Efficacy and safety of preoperative immunotherapy in patients with mismatch repair-deficient or microsatellite instability-high gastrointestinal malignancies
Ying-Jie Li,Xin-Zhi Liu,Yun-Feng Yao,Nan Chen,Zhong-Wu Li,Xiao-Yan Zhang,Xin-Feng Lin,Ai-Wen Wu
Ying-Jie Li,Xin-Zhi Liu,Nan Chen,Ai-Wen Wu,Department of Gastrointestinal Surgery,Peking University Cancer Hospital &Institute,Beijing 100142,China
Yun-Feng Yao,Gastro-intestinal Ward III,Beijing Cancer Hospital,Beijing 100142,China
Zhong-Wu Li,Department of Pathology,Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education),Peking University Cancer Hospital &Institute,Beijing 100142,China
Xiao-Yan Zhang,Department of Radiology,Peking University Cancer Hospital &Institute,Beijing 100142,China
Xin-Feng Lin,Department of Nuclear Medicine,Peking University Cancer Hospital &Institute,Beijing 100142,China
Abstract BACKGROUND Programmed death protein (PD)-1 blockade immunotherapy significantly prolongs survival in patients with metastatic mismatch repair-deficient (dMMR)/microsatellite instability-high (MSI-H) gastrointestinal malignancies such gastric and colorectal cancer.However,the data on preoperative immunotherapy are limited.AIM To evaluate the short-term efficacy and toxicity of preoperative PD-1 blockade immunotherapy.METHODS In this retrospective study,we enrolled 36 patients with dMMR/MSI-H gastrointestinal malignancies.All the patients received PD-1 blockade with or without chemotherapy of CapOx regime preoperatively.PD1 blockade 200 mg was given intravenously over 30 min on day 1 of each 21-d cycle.RESULTS Three patients with locally advanced gastric cancer achieved pathological complete response (pCR).Three patients with locally advanced duodenal carcinoma achieved clinical complete response (cCR),followed by watch and wait.Eight of 16 patients with locally advanced colon cancer achieved pCR.All four patients with liver metastasis from colon cancer reached CR,including three with pCR and one with cCR.pCR was achieved in two of five patients with nonliver metastatic colorectal cancer.CR was achieved in four of five patients with low rectal cancer,including three with cCR and one with pCR.cCR was achieved in seven of 36 cases,among which,six were selected for watch and wait strategy.No cCR was observed in gastric or colon cancer.CONCLUSION Preoperative PD-1 blockade immunotherapy in dMMR/MSI-H gastrointestinal malignancies can achieve a high CR,especially in patients with duodenal or low rectal cancer,and can achieve high organ function protection.
Key Words: Preoperative;PD-1 blockade;dMMR/MSI-H;Gastrointestinal malignancies
lNTRODUCTlON
Many solid tumors have a mismatch repair-deficient (dMMR)/microsatellite instability-high (MSI-H) ratio,and dMMR/MSI-H subtype,as a unique type of tumor,accounts for 5%-15% of solid tumor patients[1].However,the proportion of dMMR/MSI-H varies significantly among different tumor types,with a high incidence in colorectal,gastric and endometrial cancer,as well as breast cancer,liver cancer,cholangiocarcinoma,pancreatic cancer and other solid tumors[2].As a distinguished biomarker,dMMR/MSI-H status can accurately predict the efficacy of immunotherapy for many solid tumors[3,4].
MSI-H subtype is one of the molecular subtypes of gastric or colorectal cancer[5,6].MSI-H is an independent prognostic factor in gastrointestinal tumors,with good prognosis and resistance to conventional chemoradiotherapy[7,8].Locally advanced gastrointestinal malignancies or surgically resectable metastatic colorectal cancer require preoperative treatment to improve R0 resection rate.However,many studies[9,10] on locally advanced gastric cancer suggest that the pathological complete response (pCR) rate of conventional preoperative neoadjuvant chemotherapy is not high,and dMMR/MSI-H gastric cancer has a worse response to conventional chemotherapy.As with gastric cancer,pancreaticoduodenectomy (Whipple’s procedure) for duodenal cancer is a major,invasive procedure that needs to be performed even after preoperative chemotherapy.MSI-H colorectal cancer is resistant to conventional chemotherapy[11] and radiotherapy[12],and low advanced rectal cancer requires R0 resection and organ preservation.
