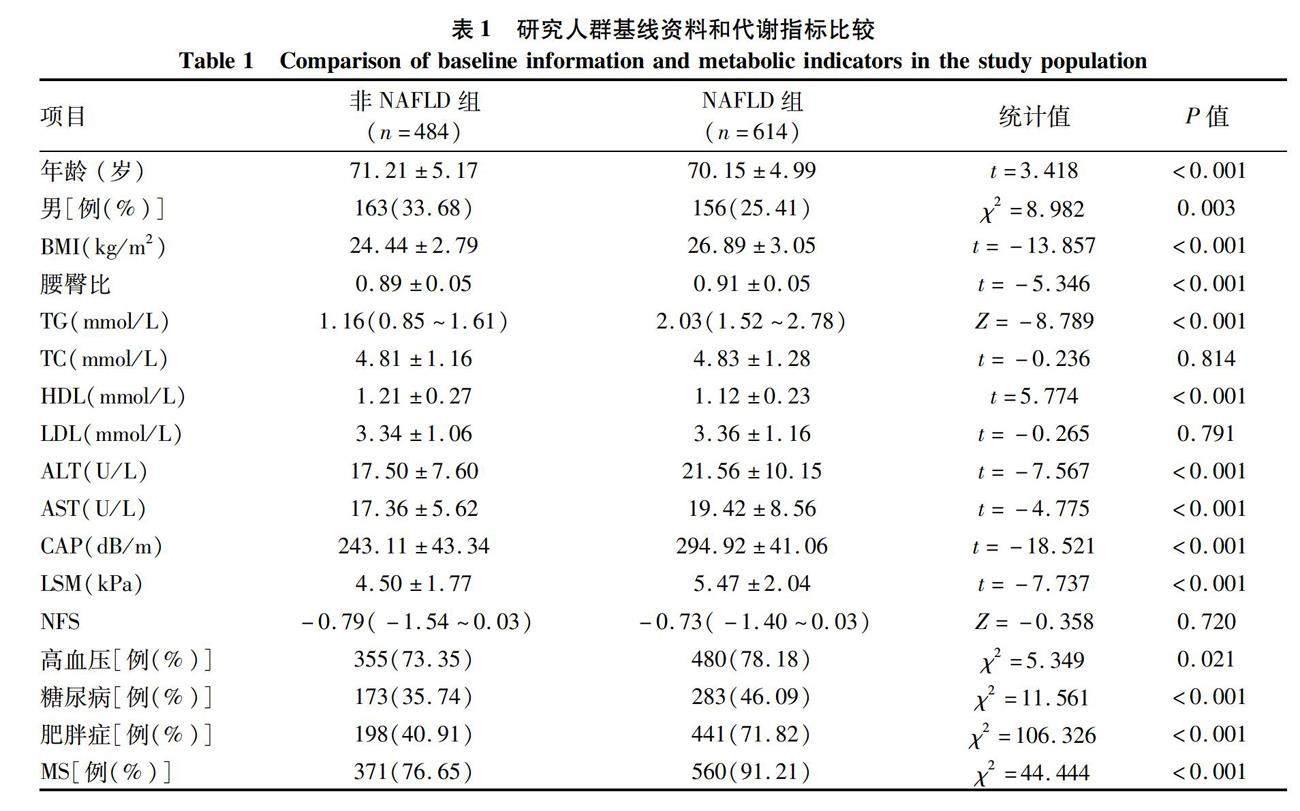
2.1 一般资料 NAFLD组共收集614例,非NAFLD组484例。两组间性别、年龄差异均有统计学意义(P值均< 0.05)。相比于非NAFLD组,NAFLD组的BMI、腰臀比、TG、ALT、AST、CAP、LSM水平均顯著提高(P值均<0.05),而HDL明显降低(P<0.05)。NAFLD组中高血压、糖尿病、肥胖及MS患者的比例也均高于非NAFLD组(P值均<0.05) (表1)。
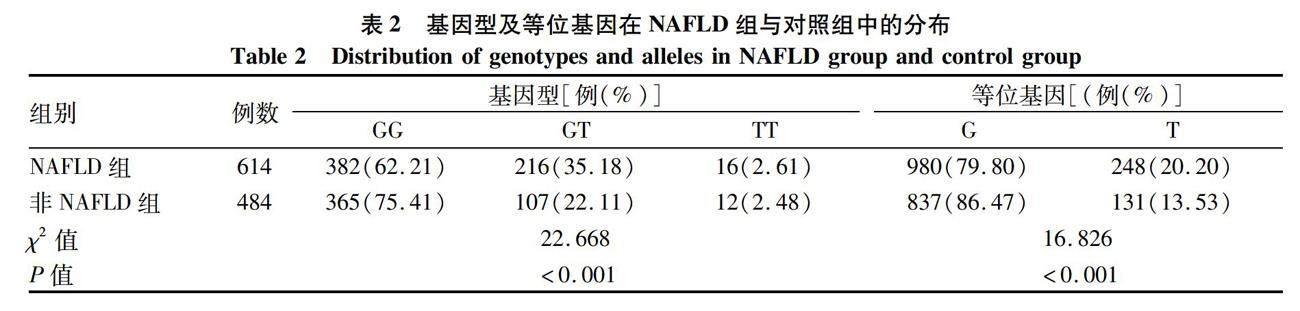
2.2 MTTP-rs1800591基因型及等位基因的分布 MTTP rs1800591多态性在对照组基因型频率分布符合Hardy-Weinberg平衡 (χ2=1.097,P=0.29)。MTTP rs1800591不同基因型及等位基因分布在NAFLD患者与对照组中均有显著性差异 (P值均<0.001)(表2)。
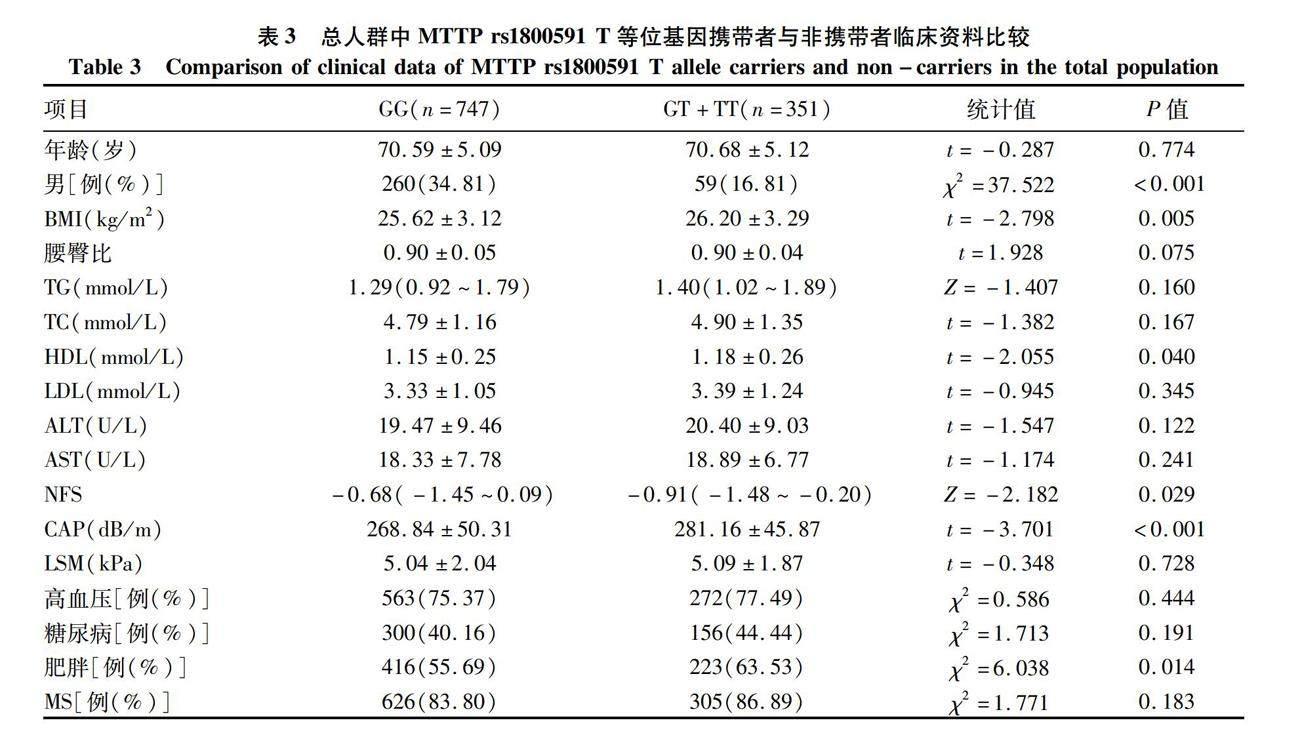
2.3 总人群中MTTP rs1800591 T等位基因携带者与非携带者临床资料比较 为明确T等位基因对NAFLD的影响,将研究对象分为T等位基因携带者 (GT+TT,n=351) 及非T等位基因携带者 (GG,n=747) 两组,并在两组间进行了临床资料比较。结果显示,总人群中T等位基因携带率在男性中比例偏低,而BMI和CAP值显著高于GG等位基因携带者(P值均<0.001)。进一步分析表明相比于非携带者,T等位基因携带者中肥胖患者比例明显提高,但NFS评分却显著降低(P值均<0.05)(表3)。
2.4 NAFLD患者中MTTP rs1800591基因多态性的临床资料比较 在NAFLD受试者中,T等位基因携带者男性比例和腰臀比显著降低(P值均<0.05), T等位基因携带者HDL高于非携带者,T等位基因携带者NFS评分仍明显低于非携带者(P值均<0.05)(表4)。
2.5 MTTP rs1800591 T等位基因与NAFLD及相关疾病风险分析 非条件logistic回归分析表明,在校正性别、年龄、BMI混杂因素后,MTTP rs1800591 GT+TT型仍显著增加了NAFLD的发生风险 (OR=1.643, 95%CI:1.226~2.203, P=0.001) (表5)。而T等位基因携带则增加了总人群中肥胖的发生风险 (OR=1.371, 95%CI:1.051~1.788, P=0.02),但与高血压、糖尿病、MS的发生风险无关 (图1)。
