甲胎蛋白应答评价索拉非尼联合卡瑞利珠单抗治疗晚期肝细胞癌的效果分析
王星 张韬
摘要:目的 探討甲胎蛋白(AFP)应答在索拉非尼联合卡瑞利珠单抗治疗晚期肝细胞癌中的临床疗效及安全性。方法 选取2020年9月—2022年2月于新疆医科大学第一附属医院接受索拉非尼联合卡瑞利珠单抗治疗的48例晚期肝细胞癌患者的临床资料,按照治疗后AFP应答水平进行分组:应答组(治疗6~8个月时,与基础AFP比较,AFP下降率>20%,n=32);无应答组(治疗6~8个月时,与基础AFP比较,AFP下降率≤20%,n=16)。计量资料组间比较采用 Mann-Whitney U检验;计数资料组间比较采用χ2检验。绘制生存曲线,行Cox单因素/多因素回归分析得到与OS相关的独立危险因素。比较两组患者无进展生存时间、总生存期与治疗效果。结果 两组患者中均未观察到达到临床缓解的病例。AFP应答组的客观有效率(21.88% vs 0)和疾病控制率(84.38% vs 43.75%)均高于无应答组(χ2值分别为2.530、6.668,P值分别为0.112、0.010)。应答组无进展生存期(9.9个月)、总生存期(13.8个月)均高于无应答组(6.8个月、11.1个月)。AFP早期无应答(HR=2.624,95%CI:1.097~6.277,P=0.030)及肝外转移(HR=0.392,95%CI:0.157~0.978,P=0.045)与较短的无进展生存期独立相关。未出现因不良反应导致停药事件的发生。结论 AFP早期应答在预测索拉非尼联合卡瑞利珠单抗治疗晚期肝细胞癌患者疗效及预后方面具有较高的临床价值。
关键词:癌, 肝细胞; 甲胎蛋白类; 索拉非尼
基金项目:新疆维吾尔自治区自然科学基金(2021D01C322)
Value of alpha-fetoprotein response in evaluating the efficacy of sorafenib combined with camrelizumab in treatment of advanced hepatocellular carcinoma
WANG Xing, ZHANG Tao. (Center of Infectious Diseases and Hepatology, The First Affiliated Hospital of Xinjiang Medical University, Urumqi 830054, China)
Corresponding author:
ZHANG Tao, zhang1tao3@163.com (ORCID:0000-0003-1272-675X)
Abstract:
Objective
To investigate the value of alpha-fetoprotein (AFP) response in evaluating the clinical efficacy and safety of sorafenib combined with camrelizumab in the treatment of advanced hepatocellular carcinoma (HCC). MethodsClinical data were collected from 48 patients with advanced HCC who were treated with sorafenib combined with camrelizumab in The First Affiliated Hospital of Xinjiang Medical University from September 2020 to February 2022, and according to the level of AFP response after treatment, they were divided into response group with 32 patients (AFP after 6-8 months of treatment was reduced by more than 20% compared with baseline AFP) and non-response group with 16 patients (AFP after 6-8 months of treatment was reduced by less than 20% compared with baseline AFP). The Mann-Whitney U test was used for comparison of continuous data between groups, and the chi-square test was used for comparison of categorical data between groups. Survival curves were plotted, and univariate and multivariate Cox regression analyses were used to investigate the independent risk factors for overall survival (OS). Progression free survival (PFS) time, OS time, and treatment outcome were compared between the two groups. Results No patient achieved clinical remission in either group. Compared with the non-response group, the response group had significantly higher objective response rate (21.88% vs 0, χ2=2.530, P=0.112) and disease control rate (84.38% vs 43.75%, χ2=6.668, P=0.010). Compared with the non-response group, the response group had longer PFS time (9.9 months vs 6.8 months) and OS time (13.8 months vs 11.1 months). Early non-response of AFP (hazard ratio [HR]=2.624, 95% confidence interval [CI]: 1.097-6.277, P=0.030) and extrahepatic metastasis (HR=0.392, 95%CI: 0.157-0.978, P=0.045) were independently associated with a shorter PFS time. No adverse event leading to drug withdrawal was observed in the study. Conclusion Early AFP response has a high clinical value in predicting the efficacy of sorafenib combined with camrelizumab in the treatment of advanced HCC and the prognosis of such patients.
