可见光催化环丙基苯胺的[3+2]环化加成反应研究
张晓斐 王尧 刘佳鑫 李丹璐
摘要:环烷烃类天然有机产物和生物医药分子的骨架构建依靠环加成反应得以实现,而环丙基苯胺能够在光催化条件下断键产生胺基阳离子自由基,继而为环化提供自由基供体,实现新的C-C键的构筑.但是此类反应通常需要使用昂贵的过渡金属光催化剂,提高了应用成本.本文使用4CzIPN这种非金属催化剂,以环丙基苯胺和肉桂酸酯类衍生物作为底物,通过光催化环化反应,以较为优异的位置选择性合成了一系列环戊烷类衍生物,为光催化自由基环化反应提供了更加经济的新方法,进一步拓宽了环丙基苯胺在光催化领域的应用.
关键词:光催化; 自由基; 环化反应
中图分类号:O62文献标志码: A
Study on visible-light-induced [3+2] cycloaddition of cyclopropylaniline
ZHANG Xiao-fei WANG Yao LIU Jia-xin LI Dan-lu(1.College of Chemistry and Chemical Engineering,Shaanxi University of Science & Technology, Xi′an 710021, China; 2.Shaanxi Key Laboratory of Chemical Additive for Industry, Shaanxi University of Science & Technology, Xi′an 710021, China)
Abstract:The skeleton construction of cycloalkane natural organic products and biomedical molecules is realized by cycloaddition reaction,while cyclopropylaniline can break bond under photocatalytic conditions to produce amino cation free radicals,and then provide free radical donors for cyclization to realize the construction of new C-C bond.However,such reactions usually require the use of expensive transition metal photocatalysts,which increases the application cost.In this paper,a series of cyclopentane derivatives were synthesized with excellent regioselectivity by using 4CzIPN,a non-metallic catalyst,with cyclopropylaniline and cinnamate derivatives as substrates through photocatalytic cyclization reaction,which provided a more economical new method for photocatalytic free radical cyclization reaction and further broadened the application of cyclopropylaniline in the field of photocatalysis.
Key words:photocatalysis; free radical; cyclization reaction
0引言
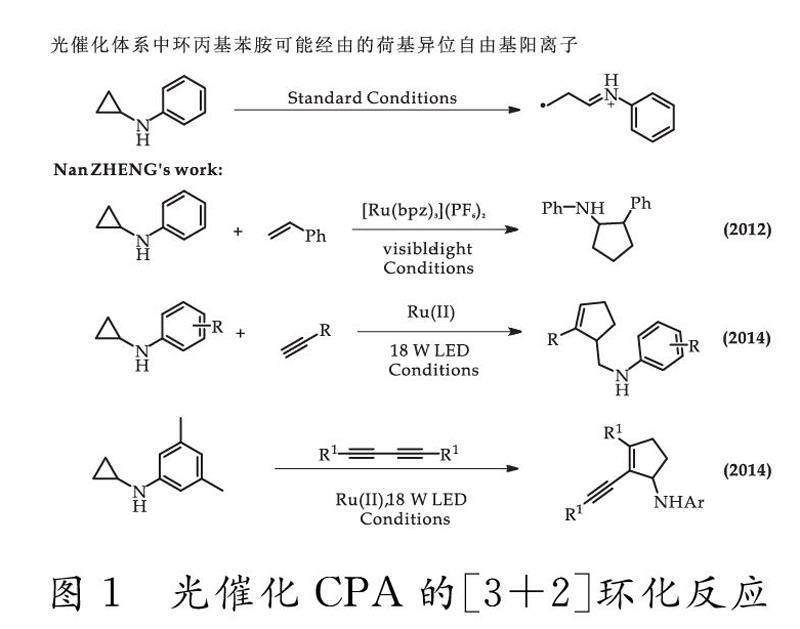
可持續发展是当今世界所面临的整体趋势.太阳能作为自然界一种清洁、丰富、可再生的天然能源,如何将其高效快捷转化为化学能,是如今科学家们所面临的巨大挑战.在有机化学合成中,大部分有机分子都不能直接吸收太阳光,从而限制了太阳能在有机合成中的应用,为解决这一问题,通常会使用贵金属络合物作为光催化剂,通过其将可见光能量导入有机分子.例如Macmillan团队[1-4]、Glorius团队[5-9]、Yoon团队[10-17]、Stephenson团队[18,19]等通过使用钌(II)、铱(III)等贵金属,在温和的条件下,以较高产率、高选择性实现各种不同杂化方式的碳碳键的构架.其中,环丙基苯胺能够在此类光催化条件下不可逆开环,同时氧化为氮基正离子,进而作为自由基供体,提供三个碳原子,完成碳碳键的构筑.
早在2012年,Zheng课题组利用可见光氧化还原催化首次实现了环丙基苯胺(CPA)与苯乙烯的分子间[3+2]环化反应.他们发现在可见光及光催化剂作用下,环丙胺能够被氧化为碳自由基阳离子且此过程不可逆,进而,能够与烯烃在一定条件下,发生环化反应形成五元碳环.该方法针对烯烃两个可能的反应位点具有较好的区域选择性,同时,在对底物拓展时具有优良的产率及官能团耐受性[20].2014年,该团队深入探究了CPA与其他类型不饱和键的反应能力[21].Zheng课题组将环丙基苯胺与端炔烃进行反应,使用不同的光催化剂,以较好或优秀的产率合成了一系列的五元环烯化合物,同时,对CPA与对称的芳香二炔、脂肪二炔的反应也进行了报道,以极好的产率和选择性得到了一系列烯炔化合物,进一步丰富了CPA的使用范畴.同年,该课题组通过更换光催化剂,突破性的提高了CPA与端炔烃反应的产率,使得N-环丙基苯胺在与各类不饱和碳的反应中均能以稳定高效的碳自由基阳离子中间体为反应提供碳源,实现环化[22],如图1所示.
2017年,Zheng团队不满足于仅猜测反应过程中所经由的中间体,而通过N-环丙基苯胺(CPA)和苯乙烯分子间[3+2]环化反应,利用电喷雾电离质谱(ESI-MS),结合在线激光照射,对二者反应中生成的瞬态胺基阳离子进行检测.他们通过高分辨质谱(HRMS)成功地检测到了反应中的还原性光催化剂Ru(I)(bpz)3+以及[3+2]环化产物阳离子2+·中间体,为Ru(II)促进的[3+2]环化反应链式反应机制提供了强有力的证据,成功确认了由CPA参与的环化反应的关键步骤,也为后续拓展CPA的其他反应提供了可靠的理论基础[23].