3 讨论
NAFLD以肝脏内脂肪累积和慢性炎症为主要表现,是一种高度异质性的疾病,其异质性来自于年龄、性别、种族、饮食、遗传因素等[21]。其中,不同种族群体中NAFLD的患病率、临床特征、组织学严重程度和预后存在显著差异,表明遗传因素发挥了重要作用[22]。SNP是NAFLD异质性的主要机制之一,在脂肪肝的发生发展约占50%的驱动作用[5]。MTTP是肝脏中脂质代谢的关键蛋白[23],在肝细胞内VLDL的组装和分泌中发挥重要作用。MTTP在脂蛋白组装的早期阶段可以催化TG转移到新生的ApoB,形成原始的VLDL颗粒,进而去除肝细胞内TG的聚集状态。研究[24-26]表明,MTTP活性降低或缺失会促进肝脏脂质聚集,导致肝脂肪变性的发生。
MTTP rs1800591是近年来被证明与NAFLD发生发展密切相关的新遗传基因SNP[27-28],其与肝脂肪变性的关系已在相关研究中得以证实。2004年,Namikawa等[11]对63例NASH患者和150例健康对照组进行MTTP rs1800591多态性研究发现,GG基因型NASH患者肝脂肪变性程度明显高于GT基因型(无TT基因型携带NASH患者)。肝活检也表明与GT基因型患者相比,GG基因型肝小叶脂肪面积更大。Bernard等[29]对217例糖尿病患者的研究中也证明,MTTP rs1800591的G等位基因可以显著增加患者肝脂肪变性的遗传易感性,GG基因型与GT+TT型肝脏脂肪变性比例分别为36%和17%。Gambino等[30]通过研究29例非肥胖非糖尿病NASH患者MTTP rs1800591多态性后发现,NASH人群中GG型携带者的TG和游离脂肪酸显著高于其他基因型。与之相反,一项涉及意大利人群的研究[12]显示rs1800591多态性与NAFLD、其临床或组织学特征之间无显著的相关性。Oliveira等[13]在巴西人群的研究也显示,GG和GT基因型携带者分别在NAFLD组与对照组,以及NAFL组和NASH组比较中无明显临床病理差异。2014年Peng等[14]在包含580例NAFLD患者[平均年龄(46.12±12.88)岁]和580例健康对照组[平均年龄(44.86±13.55)岁] 的中国汉族人群中的研究显示rs1800591与NAFLD无相关性。介于MTTP是血脂调节中的重要因子,其功能更易受到饮食和各种环境因素的影响,上述研究也提示MTTP rs1800591多态性在不同种族、地域和人群中,也许发挥着不同的功能。