In 2015[13] and 2017,Leet al[14] revealed that pembrolizumab [programmed death protein (PD) 1 blockade] achieved a high objective response rate for metastatic dMMR/MSI-H solid malignant tumors,and some patients achieved complete response (CR).In 2017,for the first time,the United States FDA approved pembrolizumab (for immunotherapy of all unresectable,metastatic dMMR/MSI-H solid tumors based on biomarkers instead of tumor types.A series of studies[15-18] on advanced or metastatic dMMR/MSI-h gastrointestinal malignancies such as gastric and colorectal cancer showed that PD1 blockade achieved better therapeutic effects.
This study aimed to evaluate the efficacy and safety of preoperative PD-1 blockade immunotherapy in patients with dMMR/MSI-H locally advanced or metastatic gastrointestinal malignancies.
MATERlALS AND METHODS
Patients
We retrospectively analyzed clinical data of all patients with dMMR/MSI-H gastrointestinal malignancies who received preoperative PD1 blockade immunotherapy with or without chemotherapy in Ward 3,Gastrointestinal Cancer Center,Peking University Cancer Hospital from January 1,2020 to September 30,2022.Preoperative PD-1 blockade immunotherapy is recommended for patients with dMMR/MSI-H detected by molecular detection before treatment.This cohort was a series of sequential cases.
The inclusion criteria were: (1) Age ≥ 18 and ≤ 90 years;(2) Eastern Collaborative Oncology Group performance status score 0-2;(3) gastrointestinal malignancies: gastric cancer,duodenal carcinoma,or colorectal cancer;(4) adenocarcinoma or mucous adenocarcinoma confirmed by endoscopic biopsy;(5) clinical stage II-IV according to imaging examinations [spiral computed tomography,magnetic resonance imaging,positron emission tomography-computed tomography (PET-CT) or ultrasound colonoscopy];and (6) MSI and MMR status confirmed by immumohistochemical staining,polymerase chain reaction (PCR) and next-generation sequencing (NGS).The exclusion criteria included: (1) Initially unresectable metastasis lesion;and (2) diseases of the immune system.This study was approved by the Ethics Committee of Peking University Cancer Hospital (2022YJZ39) and it conformed to the provisions of the Declaration of Helsinki.
Treatment and evaluation
All patients were discussed in a multidisciplinary team conference.All patients received PD1 blockade (PD1 blockade 200 mg intravenously over 30 min on day 1 of each 21-d cycle) preoperative immunotherapy with or without CapOx chemotherapy (oxaliplatin 130 mg/m2on day 1 and capecitabine 1000 mg/m2twice daily on d 1-14,repeated every 3 wk).
The primary tumor response was assessed according to the iRECIST criteria[19].Acute toxicity was graded according to the NCI Common Terminology Criteria for Adverse Events 4.0[20].After every one or two cycles of neoadjuvant immunotherapy,all patients had complete assessment including computed tomography (CT),magnetic resonance imaging,PET-CT,blood counts,renal biochemistry,hepatobiliary function,thyroid function,cardiac function,and tumor markers (carcinoembryonic antigen and carbohydrate antigen 19-9) to evaluate the general condition and treatment response.The determination of clinical complete response (cCR) was based on Memorial Sloan Kettering Cancer Center standard[21] and International Watch &Wait Database[22].The cCR was defined as no evidence of residual tumor determined by rectum MRI,abdomen/pelvis CT and chest CT,endoscopic physical examination,nomarl CEA and/or digital rectal exam.Pathological staging was based on the 8thedition of the American Joint Committee on Cancer TNM staging system[23].Post-treatment response was assessed by NCCN grading: 0=complete response (ypCR) with no detectable cancer cells;1=major response with few residual cancer cells;2=partial response;3=no or very little response[24].