Key words:
Carcinoma, Hepatocellular; alpha-Fetoproteins; Sorafenib
Research funding:Natural Science Foundation of Xinjiang Uygur Autonomous Region (2021D01C322)
肝细胞癌(hepatocellular carcinoma,HCC)为原发性肝癌中的一种,约占原发性肝癌的2/3以上。根据全球最新数据统计,因癌症导致死亡的原因中,HCC排名第四且在发病率方面排名第六[1-2]。据世界卫生组织[3]估计,截至2040年,将有100多万患者死于HCC。HCC早期临床症状缺乏特异性,70%~80%的患者确诊时已达中晚期,失去手术切除的机会或者超出肝移植适应证[4],从而无法获得根治性治疗。因此,亟需对于不能切除或晚期HCC患者的其他治疗策略。索拉非尼是一种口服多激酶抑制剂,是美国食品药品监督管理局批准用于治疗晚期肝癌的首个靶向药物,其通过阻断血管生成和RAS/MAPK信号传导介导的细胞增殖两种途径抑制肝癌的发生与发展[5],近年来,相关研究[6]逐渐发现单用索拉非尼易产生耐药现象,且常在获益后的6个月内发生,因此,联合治疗已成为趋势。考虑到HCC的发生发展和免疫逃逸有关,免疫检查点的激活可抑制自身免疫,有利于肿瘤细胞的生长和逃逸,使用针对免疫检查点的抑制剂可有效抑制肿瘤生长[7]。卡瑞利珠单抗是一种新型人源化IgG4型单克隆抗体,能够结合程序性死亡分子1并阻断其与程序性死亡分子配体1的结合,从而恢复机体免疫功能,达到抗肿瘤作用。基于一项中国晚期HCC的前瞻性、随机、平行对照、多中心的Ⅱ期临床研究[8]结果,中国国家药品监督管理局于2020年批准其用于接受过索拉非尼治疗和/或含奥沙利铂系统化疗的晚期HCC患者的治疗。其次,有相关研究[9-10]表明索拉非尼可预防免疫抑制,由于索拉非尼和卡瑞利珠单抗均为晚期肝癌推荐的治疗药物,因此,可以合理地预期,与单药治疗相比,二者联合使用将带来益处。AFP是一种约70 kDa的糖蛋白分子,由591个氨基酸组成,来源于胎儿肝细胞和卵黄囊,成熟肝脏细胞合成AFP能力不足,但是,在肝脏肿瘤中,AFP往往被重新激活并合成增多,虽然目前已有许多新的标志物被发现[11-12],但AFP因其临床易获取、安全且普及范围广,仍有其独特的优势。虽然联合治疗方案很多,但索拉非尼联合卡瑞利珠单抗治疗的相关报道较少,因此,本研究结合AFP应答评价索拉非尼联合卡瑞利珠单抗治疗晚期HCC患者疗效及预后,为HCC系统内科治疗提供临床参考。