利用当前社区老年脂肪肝病的队列发现,GT+TT基因型携带者的NAFLD发病风险显著增加,与非携带者相比T等位基因携带者CAP值显著升高,反映了T等位基因可以增加肝脏脂肪含量。同时,无论是在总人群还是NAFLD人群中,GG等位基因型携带者NFS评分都显著升高,表明GG等位基因在老年NAFLD人群中可能参与了纤维化进展。这种不同等位基因与不同脂肪肝病病理进程的相关性,可能取决于MTTP本身的功能。研究[31]表明在小鼠肝特异性敲除MTTP可以减少VLDL和ApoB-100的表达,并发展为中度脂肪肝。小鼠肝过表达MTTP会导致ApoB及VLDL的分泌增加,提高了高脂血症的发病率[32]。而采用药物抑制MTTP的表达,不仅会降低LDL-C和TG,也会存在胃肠道不良事件和肝脏脂肪含量增加的副作用[33]。MTTP在肝细胞内的动态平衡,在维持血脂和肝脏脂肪水平稳定上起重要作用。另外,有研究[34]表明T等位基因可以促进未成熟的VLDL颗粒酯化,从而减少VLDL到LDL的输入,反而导致LDL-C、TC和ApoB的水平降低,进而促进肝内脂质的聚集,这可能是T等位基因增加NAFLD发病风险的原因之一。而T等位基因是脂肪肝和MS的危险因子也被其他临床研究[35-37]所证实。此外,MTTP在胰腺和肠道等其他组织中也发挥重要的作用。Musso等[10]在NASH患者中证明相比于GT/TT基因型,GG携带者具有更严重的胰岛β细胞功能障碍。Iqbal等[38]也证明在小鼠肠道内敲除MTTP,可以增加肠道内TG水平,并减少其通过乳糜微粒的运输,这也许会进一步导致肠道菌群的紊乱,促进肝纤维化的进展。这些研究表明相比于T等位基因,G等位基因引起MTTP表达和活性降低,血脂水平持续的改变,协同其他组织的病理性变化,进而在促进肝纤维化的进展中起到主导作用。本研究是在中国北方汉族老年人群中开展,老年人常合并如糖尿病和肥胖等多种慢性疾病,NAFLD发病特征及影响因素有其自身的特点[39],这可能是在该队列中发现G等位基因与肝纤维化密切相关的主要原因。
此外,本研究还首次对rs1800591多态性与肥胖、高血压、糖尿病、MS等疾病的相关性进行探究,发现MTTP rs1800591多态性的GT+TT基因携带者肥胖症比例明显升高。非条件Logistic回归在校正性别、年龄、BMI等混杂因素后仍显示T等位基因可以增加总人群中肥胖发生的风险(OR=1.371, 95%CI:1.051~1.788),表明T等位基因携带可能是老年人群肥胖发生的独立危险因素。