Postoperative complications were classified according to the Clavien-Dindo classification[24].
Follow-up and statistical analysis
After initial treatment,all patients were re-examined every 3 mo for the first 2 years,6-mo intervals for the next 3 years,and yearly thereafter.Clinical data were obtained by laboratory examination records,imaging examination records,pathological examination records,and medical records review.Survival data were obtained from outpatient clinical visit or telephone interview.Analyses were conducted using SPSS for Windows version 19.0.Frequencies and percentages were calculated for categorical variables,and means and standard deviations for continuous variables.
RESULTS
Patient characteristics
From January 1,2020 to September 30,2022,37 patients with dMMR/MSI-H gastrointestinal malignancies finished preoperative PD1 blockade immunotherapy,and one patient with locally advanced colon cancer refused re-examination and treatment after symptom resolution.By September 30,2022,36 patients completed PD1 blockade immunotherapy and the treatment results were analyzed.
The clinical features are shown in Tables 1 and 2.Twenty-six men (26/36,72.2%) and 10 women (10/36,27.8%) were enrolled and the median age was 52 (26-87) years.Lynch syndrome was diagnosed in 16 patients.Three patients were diagnosed with initially unresectable locally advanced gastric cancer,three with locally advanced duodenal carcinoma,and 30 with colorectal cancer.Among the 30 cases of colorectal cancer,21 were locally advanced and nine were surgically resectable metastatic colorectal cancer.Twenty-seven patients received single PD-1 blockade as preoperative therapy,and nine with PD1 blockade and CapOx regimen.Of these patients,nine were initially treated with CapOx and two with radiotherapy,which was ineffective and switched to immunotherapy.
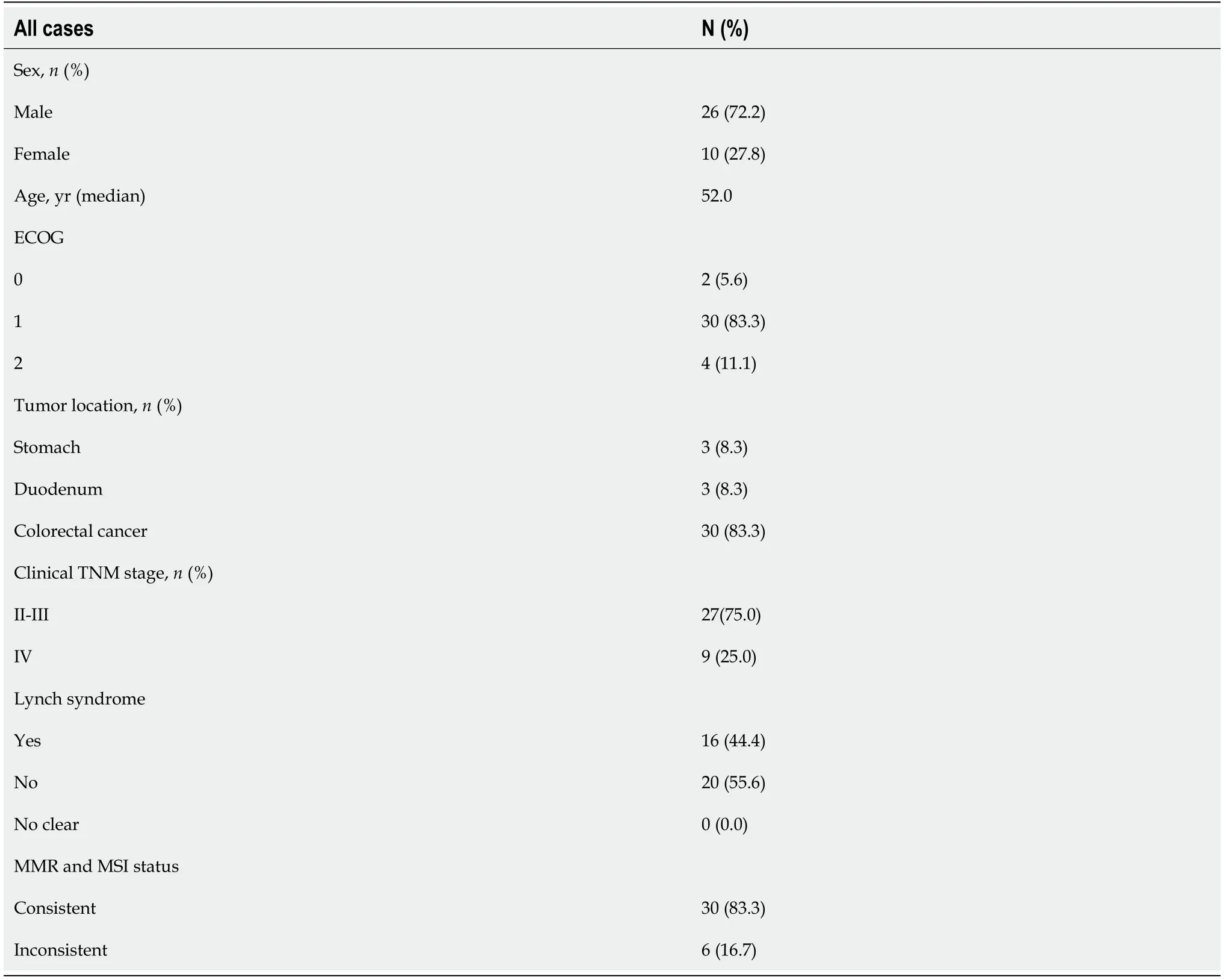
Table 1 Patient,tumor and treatment characteristics
Efficacy