1 资料与方法
1.1 研究对象 回顾性收集2020年9月—2022年2月于本院接受索拉非尼联合卡瑞利珠单抗的BCLC分期为B期或者C期的HCC患者的临床资料。研究对象纳入标准:(1)经病理活检或影像学确诊为HCC;(2)BCLC分期为B期或C期;(3)基线AFP≥20 ng/mL,接受索拉非尼联合卡瑞利珠单抗治疗≥6个月;(4)治疗前至少有1个可测量病灶;(5)Child-Pugh A/B级;(6)体力状况ECOG评分0~1分;(7)规律随访且随访资料完整。排除标准:(1)确诊为肝内胆管癌或混合型肝癌;(2)基线AFP<20 ng/mL;(3)接受索拉非尼聯合卡瑞利珠单抗治疗<6个月;(4)联合其他治疗;(5)无法规律随访或随访资料不完整。
1.2 联合治疗方案
1.2.1 甲苯磺酸索拉非尼治疗 评估患者一般情况,无药物禁忌证后给药2次/d,0.4 g/次,若出现无法耐受的不良反应,减少剂量或者停止用药,待临床症状缓解后再恢复剂量至0.8 g/d。
1.2.2 卡瑞利珠单抗治疗 索拉非尼服药后3~5 d,给予卡瑞利珠单抗200 mg溶于生理盐水250 mL中静点治疗,每3周重复给药1次。
1.3 疗效评价 影像学评价:根据实体瘤疗效评价分为以下4种标准。(1)肿瘤完全缓解(CR):所有靶病灶消失;(2)部分缓解(PR):以靶病灶直径的基线总和为基础,靶病灶直径总和≤30%;(3)稳定(SD):任何不符合PR或者肿瘤进展(PD)条件的病例;(4)PD:以自治疗开始以来记录的最小目标病灶直径之和为基础,目标病灶直径之和≥20%。计算客观缓解率(ORR)、疾病控制率(DCR)、无进展生存期(PFS)及总生存期(OS)[13-14]。
1.4 统计学方法 采用SPSS 26.0统计软件进行数据分析。计量资料采用M(P25~P75)表示,组间比较采用Mann-Whitney U检验;计数资料组间比较采用χ2检验。绘制生存曲线,Cox单因素及多因素回归分析与OS相关的独立危险因素。P<0.05为差异有统计学意义。