其可能的机制有以下两点:(1)最新研究[40]表明,在脂肪细胞中,MTTP可以与脂肪甘油三酯脂肪酶 (adipose triglyceride lipase, ATGL) 蛋白质相互作用,调节ATGL的TG水解酶活性。MTTP在小鼠脂肪细胞中特异性敲除可以提高ATGL活性,促进TG降解,抵抗饮食诱导的肥胖,而这一新功能是独立于MTTP脂质转移活性的。为此推测相比于T等位基因,G等位基因携带者脂肪组织中MTTP表达及活性减低,ATGL活性增加,进而促进脂肪降解,抵抗肥胖的发生,这可能是本研究发现T等位基因增加肥胖症发生风险的主要原因。(2)正如前文所述,MTTP 中T等位基因可能导致循环中TC,LDL-C和ApoB水平降低[41],而低水平的ApoB已被报道[42]可以促进肥胖的发生, 这可能是另一重要机制。
本研究也存在一些不足。首先,本研究为单中心横断面研究,不能代表更普遍的老年群体,要进一步明确MTTP rs1800591与NAFLD发病风险的相关性还需进行更大规模的多中心前瞻性研究;其次,纳入对象年龄较大,吸烟、饮酒、用药史及既往病史等为档案查找及患者的自我报告,存在一定回忆偏倚;鉴于本研究队列老年人群血脂异常者居多,且服用降脂药物情况复杂,基因多态性与血脂的相关性可靠性不强。最后,腹部超声对轻度脂肪肝敏感性较低,结果可能存在偏倚,且不能对NAFLD脂肪变及纤维化严重程度进一步分级。
综上所述,本研究报道了MTTP rs1800591多态性在老年人群中与NAFLD发病风险的相关性,具有T等位基因的个体可能增加NAFLD脂肪变性的風险,且T等位基因携带是老年人群肥胖症发生的独立危险因素。同时T等位基因携带者NFS评分显著降低,可能抑制了纤维化进展。上述结果不仅首次阐明了MTTP rs1800591多态性在老年NAFLD人群中的临床特征,为进一步的机制研究奠定了基础,还为NAFLD患者早期筛选提供一个可靠的遗传基因位点,促进了老年NAFLD患者的精确医疗和临床防治的发展。
伦理学声明:本研究方案于2020年10月26日经由首都医科大学附属北京佑安医院伦理委员会审批,批号:京佑科伦意[2020]-272。所纳入患者均签署知情同意书。
利益冲突声明:本研究不存在研究者、伦理委员会成员、受试者监护人以及与公开研究成果有关的利益冲突。