然而,目前已知的针对CPA的反应都是由贵金属光催化剂促进,具有成本较高、毒性强的缺点,且通常使用在与端烯、端炔这类较为特殊的底物之上,进一步限制了其在药物合成领域及工业化领域的发展.
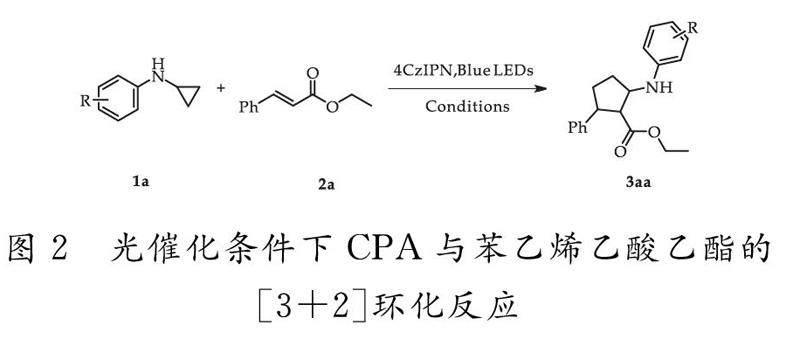
本文拟采取N-环丙基苯胺及其衍生物作为自由基供体,在有机廉价非金属光催化剂4CzIPN存在的条件下,经蓝光照射之后产生胺基阳离子自由基再发生环加成反应,得到高选择性的2-苯基-3-(苯基氨基)环戊烷羧酸乙酯衍生物,具体过程如图2所示.
1实验部分
1.1试剂和仪器
1.1.1主要试剂
溴苯、环丙氨、肉桂酸乙酯、4-甲氧基肉桂酸、膦酰基乙酸三乙酯、3-甲基肉桂酸、氢化钠、甲酸、三(2,2′-联吡啶)钌(II)二(六氟磷酸)盐、三联吡啶氯化钌六水合物、四氟间苯二腈,以上试剂均购买自萨恩化学技术(上海)有限公司;曙红Y、罗丹明、氯化铈、咔唑、4-甲基肉桂酸、4-乙基苯甲醛、4-叔丁基苯甲醛、4-三氟甲基肉桂酸、4-氟肉桂酸、4-溴苯甲醛、4-氯苯甲醛、1-溴-4-乙基苯、1-溴2-甲基苯、1-溴-3-甲基苯、1-溴-3氯苯、1-溴-4氯苯,以上试剂均购买自上海毕得医药科技有限公司;THF、DMF、DMC等溶剂均购买自广东光华科技股份有限公司;200~300目柱层析用硅胶购买自青岛海洋化工厂.
1.1.2主要仪器
SGW X-4B 型显微熔点仪,上海仪电物理光学仪器有限公司;20 W 蓝色LED实验灯,前方照明公司;AVANCE Ⅲ 400 MHz 型核磁共振仪,Impact HD Q-TOF 型高分辨质谱仪,VECTOR Ⅱ 型光谱仪,德国Bruker公司;旋轉蒸发仪RE-52AA,上海亚荣科技有限公司;加热磁力搅拌器MS-H-Pro+,SCILOGEX公司.
1.22-苯基-3-(苯基氨基)环戊烷-1-羧酸乙酯衍生物的合成
1.2.1环丙苯胺类底物的合成
根据前人的方法指导,合成一系列环丙苯胺类底物[24].将溴苯(1.9 mmol),环丙氨(2.3 mmol),醋酸钯(10 mol%),叔丁醇钾(2.7 mmol),2-(二环已基膦)-3,6-二甲氧基-2,4,6-三异丙基-1,1-联苯(0.06 mmol),甲苯(10 mL)加入反应瓶中,氮气保护并使用锡箔纸避光,80 ℃反应18 h.待反应完成后,冷却至室温,加入10 mL饱和食盐水,使用乙酸乙酯萃取洗涤(3*10 mL)之后收集有机相,减压除去溶剂,快速柱层析(PE/EA=30∶1,体积比),得到相应的N-环丙基苯胺及其衍生物.
1.2.2肉桂酸乙酯类底物的合成
方法一:将肉桂酸或其衍生物(3.0 mmol),浓硫酸(0.5 mL,98%),乙醇(3.8 mL),装入圆底烧瓶中,82 ℃下回流5 h,反应完成后冷却至室温,加入饱和碳酸氢钠中和反应液后使用乙酸乙酯(3*30 mL)萃取,收集有机相,减压除去溶剂,经快速柱层析(PE/EA=2∶1,体积比),得到对应的肉桂酸酯类衍生物.
方法二:将膦酰基乙酸三乙酯的四氢呋喃溶液(12.0 mmol,1.0 mmol/L THF)在氮气保护,0 ℃下缓慢滴入氢化钠溶液(12.0 mmol,1.0 mmol/L THF)中,搅拌15 min,再将带取代基的苯甲醛类溶液(10.0 mmol,1.0 mmol/L THF)缓慢滴入混合溶液中,0 ℃下搅拌0.5 h后,在80 ℃下回流过夜,待反应完成后冷却至室温,加入饱和食盐水,经乙酸乙酯萃取之后收集有机相,减压除去溶剂,经快速柱层析(PE/EA=10∶1,体积比),得到反应原料肉桂酸酯类衍生物.
1.2.32-苯基-3-(苯基氨基)环戊烷-1-羧酸乙酯衍生物的合成
将肉桂酸酯类衍生物(0.1 mmol),环丙苯胺类衍生物(0.2 mmol),光反应催化剂2,4,5,6-四(9-咔唑基)-间苯二睛,以下简称为4CzIPN(2.0 mol%),反应溶剂无水甲醇(2.0 mL)依次加入10 mL透光反应管中,氮气密封,在室温下经20 W蓝光照射下搅拌12 h,反应完全后,加入水溶液猝灭反应,使用乙酸乙酯(3*10 mL)萃取三次,合并有机相,无水硫酸钠干燥减压除去溶剂后进行快速柱层析(PE/EA=30∶1,体积比),得到相对应的2-苯基-5-(苯基氨基)环戊烷-1-羧酸乙酯衍生物,最后使用核磁共振,X-射线单晶衍射对产物进行表征.