With a median interval of 2 wk after neoadjuvant immunotherapy (range: 1-4 wk),all patients received imaging evaluation (Table 2).cCR was achieved in seven patients (patient 4,5,6,23,26,27,30),among which six (patient 4,5,6,23,26,30) were selected for wait and watch strategy.No cCR was observed in gastric or colon cancer.Thirty patients underwent surgery,including nine laparoscopic operations and 21 Laparotomies.Twenty-nine patients achieved R0 resection according to the postoperative pathological diagnosis (Table 2).
Three patients with locally advanced gastric cancer achieved pathological CR (pCR).Three patients with locally advanced duodenal carcinoma achieved cCR and then watch and wait (Video 1).Eight of 16 patients with locally advanced colon cancer achieved pCR.All four patients with liver metastasis from colon cancer achieved CR,including three pCR and one cCR.pCR was achieved in two of five patients with non-liver metastases from colorectal cancer.CR was achieved in four of five patients with low rectal cancer,including three with cCR and one with pCR.The CR (cCR and pCR) rate was 58.3% (21/36).
No adjuvant therapy was performed after pCR was achieved.Six patients received 2-10 cycles of adjuvant mono-immunotherapy after reaching cCR.The median follow-up was 9.4 (1-29) mo,and final follow-up time was September 30,2022.pCR and cCR patients had no recurrence during follow-up.No patient died during follow-up.
Safety
Preoperative immunotherapy was well tolerated and there was no interruption caused by immunotherapy-related toxicity.Two patients had grade 3 toxicity that presented as hypothyroidism and hematochezia,and eight patients had grade 1 or 2 toxicity including hypothyroidism,thyroid toxicity,rash,fatigue,reactive cutaneous capillary endothelial proliferation (Table 3).No surgery was delayed,but four patients experienced emergency surgery,of which two had perforated tumor,and two had colonic obstruction.There was no 30-d mortality.Four patients had grade 1 or 2 postoperative complications,including two grade 2 chylous fistula,two grade 2 anastomotic leak,and one grade 1 fever.One patient (case 24) underwent emergency surgery for intestinal obstruction,and received a second operation for postoperative abdominal bleeding (grade 3).All patients recovered well after conservative treatment and only one (anastomotic leak) stayed in hospital > 2 wk after surgery.