作者贡献声明:赵锦涵、张晶负责课题设计,资料分析,论文撰写;张洋负责修改文章关键内容;徐潇艺、勾钰淞、徐航飞、万妍参与收集数据,统计分析解释;吴剑负责拟定写作思路,指导撰写文章及最后定稿。赵锦涵和张晶对本文贡献等同,同为第一作者。
参考文献:
[1]
ZHOU J, ZHOU F, WANG W, et al. Epidemiological features of NAFLD From 1999 to 2018 in China[J]. Hepatology, 2020, 71(5): 1851-1864. DOI: 10.1002/hep.31150.
[2]
National Workshop on Fatty Liver and Alcoholic Liver Disease, Chinese Society of Hepatology, Chinese Medical Association, Fatty Liver Expert Committee, Chinese Medical Doctor Association. Guidelines of prevention and treatment for nonalcoholic fatty liver disease: A 2018 update[J]. J Clin Hepatol, 2018, 34(5): 947-957. DOI: 10.3969/j.issn.1001-5256.2018.05.007.
中華医学会肝病学分会脂肪肝和酒精性肝病学组, 中国医师协会脂肪性肝病专家委员会. 非酒精性脂肪性肝病防治指南(2018年更新版)[J]. 临床肝胆病杂志, 2018, 34(5): 947-957. DOI: 10.3969/j.issn.1001-5256.2018.05.007.
[3]LI J, YAO YS, DONG Q, et al. Characterization and factors associated with sleep quality among rural elderly in China[J]. Arch Gerontol Geriatr, 2013, 56(1): 237-243. DOI: 10.1016/j.archger.2012.08.002.
[4]ALQAHTANI SA, SCHATTENBERG JM. NAFLD in the elderly[J]. Clin Interv Aging, 2021, 16: 1633-1649. DOI: 10.2147/CIA.S295524.