产品的表征数据如下:
2-苯基-5-(苯基氨基)环戊烷-1-羧酸乙酯(3aa,淡黄色油状液体,78%):1H NMR (400 MHz,CDCl3):δ 7.34-7.20 (m,5H),7.14-7.07 (m,2H),6.67 (t,J=7.3 Hz,1H),6.52 (d,J=7.7 Hz,2H),4.08 (q,J=7.1 Hz,2H),3.95-3.86 (m,2H),3.27 (t,J=8.6 Hz,1H),2.95 (d,J=7.4 Hz,1H),2.45-2.37 (m,1H),2.27-2.07 (m,2H),1.81-1.70(m,1H),1.17 (t,J=7.1 Hz,3H).13C NMR (100 MHz,CDCl3):δ 175.33,147.66,141.54,129.18,128.71,127.55,126.97,117.34,113.38,61.54,60.68,56.19,50.68,32.50,28.08,14.18.
2-苯基-3-(苯基氨基)环戊烷-1-羧酸乙酯(4aa,淡黄色油状液体,15%):1H NMR (400 MHz,CDCl3):δ 7.32-7.17 (m,5H),7.10 (t,J=7.7 Hz,2H),6.67 (t,J=7.2 Hz,1H),6.46 (d,J=7.9 Hz,2H),4.18-4.09 (m,3H),3.81 (t,J=7.4 Hz,1H),3.42-3.30 (m,2H),2.37-2.19 (m,2H),2.08-1.97 (m,1H),1.91-1.81 (m,1H),1.23 (t,J=7.1 Hz,3H).13C NMR (100 MHz,CDCl3):δ 176.12,147.48,138.68,129.20,128.70,128.62,127.08,117.46,113.49,60.79,57.80,51.67,47.01,31.86,27.63,14.32.
2-(苯胺基)-5-(对甲苯基)环戊烷-1-羧酸乙酯(3ab,淡黄色油状液体,81%):1H NMR (400 MHz,CDCl3) δ 7.17-7.10 (m,6H),6.66 (t,J=7.28 Hz,1H),6.52 (d,J=7.84 Hz,2H),4.12-4.07 (m,2H),3.89-3.84 (m,1H),3.26 (t,J=8.72 Hz,1H),2.99-2.92 (m,1H),2.41-2.36(m,1H),2.32 (s,3H),2.25-2.06 (m,2H),1.79-1.70 (m,1H),1.18 (t,J=7.12,3H).13C NMR (100 MHz,CDCl3):δ 175.38,147.72,138.42,136.49,129.38,129.13,127.38,117.26,113.38,61.46,60.60,55.73,50.65,32.44,28.02,21.04,14.19.
3-(苯胺基)-2-(对甲苯基)环戊烷-1-羧酸乙酯(4ab,淡黄色固体,18%):1H NMR (400 MHz,CDCl3) δ 7.18-7.09 (m,6H),6.68 (t,J=8 Hz,1H),6.51 (d,J=7.8 Hz,2H),4.18-4.07 (m,3H),3.79 (t,J=7.52,1H),3.41-3.34 (m,1H),2.34 (s,3H),2.28-2.19 (m,2H),2.08-1.99 (m,1H),1.92-1.83 (m,1H),1.25 (t,J=7.12Hz,3H).13C NMR (100 MHz,CDCl3):δ 176.08,147.49,136.54,135.43,129.25,129.01,128.45,117.32,113.45,60.64,57.69,51.27,46.92,31.70,27.55,21.04,14.25.
2-(苯胺基)-5-(間甲苯基)环戊烷-1-羧酸乙酯(3ac,淡黄色油状液体,63%):1H NMR (400 MHz,CDCl3) δ 7.19 (t,J=7.68 Hz,1H),7.13-7.02 (m,5H),6.66 (t,J=7.28 Hz,1H),6.53 (d,J=8.04 Hz,2H),4.16-4.06 (m,2H),3.91-3.85 (m,1H),3.24 (t,J=8.48 Hz,1H),3.00-2.94 (m,1H),2.42-2.35 (m,1H),2.32 (s,3H),2.26-2.05 (m,2H),1.79-1.71 (m,1H),1.17 (t,J=7.12 Hz,3H).13C NMR (100 MHz,CDCl3):δ 175.36,147.69,141.49,138.20,129.12,128.55,128.31,127.73,124.53,117.27,113.42,61.50,60.60,56.13,50.67,32.50,28.14,21.50,14.17.
3-(苯胺基)-2-(间甲苯基)环戊烷-1-羧酸乙酯(4ac,淡黄色固体,29%):1H NMR (400 MHz,CDCl3) δ 7.14-7.00 (m,6H),6.69-6.49 (m,1H),6.48 (d,J=7.88 Hz,2H),4.13-4.08 (m,3H),3.78 (t,J=7.44 Hz,1H),3.42-3.36 (m,1H),2.36-2.28,(m,1H),2.32 (s,3H),2.26-2.19 (m,1H),2.07-1.98 (m,1H),1.92-1.83,(m,1H),1.24 (t,J=7.08 Hz,3H).13C NMR (100 MHz,CDCl3):δ 176.04,147.46,138.10,129.57,129.07,128.38,127.76,125.39,117.34,113.48,60.62,57.69,51.55,46.78,31.73,27.59,21.49,14.23.
2-(4-甲氧基苯基)-5-(苯胺基)环戊烷-1-羧酸乙酯(3ad,淡黄色油状液体,62%):1H NMR (400 MHz,CDCl3) δ 7.18 (d,J=8.72 Hz,2H),7.11 (t,J=8.04 Hz,2H),6.83 (d,J=8.6 Hz,2H),6.65 (t,J=7.28 Hz,1H),6.51 (d,J=7.84 Hz,2H),4.11-4.06 (m,2H),3.86-3.80 (m,1H),3.77 (s,3H),3.22 (t,J=8.04 Hz,1H),2.95-2.89 (m,1H),2.41-2.33 (m,1H),2.23-2.04 (m,2H),1.77-1.69 (m,1H),1.17 (t,J=7.12 Hz,3H).13C NMR (100 MHz,CDCl3):δ 175.37,158.55,147.76,133.37,129.15,128.50,117.30,114.10,113.38,61.45,60.60,55.44,55.26,50.71,32.38,27.88,14.20.