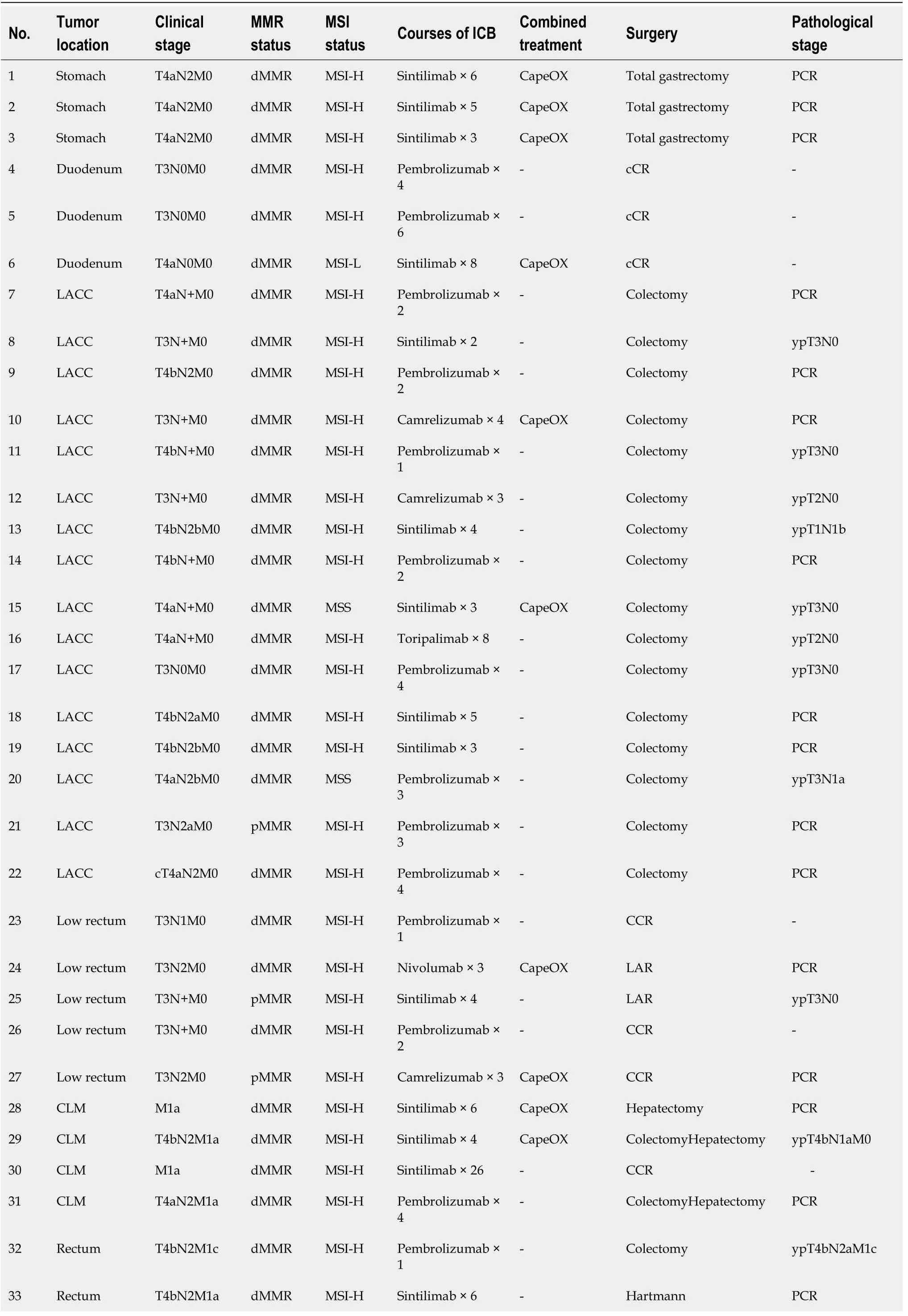
Table 2 Details of the 36 patients with neoadjuvant programmed death protein 1 blockade immunotherapy

cCR: Clinical complete response;LACC: Locally Advanced Colon Cancer;pMMR: Proficient mismatch repair;dMMR: Different mismatch repair;ICB: Immune checkpoint inhibitor;CLM: Colorectal Liver Metastases;LAR: Low Anterior Resection;PCR: Polymerase chain reaction.