2 结果
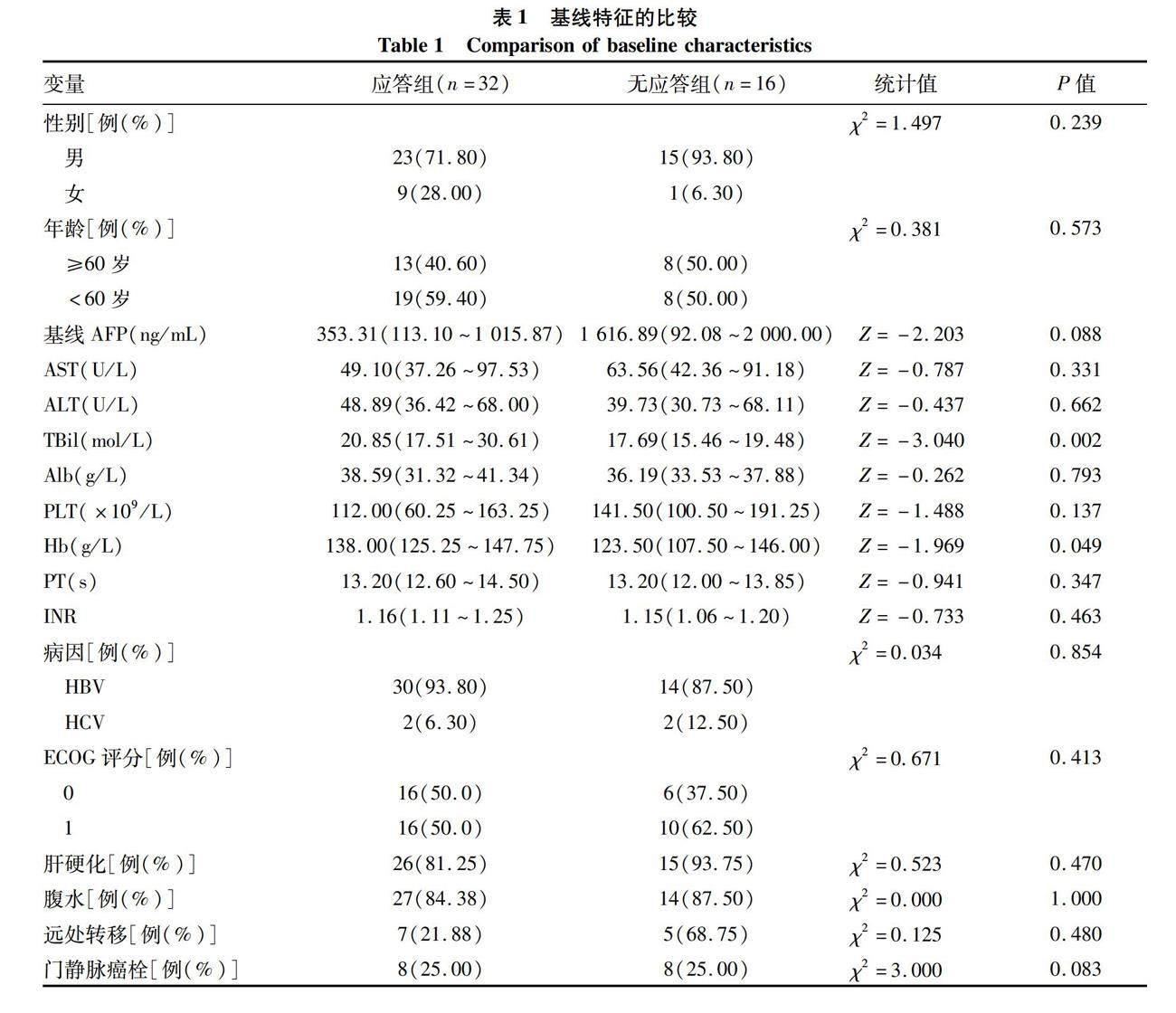
2.1 一般资料 共收集患者48例,按照治疗后AFP应答水平分为应答组(治疗6~8个月时,与基础AFP比较,AFP下降率>20%);无应答组(治疗6~8个月时,与基础AFP比较,AFP下降率≤20%)。应答组和无应答组患者TBil及Hb水平比较,差异均有统计学意义(P值均<0.05);其余基线指标比较,差异均无统计学意义(P值均>0.05)(表1)。
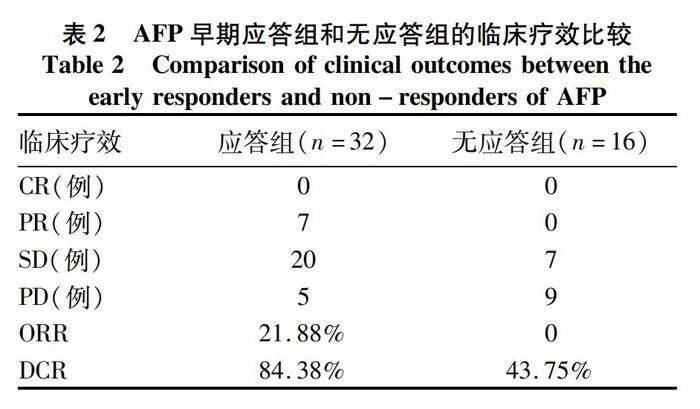
2.2 两组患者临床疗效评价 AFP应答组的ORR和DCR均高于无应答组(χ2值分别为2.530、6.668,P值分别为0.112、0.010)(表2)。
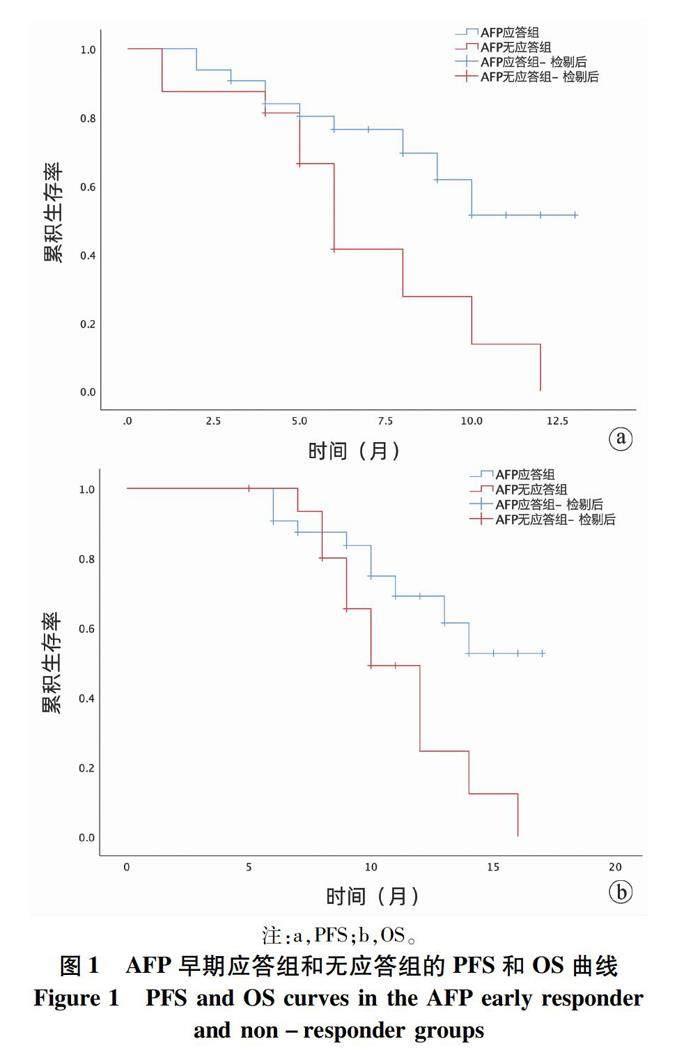
2.3 两组患者生存情况比较 截至本研究末次随访(2022年1月),应答组PFS为9.90个月(95%CI:9.34~11.40);无应答组PFS为6.80个月(95%CI:4.90~8.62);应答组OS为13.80个月(95%CI:12.25~15.37);无应答组OS为11.10个月(95%CI:9.51~12.70)(图1)。21例患者死亡(AFP早期应答组10例,无应答组11例)。
2.4 单因素和多因素分析 PFS相关单因素分析结果显示,AFP早期无应答、肝外转移患者的PFS较差;多因素分析结果显示,AFP早期无应答及肝外转移与较短的PFS独立相关(表3)。 OS相关单因素分析显示,AFP早期无应答、肝外转移和ECOG评分1分患者的OS更差;多因素分析结果显示,肝外转移、ECOG 评分1分与较短的OS独立相关。AFP早期无应答组多因素分析结果P值接近统计学临界值,但未达到,考虑与样本量含量及随访时间较短有关(表4)。
3 讨论
HCC是世界上最常见的癌症之一,与其他常见癌症(肺癌、乳腺癌和前列腺癌)逐渐下降的病死率相比,HCC的病死率持续以每年约23%的速度增长,正是因为其持续升高的病死率,各种抗肿瘤药物(索拉非尼、仑伐替尼、瑞戈非尼、多纳非尼)及联合治疗(阿替丽珠单抗+贝伐珠单抗、仑伐替尼+帕博利珠单抗)正活跃在肝癌系统治疗领域。