[5]LOOMBA R, SCHORK N, CHEN CH, et al. Heritability of hepatic fibrosis and steatosis based on a prospective twin study[J]. Gastroenterology, 2015, 149(7): 1784-1793. DOI: 10.1053/j.gastro.2009.03.065
[6]PEREIRA IV, STEFANO JT, OLIVEIRA CP. Microsomal triglyceride transfer protein and nonalcoholic fatty liver disease[J]. Expert Rev Gastroenterol Hepatol, 2011, 5(2): 245-251. DOI: 10.1586/egh.11.22.
[7]WETTERAU JR, AGGERBECK LP, BOUMA ME, et al. Absence of microsomal triglyceride transfer protein in individuals with abetalipoproteinemia[J]. Science, 1992, 258(5084): 999-1001. DOI: 10.1126/science.1439810.
[8]BRADBURY P, MANN CJ, KCHL S, et al. A common binding site on the microsomal triglyceride transfer protein for apolipoprotein B and protein disulfide isomerase[J]. J Biol Chem, 1999, 274(5): 3159-3164. DOI: 10.1074/jbc.274.5.3159.
[9]GOUDA W, ASHOUR E, SHAKER Y, et al. MTP genetic variants associated with non-alcoholic fatty liver in metabolic syndrome patients[J]. Genes Dis, 2017, 4(4): 222-228. DOI: 10.1016/j.gendis.2017.09.002
[10]MUSSO G, GAMBINO R, CASSADER M. Lipoprotein metabolism mediates the association of MTP polymorphism with beta-cell dysfunction in healthy subjects and in nondiabetic normolipidemic patients with nonalcoholic steatohepatitis[J]. J Nutr Biochem, 2010, 21(9): 834-840. DOI: 10.1016/j.jnutbio.2009.06.007.
[11]NAMIKAWA C, SHU-PING Z, VYSELAAR JR, et al. Polymorphisms of microsomal triglyceride transfer protein gene and manganese superoxide dismutase gene in non-alcoholic steatohepatitis[J]. J Hepatol, 2004, 40(5): 781-786. DOI: 10.1016/j.jhep.2004.01.028.
[12]CARULLI L, CANEDI I, RONDINELLA S, et al. Genetic polymorphisms in non-alcoholic fatty liver disease: interleukin-6-174G/C polymorphism is associated with non-alcoholic steatohepatitis[J]. Dig Liver Dis, 2009, 41(11): 823-828. DOI:10.1016/j.dld.2009.03.005.
[13]OLIVEIRA CP, STEFANO JT, CAVALEIRO AM, et al. Association of polymorphisms of glutamate-cystein ligase and microsomal triglyceride transfer protein genes in non-alcoholic fatty liver disease[J]. J Gastroenterol Hepatol, 2010, 25(2): 357-361. DOI: 10.1111/j.1440-1746.2009.06001.x.
[14]PENG XE, WU YL, LU QQ, et al. MTTP polymorphisms and susceptibility to non-alcoholic fatty liver disease in a Han Chinese population[J]. Liver Int, 2014, 34(1): 118-128. DOI: 10.1111/liv.12220.
[15]ALQAHTANI SA, SCHATTENBERG JM. NAFLD in the Elderly[J]. Clin Interv Aging, 2021, 16: 1633-1649. DOI: 10.2147/CIA.S295524.
[16]ANGULO P, HUI JM, MARCHESINI G, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD[J]. Hepatology, 2007, 45(4): 846-854. DOI: 10.1002/hep.21496.
[17]WILLIAMS B, MANCIA G, SPIERING W, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension[J]. J Hypertens, 2018, 36(10): 1953-2041. DOI: 10.1097/HJH.0000000000001940.
[18]Chinese Diabetes Society. Guidelines for the prevention and control of type2 diabates in China(2017 Edition)[J]. Chin J Pract Intern Med, 2018, 38(4): 292-344. DOI: 10.19538/j.nk2018040108.
中華医学会糖尿病学分会. 中国2型糖尿病防治指南(2017年版)[J]. 中国实用内科杂志, 2018, 38(4): 292-344. DOI: 10.19538/j.nk2018040108.