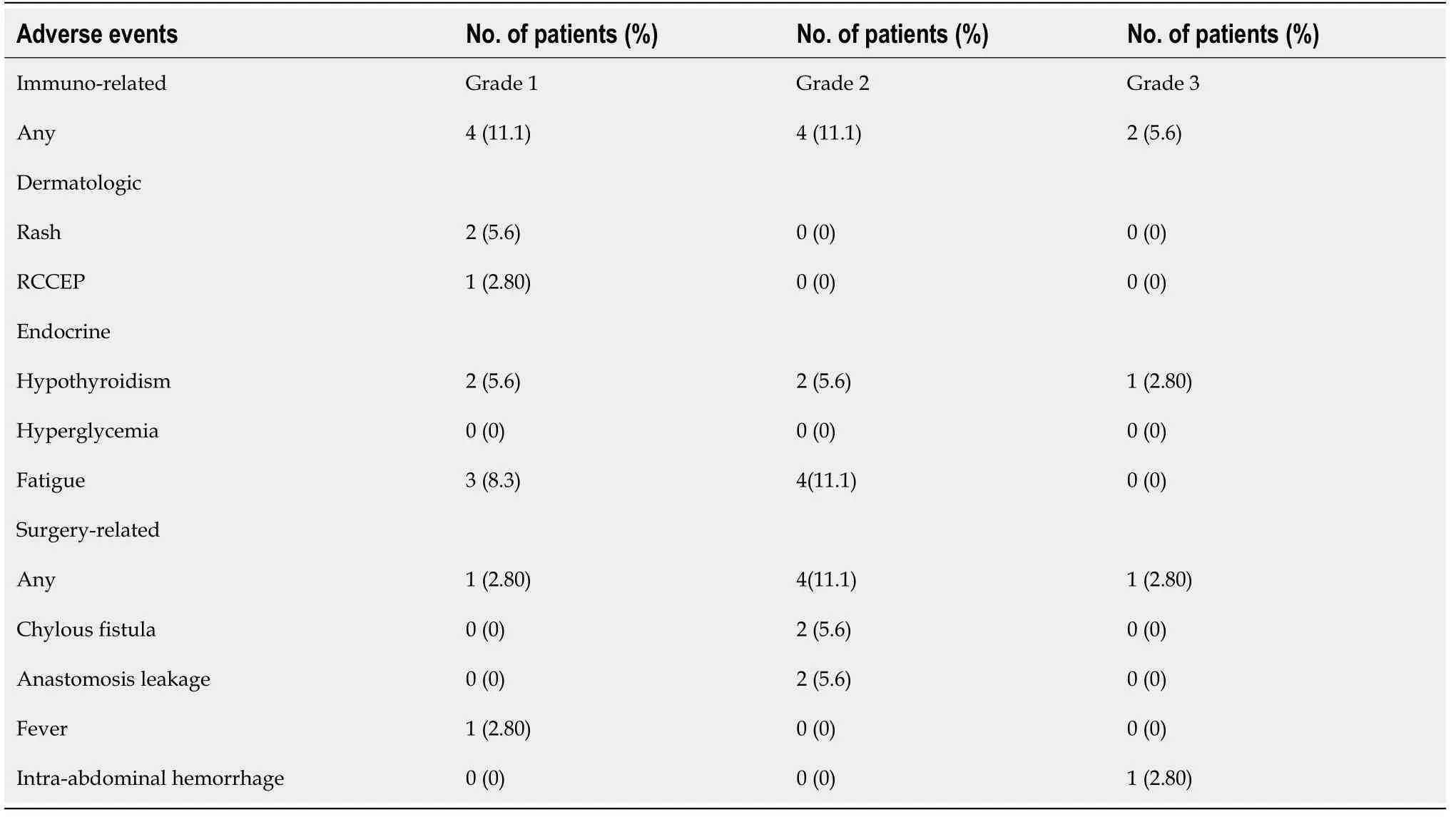
Table 3 Adverse events
DlSCUSSlON
This retrospective study is one of the few to summarize dMMR/MSI-H gastric,duodenal and colorectal cancers for preoperative immunotherapy.The cohort was a sequential case analysis and only one patient was excluded because her symptoms disappeared after PD1 therapy and she refused further examination and treatment.