2-(4-甲氧基苯基)-3-(苯胺基)环戊烷-1-羧酸乙酯(4ad,淡黄色固体,21%):1H NMR (400 MHz,CDCl3) δ 7.12-7.09 (m,4H),6.84 (d,J=8.6 Hz,2H),6.66 (t,J=7.28 Hz,1H),6.47 (d,J=7.88 Hz,2H),4.17-4.11 (m,2H),4.08-4.04 (m,1H),3.79 (s,3H),3.76-3.73 (m,1H),3.35-3.29 (m,1H),2.33-2.18 (m,2H),2.05-1.98 (m,1H),1.88-1.80 (m,1H),1.23 (t,J=7.12 Hz,3H).13C NMR (100 MHz,CDCl3):δ 176.05,147.47,130.45,129.56,129.08,117.33,113.92,113.43,60.63,57.67,55.24,50.92,47.12,31.73,27.52,14.22.
2-(4-乙基苯基)-5-(苯基胺基)環戊烷-1-羧酸乙酯(3ae,淡黄色油状液体,69%):1H NMR (400 MHz,CDCl3) δ 7.24-7.14 (m,6H),6.71 (t,J=7.28 Hz,1H),6.57 (d,J=7.72 Hz,2H),4.17-4.11 (m,2H),3.94-3.89 (m,1H),3.31 (t,J=8.6 Hz,1H),2.69-2.64 (m,2H),2.42-2.38 (m,1H),2.29-2.13 (m,2H),1.81-1.76 (m,1H),1.29-1.20 (m,6H).13C NMR (100 MHz,CDCl3):δ 175.48,147.78,142.89,138.71,129.19,128.23,127.48,117.31,113.44,61.52,60.66,55.78,50.66,32.48,30.65,28.50,19.27,15.58,14.24.
2-(4-乙基苯基)-3-(苯基胺基)环戊烷-1-羧酸乙酯(4ae,淡黄色固体,23%):1H NMR (400 MHz,CDCl3) δ 7.15-7.01 (m,6H),6.66 (t,J=7.32 Hz,1H),6.48 (d,J=7.84 Hz,2H),4.17-4.08 (m,3H),3.78 (t,J=7.16 Hz,1H),3.40-3.34 (m,2H),2.66-2.60 (m,2H),2.35-2.16 (m,2H),2.06-1.97 (m,1H),1.91-1.82 (m,1H),1.25-1.21 (m,6H).13C NMR (100 MHz,CDCl3):δ 176.10,147.47,142.87,135.62,129.07,128.48,128.02,117.29,113.44,60.61,57.68,51.27,46.84,31.64,28.41,27.53,15.49,14.22.
2-(4-(叔丁基)苯基)-5-(苯胺基)环戊烷-1-羧酸乙酯(3af,淡黄色油状液体,67%):1H NMR (400 MHz,CDCl3) δ 7.35 (d,J=7.04 Hz,2H),7.24-7.16 (m,4H),6.70 (t,J=6.68 Hz,1H),6.57 (d,J=7.28 Hz,2H),4.14-4.13 (m,2H),3.92-3.90 (m,1H),3.32 (t,J=7.92 Hz,1H),3.05-2.99 (m,1H),2.41-2.15 (m,3H),1.50-1.49 (m,1H),1.34 (s,9H),1.21 (t,J=6.84 Hz,3H).13C NMR (100 MHz,CDCl3):δ 175.48,149.66,147.71,138.39,129.13,127.11,125.57,117.24,113.38,61.42,60.60,55.50,50.46,32.38,31.35,28.09,14.17.
2-(4-(叔丁基)苯基)-3-(苯胺基)环戊烷-1-羧酸乙酯(4af,淡黄色固体,31%):1H NMR (400 MHz,CDCl3) δ 7.40 (d,J=7.92 Hz,2H),7.20 (t,J=8.52 Hz,4H),6.75 (t,J=7.04 Hz,1H),6.56 (d,J=8 Hz,2H),4.24-4.18 (m,3H),3.87 (t,J=7.2 Hz,1H),3.50-3.44 (m,1H),2.44-2.25 (m,2H),2.14-2.10 (m,1H),2.00-1.94 (m,1H),1.40 (s,9H),1.32 (t,J=7.08 Hz,3H).13C NMR (100 MHz,CDCl3):δ 176.14,149.75,147.49,135.34,129.07,128.21,125.43,117.30,113.47,60.61,57.70,51.18,46.75,34.43,31.59,31.33,27.56,14.22.
2-(苯基胺基)-5-(4-(三氟甲基)苯基)环戊烷-1-羧酸乙酯(3ag,淡黄色油状液体,63%):1H NMR (400 MHz,CDCl3) δ 7.60 (d,J=8.08 Hz,2H),7.43 (d,J=8.04 Hz,2H),7.15 (t,J=7.68 Hz,2H),6.72 (t,J=7.24 Hz,1H),6.53 (d,J=8.08 Hz,2H),4.17-4.11 (m,2H),4.00-3.95 (m,1H),3.38 (t,J=8.76 Hz,1H),3.05-2.99 (m,1H),2.47-2.40 (m,1H),2.33-2.15 (m,2H),1.85-1.75 (m,1H),1.22 (t,J=7.12 Hz,3H).13C NMR (100 MHz,CDCl3):δ 174.81,147.29,145.84,129.22,127.91,125.66,125.62,117.66,113.41,61.60,60.83,55.91,50.55,32.52,27.99,14.15.
3-(苯基胺基)-2-(4-(三氟甲基)苯基)环戊烷-1-羧酸乙酯(4ag,淡黄色油狀液体,21%):1H NMR (400 MHz,CDCl3) δ 7.56 (d,J=8 Hz,2H),7.32 (t,J=7.52 Hz,2H),7.14 (t,J=7.76 Hz,2H),6.71 (t,J=7.4 Hz,1H),6.48 (d,J=8.16 Hz,2H),4.23-4.16 (m,3H),3.89 (t,J=7.32 Hz,1H),3.42-3.36 (m,1H),2.42-2.26 (m,2H),2.13-2.04 (m,1H),1.92-1.83 (m,1H),1.27 (t,J=7.12 Hz,3H).13C NMR (100 MHz,CDCl3):δ 175.50,146.97,143.12,129.14,128.99,125.25,125.22,117.64,113.31,60.85,57.82,51.27,47.19,31.96,27.30,14.18.
2-(4-氟苯基)-5-(苯胺基)环戊烷-1-羧酸乙酯(3ah,淡黄色油状液体,64%):1H NMR (400 MHz,CDCl3) δ 7.30-7.26 (m,2H),7.16 (t,J=7.52 Hz,2H),7.04 (t,J=8.64 Hz,2H),6.72 (t,J=7.28 Hz,1H),6.55 (d,J=8.2 Hz,2H),4.17-4.11 (m,2H),3.93-3.88 (m,1H),3.30 (t,J=8.04 Hz,1H),3.01-2.94 (m,1H),2.46-2.39 (m,1H),2.30-2.11 (m,2H),1.83-1.75 (m,1H),1.22 (t,J=7.12 Hz,3H).13C NMR (100 MHz,CDCl3):δ 175.06,163.05,147.52,137.12,129.17,129.01,117.46,115.39,113.36,61.55,60.68,55.49,50.73,32.38,27.82,14.16.