[19]
WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies[J]. Lancet, 2004, 363(9403): 157-163. DOI: 10.1016/S0140-6736(03)15268-3.
[20]CHITTURI S, FARRELL GC, HASHIMOTO E, et al. Non-alcoholic fatty liver disease in the Asia-Pacific region: definitions and overview of proposed guidelines[J]. J Gastroenterol Hepatol, 2007, 22(6): 778-787. DOI: 10.1111/j.1440-1746.2007.05001.x.
[21]WANG CE, XU WT, GONG J, et al. Research progress in the treatment of nonalcoholic fatty liver disease[J]. Clin J Med Offic, 2022, 50(9): 897-899,903. DOI: 10.16680/j.1671-3826.2022.09.06.
王彩娥, 许文涛, 宫建, 等. 非酒精性脂肪性肝病治疗研究进展[J]. 临床军医杂志, 2022, 50(9): 897-899, 903. DOI: 10.16680/j.1671-3826.2022.09.06.
[22]BROUWERS MC, van GREEVENBROEK MM, CANTOR RM. Heritability of nonalcoholic fatty liver disease[J]. Gastroenterology, 2009, 137(4): 1536. DOI: 10.1053/j.gastro.2009.03.065.
[23]HUSSAIN MM, SHI J, DREIZEN P. Microsomal triglyceride transfer protein and its role in apoB-lipoprotein assembly[J]. J Lipid Res, 2003, 44(1): 22-32. DOI: 10.1194/jlr.r200014-jlr200.
[24]KIM DH, ZHANG T, LEE S, et al. FoxO6 integrates insulin signaling with MTP for regulating VLDL production in the liver[J]. Endocrinology, 2014, 155(4): 1255-1267. DOI: 10.1210/en.2013-1856.
[25]RAABE M, VNIANT MM, SULLIVAN MA, et al. Analysis of the role of microsomal triglyceride transfer protein in the liver of tissue-specific knockout mice[J]. J Clin Invest, 1999, 103(9): 1287-1298. DOI: 10.1172/JCI6576.
[26]ZHANG Y, XIN YN, CHENG YT, et al. The association between polymorphism rs1800591 in MTTP and non-alcoholic fatty liver disease[J/CD]. Chin J Liver Dis (Electronic Version), 2015, 7(3): 77-80. DOI: 10.3969/j.issn.1674-7380.2015.03.014.
張旸, 辛永宁, 程钰婷, 等. MTTP基因rs1800591位点多态性与非酒精性脂肪性肝病的相关性研究[J/CD]. 中国肝脏病杂志(电子版), 2015, 7(3): 77-80. DOI: 10.3969/j.issn.1674-7380.2015.03.014.
[27]LI L, WANG SJ, SHI K, et al. Correlation between MTP -493G>T polymorphism and non-alcoholic fatty liver disease risk: a meta-analysis[J]. Genet Mol Res, 2014, 13(4): 10150-10161. DOI: 10.4238/2014.
[28]TAN J, ZHANG J, ZHAO Z, et al. The association between SNPs rs1800591 and rs3816873 of the MTTP gene and nonalcoholic fatty liver disease: A meta-analysis[J]. Saudi J Gastroenterol, 2020, 26(4): 171-178. DOI: 10.4103/sjg.SJG_201_20.
[29]BERNARD S, TOUZET S, PERSONNE I, et al. Association between microsomal triglyceride transfer protein gene polymorphism and the biological features of liver steatosis in patients with type II diabetes[J]. Diabetologia, 2000, 43(8): 995-999. DOI: 10.1007/s001250051481.
[30]GAMBINO R, CASSADER M, PAGANO G, et al. Polymorphism in microsomal triglyceride transfer protein: a link between liver disease and atherogenic postprandial lipid profile in NASH?[J]. Hepatology, 2007, 45(5): 1097-1107. DOI: 10.1002/hep.21631.
[31]RAABE M, VNIANT MM, SULLIVAN MA, et al. Analysis of the role of microsomal triglyceride transfer protein in the liver of tissue-specific knockout mice[J]. J Clin Invest, 1999, 103(9): 1287-1298. DOI: 10.1172/JCI6576.