索拉非尼是众多酪氨酸酶抑制剂中的一种,具有抑制肿瘤血管生成和肿瘤增殖的作用,并在多个肿瘤预测模型当中使肿瘤凋亡率显著升高[15-17],国外指南[18-19]一致推荐其作为无法进行手术、局部消融或局部治疗的晚期患者的首选药物。随着免疫药物的研发上市,联合治疗体现出其优势。KEYNOTE-524研究[20]中,对接受仑伐替尼联合帕博利珠单抗治疗的患者进行随访研究,结果显示OS达到22个月。IMBRAVE-150临床研究[21]结果显示,336例接受阿替利珠单抗联合贝伐单抗治疗的患者12个月总生存率为67.2%,高于对照组54.6%(P<0.05); 两组PFS分别为6.8个月、4.3个月。AFP是HCC最具有特征性的肿瘤标志物,不仅可以筛查和诊断HCC,其评估HCC患者疗效的预后价值也逐渐被多项研究发现。虽对AFP应答国际上暂未有明确的临界值,但大部分研究将AFP在治疗后较基线水平下降超过20%定义为AFP应答[22-26]。多项研究将AFP应答同影像学结果相结合进行分析。Park[27]的研究结果显示,索拉非尼治疗的患者完全缓解率为1.34%。研究[28]显示297例接受索拉非尼治疗的患者,7例出现PR,211例处于SD。Chen等[29]对42例基线AFP>20 ng/mL、接受沙利度胺治疗晚期HCC患者进行回顾性分析指出,AFP应答组获得PR和SD的患者顯著多于无应答组。本研究结果显示,AFP应答组ORR及DCR均显著高于AFP无应答组,因此AFP应答与影像学(基于RECIST 1.1标准)应答密切相关,这与既往相关研究结论一致。目前,国内外关于AFP应答与索拉非尼联合卡瑞利珠单抗治疗晚期HCC患者生存的关系的相关报道较少。本研究结果表明AFP早期应答组的PFS为9.90个月,AFP早期无应答组的PFS为6.80个月,AFP早期应答组的OS为13.80个月,AFP早期无应答组的OS为11.10个月;与AFP应答组相比,无应答组的具有较短的PFS及OS,且单因素、多因素分析提示AFP应答与PFS独立相关,验证了AFP应答在治疗早期的预测能力,虽然在OS多因素分析中,AFP应答未能得到统计学意义,但其模型整体表现为有意义,在此认为原因可能是样本量较少引起的,进一步扩大样本量及延长随访时间预计可得到预期结果。在本研究中,据临床观察及患者主观反馈,AFP应答组患者表示其在生活中精神状态、食欲、睡眠、疼痛、体力等方面虽与患病前相比均有所下降,但明显强于AFP不应答组患者,此为临床医师观察及患者主观描述,误差不可避免。后可行相关量表开展进一步分析研究。