The GERCOR NEONIPIGA study[25] reported neoadjuvant nivolumab plus ipilimumab in patients with localized dMMR/MSI-H esophagogastric adenocarcinoma showed a pCR rate of 58.6%.The NICHE study[26] and PICC study[27] of dMMR/MSI-H colon cancer showed a pCR rate of 60% and 65%,respectively.Cerceket al[28] reported 14 patients with dMMR/MSI-H rectal cancer using dostarlimab (PD1 blockade);all of whom achieved cCR and avoided surgical treatment.
MSI-H/dMMR tumors are currently the best predictive markers for cancer immunotherapy,but MSIH tumors are also heterogeneous,and show different incidence,differences in clinical and radiographic responses to immunotherapy in different solid tumors,primary immunotherapy resistance in some patients,and heterogeneity of different stages of the same tumor[1,29-31].In our cohort three patients with locally advanced gastric cancer achieved pCR.Three patients with locally advanced duodenal carcinoma evaluated as cCR received the watch and wait strategy.Eight of 16 patients with locally advanced colon cancer achieved pCR.All four patients with liver metastatic lesions from colon cancer reached CR.pCR was achieved in two of five patients with non-liver metastatic colorectal cancer.CR was achieved in four of five patients with low rectal cancer,including three with cCR and one with pCR.No cCR was observed in gastric or colon cancer.Duodenal and low rectal cancers have a high cCR rate,and do not require surgery and achieve organ preservation.Based on such high pCR and cCR rates,some patients can avoid surgery.Eleven of 36 patients in our study underwent routine preoperative chemotherapy and/or radiotherapy,which was unnecessary.Neoadjuvant therapy should be effective,moderate and accurate based on the treatment target.It is necessary to determine the molecular status of patients before treatment.
Different from the prospective clinical study,patients in our cohort underwent surgery and achieved R0 resection on imaging examination.Five patients achieved cCR or pCR within one or two cycles,and 15 patients who achieved pCR did not receive postoperative adjuvant therapy.None of the patients with pCR or cCR had recurrence or metastasis during follow-up.The Keynote 016[13],Keynote 177[2] and CHECKMATE 142[16] studies showed that if MSI-H tumors were highly sensitive to PD1 inhibitors or CTLA4 (cytotoxic T lymphocyte-associated antigen-4,CTLA-4) antibody,the response was rapid,and there was a significant tail effect of immunotherapy after long follow-up.Because of the inaccuracy of conventional imaging in the assessment of immunotherapy,the development of new tests that reflect the pathological response (e.g.,metabolic imaging) or molecular features (e.g.,liquid biopsy) is needed to better assess the response to treatment[32].For MSI-H tumors sensitive to PD1 immunotherapy,early and short-term PD1 treatment can achieve a curative effect.It is necessary to clarify the characteristics and immune microenvironment of MSI-H tumors.