2-(4-氟苯基)-3-(苯胺基)环戊烷-1-羧酸乙酯(4ah,淡黄色油状液体,19%):1H NMR (400 MHz,CDCl3) δ 7.22-7.14 (m,4H),7.03 (t,J=8.68 Hz,2H),6.72 (t,J=7.4 Hz,1H),6.50 (d,J=7.8 Hz,2H),4.22-4.15 (m,3H),3.83 (t,J=7.56 Hz,1H),3.39-3.33 (m,1H),2.39-2.25 (m,2H),2.12-2.05 (m,1H),1.93-1.86 (m,1H),1.28 (t,J=7.12 Hz,3H).13C NMR (100 MHz,CDCl3):δ 175.75,163.01,147.22,134.39,130.12,129.11,117.49,115.37,115.16,113.34,60.73,57.69,50.93,47.38,27.40,14.20.
2-(4-氯苯基)-5-(苯胺基)环戊烷-1-羧酸乙酯(3ai,淡黄色油状液体,63%):1H NMR (400 MHz,CDCl3) δ 7.32-7.23 (m,4H),7.15 (t,J=7.52 Hz,2H),6.71 (t,J=7.28 Hz,1H),6.54 (d,J=7.76 Hz,2H),4.16-4.10 (m,2H),3.93-3.87 (m,1H),3.28 (t,J=8.76 Hz,1H),2.99-2.93 (m,1H),2.46-2.37 (m,1H),2.29-2.10 (m,2H),1.82-1.74 (m,1H),1.22 (t,J=7.12 Hz,3H).13C NMR (100 MHz,CDCl3):δ 174.96,147.44,140.05,130.93,129.19,128.88,128.82,117.52,113.38,60.73,55.55,50.62,32.44,27.89,19.19,14.17.
2-(4-氯苯基)-3-(苯胺基)环戊烷-1-羧酸乙酯(4ai,淡黄色油状液体,16%):1H NMR (400 MHz,CDCl3) δ 7.31 (d,J=8.04 Hz,2H),7.19-7.15 (m,4H),6.73 (t,J=7.32 Hz,1H),6.51 (d,J=7.84 Hz,2H),4.23-4.17 (m,3H),3.82 (t,J=7.24 Hz,1H),3.39-3.33 (m,1H),2.41-2.25 (m,2H),2.13-2.05 (m,1H),1.93-1.83 (m,1H),1.29 (t,J=7.12 Hz,3H).13C NMR (100 MHz,CDCl3):δ 175.67,147.15,137.30,132.73,129.97,129.14,128.54,117.54,113.35,60.78,57.70,50.93,47.23,31.88,27.35,14.22.
2-(4-溴苯基)-5-(苯胺基)环戊烷-1-羧酸乙酯(3aj,淡黄色油状液体,67%):1H NMR (400 MHz,CDCl3) δ 7.40 (d,J=7.64 Hz,2H),7.13-7.08 (m,4H),6.65 (t,J=7.2 Hz,1H),6.48 (d,J=7.84 Hz,2H),4.10-4.04 (m,2H),3.87-3.81 (m,1H),3.21 (t,J=8.76 Hz,1H),2.93-2.87 (m,1H),2.40-2.31 (m,1H),2.21-2.07 (m,2H),1.76-1.68 (m,1H),1.16 (t,J=6.88 Hz,3H).13C NMR (100 MHz,CDCl3):δ 174.95,147.42,140.60,131.76,129.27,129.19,120.73,117.53,113.38,61.47,60.75,55.59,50.57,32.45,27.91,14.17.
2-(4-溴苯基)-3-(苯胺基)环戊烷-1-羧酸乙酯(4aj,淡黄色固体,27%):1H NMR (400 MHz,CDCl3) δ 7.37 (t,J=7.36 Hz,2H),7.32-7.28 (m,3H),7.16 (t,J=7.44 Hz,1H),6.99 (d,J=7.24 Hz,1H),6.70-6.67 (m,2H),4.23-4.14 (m,3H),3.91 (t,J=7.12 Hz,1H),3.56-3.56 (m,1H),2.44-2.30 (m,2H),2.16-2.08 (m,1H),2.01-1.94 (m,1H),1.28 (t,J=7.12 Hz,3H).13C NMR (100 MHz,CDCl3):δ 175.89,145.43,138.46,129.95,128.64,128.49,122.64,116.96,110.37,60.66,57.67,52.08,46.76,32.09,27.98,14.24.
2-((4-乙基苯基)胺基)-5-苯基环戊烷-1-羧酸乙酯(3ba,淡黄色油状液体,68%):1H NMR (400 MHz,CDCl3) δ 7.38-7.28 (m,5H),7.02 (d,J=8.04 Hz,2H),6.53 (d,J=8.36 Hz,2H),4.17-4.12 (m,2H),3.95-3.90 (m,1H),3.34 (t,J=8.52 Hz,1H),3.07-3.00 (m,1H),2.60-2.54 (m,2H),2.48-2.39 (m,1H),2.29-2.15 (m,2H),1.86-1.76 (m,1H),1.25-1.21 (m,6H).13C NMR (100 MHz,CDCl3):δ 175.31,145.62,141.59,133.23,128.67,128.47,127.55,126.91,113.55,61.81,60.60,56.15,50.65,32.56,28.07,27.89,15.97,14.17.
3-((4-乙基苯基)胺基)-2-苯基环戊烷-1-羧酸乙酯(4ba,淡黄色固体,28%):1H NMR (400 MHz,CDCl3) δ 7.37-7.30 (m,5H),7.01 (d,J=8.24 Hz,2H),6.47 (d,J=8.32 Hz,2H),4.22-4.11 (m,3H),3.85 (t,J=7.24 Hz,1H),3.48-3.42 (m,1H),2.60-2.54 (m,2H),2.40-2.23 (m,2H),1.98-1.89 (m,1H),1.83-1.76 (m,1H),1.32-1.21 (m,6H).13C NMR (100 MHz,CDCl3):δ 176.04,145.35,138.56,130.94,128.58,128.51,128.40,126.95,113.56,60.62,58.05,51.83,46.75,31.81,27.90,19.21,15.99,14.22.