本研究也具有一定的局限性。首先是样本量较小,不排除数据结果的偏倚;其次是随访时间较短,在影响OS的多因素分析当中,虽然预测模型有效,但AFP应答未能同其他研究结果一致,可能为样本量太小导致,进一步扩大样本量及随访时间预计可达到预期结果;最后,本研究是基于单药较为安全且获益于患者前提下的临床联合用药,即使整体上OS未有明显提高,但患者总体临床疗效较好,可作为肝癌治疗方面的临床参考。综上所述,AFP作为一个安全、无创、临床上易获得、灵敏度高的实验室指标,其早期应答在索拉非尼联合卡瑞利珠单抗治疗晚期HCC患者疗效及预测方面具有较高的临床指导意义,未来需在多种标志物联合方面进一步努力,可探索外周循环遗传物质等新的标志物。
伦理学声明:本研究方案经由新疆医科大学第一附属医院伦理委员会审批,批号:K202212-10,所纳入患者均签署知情同意书。
利益冲突声明:本研究不存在研究者、伦理委员会成员、受试者监护人以及公开研究成果有关的利益冲突。
作者贡献声明:王星负责课题设计,资料分析,撰写论文;张韬负责指导撰写文章并最后定稿。
参考文献:
[1]
BRAY F, FERLAY J, SOERJOMATARAM I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2018, 68(6): 394-424. DOI: 10.3322/caac.21492.
[2]WU T, CHEN L. New advances in the precision diagnosis and treatment of liver cancer[J]. J Clin Hepatol, 2022, 38(3): 497-498. DOI: 10.3969/j.issn.1001-5256.2022.03.001.
吴彤, 陈磊. 肝癌精准诊疗新进展[J]. 临床肝胆病杂志, 2022, 38(3): 497-498. DOI: 10.3969/j.issn.1001-5256.2022.03.001.