[32]TIETGE UJ, BAKILLAH A, MAUGEAIS C, et al. Hepatic overexpression of microsomal triglyceride transfer protein (MTP) results in increased in vivo secretion of VLDL triglycerides and apolipoprotein B[J]. J Lipid Res, 1999, 40(11): 2134-2139.
[33]CUCHEL M, RADER DJ. Microsomal transfer protein inhibition in humans[J]. Curr Opin Lipidol, 2013, 24(3): 246-250. DOI: 10.1097/MOL.0b013e32836139df.
[34]GARCA-GARCA AB, GONZLEZ C, REAL JT, et al. Influence of microsomal triglyceride transfer protein promoter polymorphism-493 GT on fasting plasma triglyceride values and interaction with treatment response to atorvastatin in subjects with heterozygous familial hypercholesterolaemia[J]. Pharmacogenet Genomics, 2005, 15(4): 211-218. DOI: 10.1097/01213011-200504000-00004.
[35]GOUDA W, ASHOUR E, SHAKER Y, et al. MTP genetic variants associated with non-alcoholic fatty liver in metabolic syndrome patients[J]. Genes Dis, 2017, 4(4): 222-228. DOI: 10.1016/j.gendis.2017.09.002.
[36]ZK A, JCHYMOV M, TVRZICK E, et al. The influence of polymorphism of -493G/T MTP gene promoter and metabolic syndrome on lipids, fatty acids and oxidative stress[J]. J Nutr Biochem, 2008, 19(9): 634-641. DOI: 10.1016/j.jnutbio.2007.09.001.
[37]KARPE F, LUNDAHL B, EHRENBORG E, et al. A common functional polymorphism in the promoter region of the microsomal triglyceride transfer protein gene influences plasma LDL levels[J]. Arterioscler Thromb Vasc Biol, 1998, 18(5): 756-761. DOI: 10.1161/01.atv.18.5.756.
[38]IQBAL J, BOUTJDIR M, RUDEL LL, et al. Intestine-specific MTP and global ACAT2 deficiency lowers acute cholesterol absorption with chylomicrons and HDLs[J]. J Lipid Res, 2014, 55(11): 2261-2275. DOI: 10.1194/jlr.M047951.
[39]YIN HJ, LI XL, XU C, et al. Clinical characteristics and influencing factors of non-alcoholic fatty liver disease in the elderly at a district in Beijing[J]. Chin J Mult Organ Dis Elderly, 2022, 21(9): 651-654. DOI: 10.11915 /j.issn.1671-5403.2022.09.141.
尹慧君, 李晓利, 徐成, 等. 北京某地区老年人群非酒精性脂肪性肝病的临床特征及影响因素[J]. 中华老年多器官疾病杂志, 2022, 21(9): 651-654. DOI: 10.11915 /j.issn.1671-5403.2022.09.141.
[40]RAJAN S, HOFER P, CHRISTIANO A, et al. Microsomal triglyceride transfer protein regulates intracellular lipolysis in adipocytes independent of its lipid transfer activity[J]. Metabolism, 2022, 137: 155331. DOI: 10.1016/j.metabol.2022.155331.
[41]GARCA-GARCA AB, GONZLEZ C, REAL JT, et al. Influence of microsomal triglyceride transfer protein promoter polymorphism -493 GT on fasting plasma triglyceride values and interaction with treatment response to atorvastatin in subjects with heterozygous familial hypercholesterolaemia[J]. Pharmacogenet Genomics, 2005, 15(4): 211-218. DOI: 10.1097/01213011-200504000-00004.
[42]di FILIPPO M, MOULIN P, ROY P, et al. Homozygous MTTP and APOB mutations may lead to hepatic steatosis and fibrosis despite metabolic differences in congenital hypocholesterolemia[J]. J Hepatol, 2014, 61(4): 891-902. DOI: 10.1016/j.jhep.2014.05.023.
收稿日期:
2022-11-18;錄用日期:2023-01-06
本文编辑:林姣