2-苯基-5-(邻甲苯胺基)环戊烷-1-羧酸乙酯(3ca,淡黄色油状液体,70%):1H NMR (400 MHz,CDCl3) δ 7.40-7.33 (m,5H),7.17-7.10 (m,2H),6.72 (t,J=7.04 Hz,1H),6.60 (d,J=7.72 Hz,2H),4.21-4.17 (m,2H),4.01-3.96 (m,1H),3.45 (t,J=7.84 Hz,1H),3.17-3.10 (m,1H),2.54-2.48 (m,1H),2.35-2.25 (m,2H),2.16 (s,3H),1.91-1.83 (m,1H),1.27 (t,J=7.04 Hz,3H).13C NMR (100 MHz,CDCl3):δ 175.64,147.12,137.84,131.46,130.92,130.33,129.13,128.84,120.83,117.53,113.34,60.78,57.64,50.94,47.16,31.85,27.32,17.50,14.21.
2-苯基-3-(鄰甲苯胺基)环戊烷-1-羧酸乙酯(4ca,淡黄色油状液体,25%):1H NMR (400 MHz,CDCl3) δ 7.35-7.29 (m,5H),7.13-7.06,(m,2H),6.68 (t,J=7.24 Hz,1H),7.07 (d,J=7.52 Hz,2H),4.16-4.14 (m,2H),3.95-3.93 (m,1H),3.40 (t,J=7.92 Hz,1H),3.12-3.06 (m,1H),2.48-2.46 (m,2H),2.12 (s,3H),1.83-1.75 (m,2H),1.52-1.50 (m,1H),1.23 (t,J=7.28 Hz,3H).13C NMR (100 MHz,CDCl3):δ 175.40,145.68,141.54,130.95,130.09,128.78,127.50,126.99,122.23,116.92,110.58,61.72,60.65,56.34,50.35,32.71,28.18,17.51,14.18.
2-苯基-5-(间甲苯胺基)环戊烷-1-羧酸乙酯(3da,淡黄色油状液体,56%):1H NMR (400 MHz,CDCl3) δ 7.36-7.29 (m,5H),7.06 (t,J=7.56 Hz,1H),6.54 (d,J=7.28 Hz,1H),6.39 (d,J=7.12 Hz,2H),4.17-4.12 (m,2H),3.97-3.91 (m,1H),3.33 (t,J=8.4 Hz,1H),3.07-3.01 (m,1H),2.46-2.40 (m,1H),2.27 (s,3H),2.24-2.11 (m,2H),1.85-1.77 (m,1H),1.22 (t,J=7.04 Hz,3H).13C NMR (100 MHz,CDCl3):δ 175.32,147.69,141.64,138.92,129.03,128.68,127.56,126.93,118.24,114.19,110.51,61.57,60.63,56.24,50.69,32.60,28.09,21.59,14.18.
2-苯基-3-(间甲苯胺基)环戊烷-1-羧酸乙酯(4da,淡黄色油状液体,41%):1H NMR (400 MHz,CDCl3) δ 7.37-7.27 (m,5H),7.07 (t,J=7.08 Hz,1H),6.56 (d,J=7.12 Hz,1H),6.35-6.33 (m,2H),4.22-4.18 (m,3H),3.87 (t,J=6.68 Hz,1H),3.46-3.42 (m,1H),2.39-2.35 (m,2H),2.30 (s,3H),2.12-2.07 (m,1H),1.95-1.93 (m,1H),1.30 (t,J=6.88 Hz,3H).13C NMR (100 MHz,CDCl3):δ 176.01,147.45,138.84,130.96,128.98,128.61,128.54,126.98,118.32,114.24,110.50,60.66,57.79,51.78,46.86,31.87,27.59,21.61,14.24.
2-((3-氯苯基)胺基)-5-苯基环戊烷-1-羧酸乙酯(3ea,淡黄色油状液体,55%):1H NMR (400 MHz,CDCl3) δ 7.35-7.30 (m,5H),7.03 (t,J=7.56 Hz,1H),6.69-6.63 (m,1H),6.50 (s,1H),6.39 (d,J=7.96 Hz,1H),4.14-4.12 (m,2H),3.92-3.89 (m,1H),3.30 (t,J=7.72 Hz,1H),3.04-3.02 (m,1H),2.41-2.09 (m,3H),1.78-1.77 (m,1H),1.20 (t,J=6.84 Hz,3H).13C NMR (100 MHz,CDCl3):δ 175.24,148.72,141.27,134.89,130.04,128.76,127.46,127.08,117.08,112.84,111.69,61.44,60.70,56.15,50.53,32.30,27.99,14.15.
3-((3-氯苯基)胺基)-2-苯基环戊烷-1-羧酸乙酯(4ea,淡黄色固体,27%):1H NMR (400 MHz,CDCl3) δ 7.34-7.30 (m,5H),7.02 (t,J=6.8 Hz,1H),6.65 (d,J=7.6 Hz,1H),6.46 (s,1H),6.34 (d,J=7.88 Hz,1H),4.19-4.12 (m,3H),3.84 (t,J=7.88 Hz,1H),3.45-3.38 (m,1H),2.36-2.27 (m,2H),2.09-2.04 (m,1H),1.88-1.87 (m,1H),1.27 (t,J=7.04 Hz,3H).13C NMR (100 MHz,CDCl3):δ 175.76,148.51,138.38,134.81,130.01,128.59,128.56,127.12,117.16,112.97,111.61,60.73,57.55,51.47,47.02,31.74,27.50,14.21.
2-((4-氯苯基)胺基)-5-苯基环戊烷-1-羧酸乙酯(3fa,淡黄色油状液体,75%):1H NMR (400 MHz,CDCl3) δ 7.35-7.30 (m,5H),7.08 (d,J=7.48 Hz,2H),6.46 (d,J=7.76 Hz,2H),4.14-4.13 (m,2H),3.97-3.89 (m,1H),3.31 (t,J=7.84 Hz,1H),3.04-3.02 (m,1H),2.40-2.11 (m,3H),1.51-1.49 (m,1H),1.21 (t,J=7.24 Hz,3H).13C NMR (100 MHz,CDCl3):δ 175.25,146.13,141.46,128.90,128.74,127.45,127.02,114.43,61.74,60.67,56.17,50.63,32.34,28.00,14.14.