[3]RUMGAY H, ARNOLD M, FERLAY J, et al. Global burden of primary liver cancer in 2020 and predictions to 2040[J]. J Hepatol, 2022, 77(6):1598-1606. DOI: 10.1016/j.jhep.2022.08.021.
[4]European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma[J]. J Hepatol, 2018, 69(1): 182-236. DOI: 10.1016/j.jhep.2018.03.019.
[5]WILHELM SM, CARTER C, TANG L, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis[J]. Cancer Res, 2004, 64(19): 7099-7109. DOI: 10.1158/0008-5472.CAN-04-1443.
[6]GAO R, KALATHUR R, COTO-LLERENA M, et al. YAP/TAZ and ATF4 drive resistance to Sorafenib in hepatocellular carcinoma by preventing ferroptosis[J]. EMBO Mol Med, 2021, 13(12): e14351. DOI: 10.15252/emmm.202114351.
[7]ZHOU YY, ZHAO XX, CHEN YY, et al. Tumor immune checkpoint inhibitor and its combination research progress of combination therapy[J]. China pharmacy, 2020, 31 (7): 890-896. DOI: 10.6039/j.issn.1001-0408.2020.07.24.
周姚邑, 趙新新, 陈沅沅, 等. 肿瘤免疫检查点抑制剂及其联合疗法的研究进展[J]. 中国药房, 2020, 31(7): 890-896. DOI: 10.6039/j.issn.1001-0408.2020.07.24.
[8]QIN S, REN Z, MENG Z, et al. Camrelizumab in patients with previously treated advanced hepatocellular carcinoma: a multicentre, open-label, parallel-group, randomised, phase 2 trial[J]. Lancet Oncol, 2020, 21(4): 571-580. DOI: 10.1016/S1470-2045(20)30011-5.
[9]KUDO M. Immune checkpoint blockade in hepatocellular carcinoma: 2017 update[J]. Liver Cancer, 2016, 6(1): 1-12. DOI: 10.1159/000449342.
[10]ZHAO X, CHEN Q, LIU W, et al. Codelivery of doxorubicin and curcumin with lipid nanoparticles results in improved efficacy of chemotherapy in liver cancer[J]. Int J Nanomedicine, 2014, 10: 257-270. DOI: 10.2147/IJN.S73322.
[11]GAO YX, YANG TW, YIN JM, et al. Progress and prospects of biomarkers in primary liver cancer (Review)[J]. Int J Oncol, 2020, 57(1): 54-66. DOI: 10.3892/ijo.2020.5035.
[12]HAO X, FAN R, HOU JL. Early warning and accurate screening for the high-risk population of hepatocellular carcinoma[J]. J Clin Hepatol, 2022, 38(3): 499-504. DOI: 10.3969/j.issn.1001-5256.2022.03.002.
郝新, 樊蓉, 侯金林. 原发性肝癌高危人群的早期预警和精准筛查[J]. 临床肝胆病杂志, 2022, 38(3): 499-504. DOI: 10.3969/j.issn.1001-5256.2022.03.002.
[13]LENCIONI R, LLOVET JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma[J]. Semin Liver Dis, 2010, 30(1): 52-60. DOI: 10.1055/s-0030-1247132.
[14]SHIBA S, OKUSAKA T, IKEDA M, et al. Characteristics of 18 patients with hepatocellular carcinoma who obtained a complete response after treatment with sorafenib[J]. Hepatol Res, 2014, 44(13): 1268-1276. DOI: 10.1111/hepr.12297.
[15]LIU YC, CHENG TC, BIAN ZL.Progress and challenges of combined immune checkpoint inhibitors in the treatment of hepatocellular carcinoma[J/OL]. Chin J Immunol, 2023. [Online ahead of print]
劉一村, 程苕莼, 卞兆连. 联合免疫检查点抑制剂治疗肝细胞癌的进展与挑战[J/OL]. 中国免疫学杂志, 2023. [网络首发]
[16]CHANG YS, ADNANE J, TRAIL PA, et al. Sorafenib (BAY 43-9006) inhibits tumor growth and vascularization and induces tumor apoptosis and hypoxia in RCC xenograft models[J]. Cancer Chemother Pharmacol, 2007, 59(5): 561-574. DOI: 10.1007/s00280-006-0393-4.