3-((4-氯苯基)胺基)-2-苯基環戊烷-1-羧酸乙酯(4fa,淡黄色固体,23%):1H NMR (400 MHz,CDCl3) δ 7.31-7.05 (m,8H),6.39 (d,J=7.88 Hz,1H),4.17-4.10 (m,3H),3.82 (t,J=7.04 Hz,1H),3.38-3.36 (m,1H),2.34-2.25 (m,2H),2.07-2.02 (m,1H),1.83-1.73 (m,1H),1.25 (t,J=7.24 Hz,3H).13C NMR (100 MHz,CDCl3):δ 175.85,145.94,138.53,128.86,128.58,128.52,127.04,114.41,60.70,57.79,51.31,47.10,31.68,27.42,14.20.
2结果与讨论
2.1条件优化
在文献调研基础上,本文以环丙苯胺和肉桂酸乙酯为模板底物,通过对光催化剂和溶剂的筛选对反应条件进行优化,如图3和表1所示.
首先,对Ru(bpy)3Cl2·6H2O,Ru(bpy)3·2PF6,以及Eosin Y等光催化剂进行了考察,实验结果表明,4CzIPN的催化效果较好,不仅以较高的收率(3aa收率:57%)得到目标产物,同时,使得产物同分异构体的收率比例提高至2.2∶1.
在1~8條目对光催化剂的选择中,以4CzIPN的催化效果最好(3aa收率为57%),因此本文在该催化剂的参与下,又考察了常见有机溶剂对反应结果的影响.本文使用了甲醇、乙醇、TFE、DMC、甲苯等作为溶剂(条目9~17)发现,醇类溶剂会使反应产率进一步提高,特别是使用甲醇作溶剂时,反应以78%的产率得到了3aa,且其同分异构体4aa较少.使用甲醇作为溶剂,本文重新考察了过度金属催化剂[Ir(dtb-bpy)(ppy)2]PF6的催化能力,结果显示与4CzIPN相比,产率并没有较大提升.最终,本文确定了最优反应条件为:1a(0.2 mmol),2a(0.1 mmol),4CzIPN(2 mol%),以甲醇(2 mL)为溶剂在20 W蓝光照射下,常温反应12 h.
为确定其同分异构体3aa和4aa的结构,本文利用结晶度最好的4af对其结构进行确认,其结果如图4和表2所示.表2所示的精修参数的处理方法为:对4af进行重结晶操作,在室温下,将4af的晶体置于显微镜下,选取形状规则透亮,尺寸适用的单晶,放置于X-射线衍射仪上,石墨单色器单色纯化的Mo-Kα射线照射后,扫描该物质,收集单晶衍射数据,所收集的数据运用SAINT程序进行数据修整还原,然后利用SHELXL-2014程序解析初始结构,异性精修非氢原子,同性精修氢原子,骨架结构中的氢原子位置确定则全部利用理论加氢的方式进行.
2.2底物适用性考察
在最优条件下,本文对反应的底物适用性进行了考察,其结果如图5所示.实验结果表明,该反应对吸电子和给电子基团都表现出了较好的耐受性.当在肉桂酸酯的芳环对位上引入强给电子基团甲氧基(如:3ad)时,仍然以62%的收率得到了产物,其他弱给电子基团,例如甲基(3ab),乙基(3ae),叔丁基(3af)都取得了67%~81%的良好收率.当吸电子基团肉桂酸酯芳环对位上出现时,例如三氟甲基(3ag),氟原子(3ah),收率为63%~64%,略有降低但仍然平稳,当卤素氯原子(3ai)、溴原子(3aj)取代了苯环对位氢时,仍然以63%~75%的良好收率结束.当吸电子基团甲基取代在苯环对位(3ab)、间位(3ac,)时,对反应也没有太大影响,也取得63%~81%的收率,当CPA芳环对位上取代吸电子基团乙基(3ba)、卤素氯原子(3fa),其邻位取代吸电子基团甲基(3ca)时,其产生自由基的效用仍未受到影响,以68%、75%和70%的良好收率结束,但是当CPA的间位上取代吸电子基团(3da)、卤素氯原子(3ea)时,收率降低到55%~56%.总体来看,该反应能适应大部分底物,均能取得较好收率.
2.3控制实验与可能性反应机理
为探究其可能的反应机理,本文分别设置了两组空白对照实验,其结果如图6所示.在无催化剂或无光照,且其他条件不变的条件下进行反应,反应中几乎没有产物生成,表明此反应光照和光催化剂不可或缺.另外,在标准条件下,向反应中加入TEMPO试剂(0.3 mmol)时,反应中并无产物产生,说明该反应可能经由自由基过程.
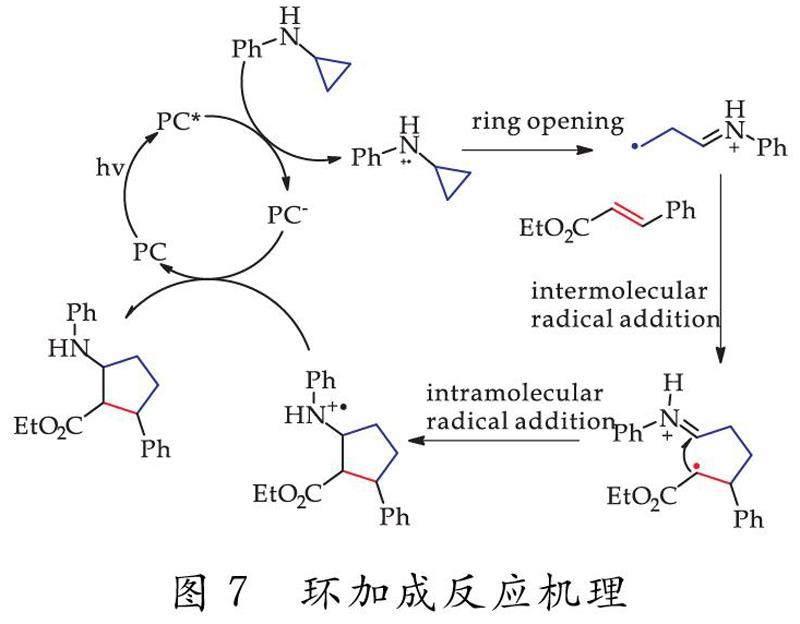
鉴于空白对照结果和对相关文献的研究,猜想其可能的反应机理如下:光催化剂(PC)在受到可见光照射时,跃升为激发态光催化剂(PC*),而激发态光催化剂(PC*)可以参与双分子电子转移或在分子中轻松捕获一个电子,进而被还原为(PC-),其环丙氨类原料则失去一个电子,诱导其三圆环断裂形成胺基阳离子自由基,其胺基阳离子自由基进攻肉桂酸类原料中sp2碳原子,诱导其双键断裂,再进一步关环,形成环戊烷类衍生物,如图7所示.