[17]FU Y, WEI X, LIN L, et al. Adverse reactions of sorafenib, sunitinib, and imatinib in treating digestive system tumors[J]. Thorac Cancer, 2018, 9(5): 542-547. DOI: 10.1111/1759-7714.12608.
[18]European Association for the Study of the Liver, European Organisation for Research and Treatment of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma[J]. J Hepatol, 2012, 56(4): 908-943. DOI: 10.1016/j.jhep.2011.12.001.
[19]MOTZER RJ, AGARWAL N, BEARD C, et al. NCCN clinical practice guidelines in oncology: kidney cancer[J]. J Natl Compr Canc Netw, 2009, 7(6): 618-630. DOI: 10.6004/jnccn.2009.0043.
[20]FINN RS, IKEDA M, ZHU AX, et al. Phase Ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma[J]. J Clin Oncol, 2020, 38(26): 2960-2970. DOI: 10.1200/JCO.20.00808.
[21]FINN RS, QIN S, IKEDA M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma[J]. N Engl J Med, 2020, 382(20): 1894-1905. DOI: 10.1056/NEJMoa1915745.
[22]KAO WY, CHIOU YY, HUNG HH, et al. Serum alpha-fetoprotein response can predict prognosis in hepatocellular carcinoma patients undergoing radiofrequency ablation therapy[J]. Clin Radiol, 2012, 67(5): 429-436. DOI: 10.1016/j.crad.2011.10.009.
[23]SHAO YY, LIN ZZ, HSU C, et al. Early alpha-fetoprotein response predicts treatment efficacy of antiangiogenic systemic therapy in patients with advanced hepatocellular carcinoma[J]. Cancer, 2010, 116(19): 4590-4596. DOI: 10.1002/cncr.25257.
[24]SHAO YY, LIU TH, HSU C, et al. Early alpha-foetoprotein response associated with treatment efficacy of immune checkpoint inhibitors for advanced hepatocellular carcinoma[J]. Liver Int, 2019, 39(11): 2184-2189. DOI: 10.1111/liv.14210.
[25]PAUL SB, SAHU P, SREENIVAS V, et al. Prognostic role of serial alpha-fetoprotein levels in hepatocellular carcinoma treated with locoregional therapy[J]. Scand J Gastroenterol, 2019, 54(9): 1132-1137. DOI: 10.1080/00365521.2019.1660403.
[26]SNCHEZ A, ROCES LV, GARCA IZ, et al. Value of α-fetoprotein as an early biomarker for treatment response to sorafenib therapy in advanced hepatocellular carcinoma[J]. Oncol Lett, 2018, 15(6): 8863-8870. DOI: 10.3892/ol.2018.8400.
[27]PARK JG. Long-term outcomes of patients with advanced hepatocellular carcinoma who achieved complete remission after sorafenib therapy[J]. Clin Mol Hepatol, 2015, 21(3): 287-294. DOI: 10.3350/cmh.2015.21.3.287.
[28]LLOVET JM, RICCI S, MAZZAFERRO V, et al. Sorafenib in advanced hepatocellular carcinoma[J]. N Engl J Med, 2008, 359(4): 378-390. DOI: 10.1056/NEJMoa0708857.
[29]CHEN LT, LIU TW, CHAO Y, et al. alpha-fetoprotein response predicts survival benefits of thalidomide in advanced hepatocellular carcinoma[J]. Aliment Pharmacol Ther, 2005, 22(3): 217-226. DOI: 10.1111/j.1365-2036.2005.02547.x.
收稿日期:
2022-08-31;錄用日期:2022-10-27
本文编辑:林姣