3结论
本文开发了一种利用光催化剂自由基环化合成2-苯基-3-(苯基氨基)环戊烷-1-羧酸乙酯衍生物的新方法,利用该方法均以较好的收率及位置选择性得到了18种2-苯基-3-(苯基氨基)环戊烷-1-羧酸乙酯衍生物及其同分异构体.该策略反应条件温和,使用有机非金属光催化剂代替了昂贵的过渡金属催化剂,大大提升经济效益,为环戊烷类化合物的合成提供了新的途径,同时也为光诱导催化的自由基加成环化反应提供了新的思路.
参考文献
[1] Dow N W,Cabre A,Mac Millan D W C.A general N-alkylation platform via copper metallaphotoredox and silyl radical activation of alkyl halides[J].Chem.,2021,7(7):1 827-1 842.
[2] Dong Z,Mac Millan D W C.Metallaphotoredox-enabled deoxygenative arylation of alcohols[J].Nature,2021,598:451-456.
[3] Li B X,Kim D K,Bloom S,et al.Site-selective tyrosine bioconjugation via photoredox catalysis for native-to-bioorthogonal protein transformation[J].Nature Chemistry,2021,13:902-908.
[4] Sarver P J,Bissonnette N B,Mac Millan D W C.Decatungstate-catalyzed C(sp(3))-H sulfinylation:Rapid access to diverse organosulfur functionality[J].Journal of the American Chemical Society,2021,143(26):9 737-9 743.
[5] Huang H M,Bellotti P,Chen P P,et al.Allylic C(sp3)-H arylation of olefins via ternary catalysis[J].Nature Synthesis,2022,1:59-68.
[6] Paulisch T O,Mai L A,Strieth Kalthoff F,et al.Dynamic kinetic sensitization of beta-dicarbonyl compounds-access to medium-sized rings by de mayo-type ring expansion[J].Angewandte Chemie (International Edition in English),2022,61(5):e202 112 695.
[7] Cembellin S,Maisuls I,Daniliuc C G,et al.One-step synthesis of indolizino[3,4,5-ab]isoindoles by manganese(I)-catalyzed C-H activation:Structural studies and photophysical properties[J].Organic & Biomolecular Chemistry,2022,20:796-800.
[8] Ye J H,Bellotti P,Heusel C,et al.Photoredox-catalyzed defluorinative functionalizations of polyfluorinated aliphatic amides and esters[J].Angewandte Chemie (International Edition in English),2022,61(9):e202 115 456.
[9] Bellotti P,Koy M,Hopkinson M N,et al.Recent advances in the chemistry and applications of N-heterocyclic carbenes[J].Nature Reviews Chemistry,2021,5:711-725.
[10] Reed N L,Lutovsky G A,Yoon T P.Copper-mediated radical-polar crossover enables photocatalytic oxidative functionalization of sterically bulky alkenes[J].Journal of the American Chemical Society,2021,143(16):6 065-6 070.
[11] Li Q Y,Gockel S N,Lutovsky G A,et al.Decarboxylative cross-nucleophile coupling via ligand-to-metal charge transfer photoexcitation of Cu(II) carboxylates[J].Nature Chemistry,2022,14:94-99.
[12] Zheng J,Dong X,Yoon T P.Divergent photocatalytic reactions of alpha-ketoesters under triplet sensitization and photoredox conditions[J].Organic Letters,2020,22(16):6 520-6 525.
[13] Dong X,Li Q Y,Yoon T P.Enantioselective synthesis of gamma-oxycarbonyl motifs by conjugate addition of photogenerated alpha-alkoxy radicals[J].Organic Letters,2021,23(15):5 703-5 708.
[14] Gravatt C S,Melecio Zambrano L,Yoon T P.Olefin-supported cationic copper catalysts for photochemical synthesis of structurally complex cyclobutanes[J].Angewandte Chemie (International Edition in English),2021,60(8):3 989-3 993.
[15] Amador A G,Sherbrook E M,Yoon T P.A redox auxiliary strategy for pyrrolidine synthesis via photocatalytic [3+2] cycloaddition[J].Asian Journal of Organic Chemistry,2019,8(7):978-985.
[16] Reed N L,Herman M I,Miltchev V P,et al.Tandem copper and photoredox catalysis in photocatalytic alkene difunctionalization reactions[J] Beilstein Journal of Organic Chemistry,2019,15:351-356.
[17] Daub M E,Jung H,Lee B J,et al.Enantioselective [2+2] cycloadditions of cinnamate esters:Generalizing lewis acid catalysis of triplet energy transfer[J].Journal of the American Chemical Society,2019,141(24):9 543-9 547.
[18] Doulcet J,Stephenson G R.Novel asymmetric formylation of aromatic compounds:enantioselective synthesis of formyl 7,8-dipropyltetrathia [7] helicenes[J].Chemistry,2015,21(38):13 431-13 436.
[19] Stephenson G,Bulman Page P,Harvey J,et al.Ruthenium-free preparation of 1,5-disubstituted triazoles by alkylative debenzylation of 1,4-disubstituted triazoles[J].Synlett,2016,27(17):2 500-2 504.
[20] Maity S,Zhu M,Shinabery R S,et al.Intermolecular [3+2] cycloaddition of cyclopropylamines with olefins by visible-light photocatalysis[J].Angewandte Chemie (International Edition in English),2012,51:226-230.
[21] Hu J,Wang J,Nguyen T H,et al.The chemistry of amine radical cations produced by visible light photoredox catalysis[J].Beilstein Journal of Organic Chemistry,2013,9:1 977-2 001.
[22] Nguyen T H,Maity S,Zheng N.Visible light mediated intermolecular [3+2] annulation of cyclopropylanilines with alkynes[J].Beilstein Journal of Organic Chemistry,2014,10:975-980.
[23] Cai Y,Wang J,Zhang Y,et al.Detection of fleeting amine radical cations and elucidation of chain processes in visible-light-mediated [3+2] annulation by online mass spectrometric techniques[J].Journal of the American Chemical Society,2017,139(35):12 259-12 266.
[24] Kuang Y,Ning Y,Zhu J,et al.Dirhodium(II)-catalyzed (3+2) cycloaddition of the N-arylaminocyclopropane with alkene derivatives[J].Organic Letters,2018,20(9):2 693-2 697.
【責任编辑:陈佳】