Our study and others have shown that preoperative immunotherapy is safe for most patients[33,34].However,we have seen that there are some hidden dangers in the use of immunotherapy for such patients before surgery,such as stenosis,obstruction and perforation during the treatment of colon cancer.Previous studies[35,36] of neoadjuvant chemotherapy have suggested that obstruction and perforation reflect the poor effect of neoadjuvant therapy and are associated with tumor progression and poor prognosis.Due to immune cell infiltration and other reasons,many patients did not observe tumor remission on imaging-maintained stability or even some enlargement,but pathological examination will find a large number of necrosis tumors and inflammatory immune response[37].Our study also observed this phenomenon.Our cohort reported four patients with MSI-H colon cancer with obstruction and perforation during PD1 treatment;all of whom had some tumor pathological response after emergency surgery,including one who achieved pCR and three with pathological ratings of TRG2-3.We also observed five patients with regional lymph node enlargement during treatment.Although no trace of metastasis was found after lymph node dissection,it increased the difficulty of surgery and was prone to complications of lymphatic leakage.The incidence of thyroid dysfunction was 22% and three patients required long-term oral thyroxine replacement therapy.
Many studies[2,3,14-18] have reported that 10%-40% of patients with dMMR/MSI-H colorectal cancer developed primary drug resistance when receiving immunotherapy.Cohenet al[38] suggested that primary resistance to PD-1 blockades in dMMR/MSI-H metastatic colorectal cancer can largely be attributed to misdiagnosis of MMR/MSI status.In our cohort,16.7% (6/36) of patients had MMR and MSI discordance[39,40].We found that this discordance was test related and in some patients it was related to examination methods,tissue volume,tumor heterogeneity,and examination quality control.Six patients were rediagnosed with MMR and MSI status,and three were found to be consistent (Table 4).Our study and many others have found that intrinsic resistance exists even when MSI is consistent with MMR status[39,40].Wanget al[41] found that combination of AKT1 and CDH1 mutations predicted primary resistance to immunotherapy in dMMR/MSI-H gastrointestinal cancer.
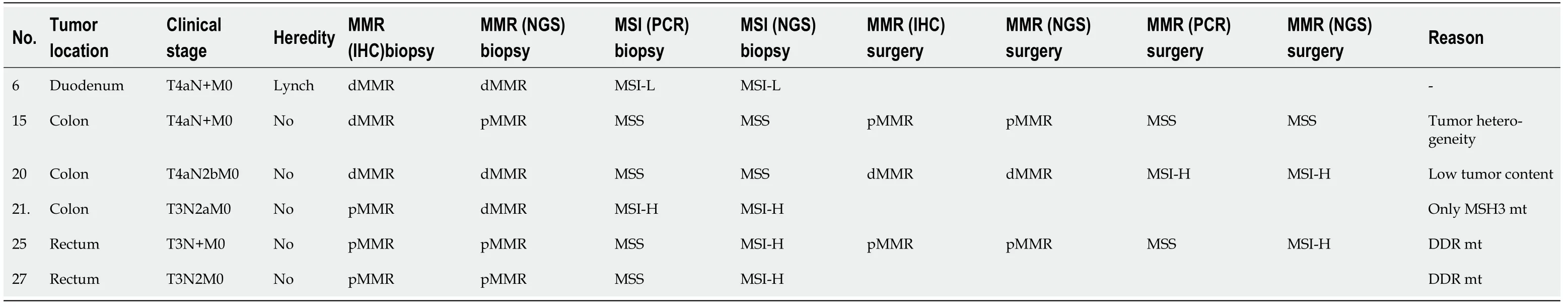
Table 4 Details of the 6 patients with inconsistency of dMMR and MSl-H
The limitations of this study included its small sample size,retrospective design,short follow-up time,and different neoadjuvant regimens and cycles.However,our study was a real-world clinical study in patients who required preoperative immunotherapy,and the group was a continuous case cohort,with the exception of one patient who declined to be enrolled because of resolution of symptoms.This study compared preoperative immunotherapy for dMMR/MSI-H in different gastrointestinal tumors,and showed that specific treatment strategies could be used for different tumor sites.It was encouraging to find that a high proportion of dMMR/MSI-H duodenal and low rectal cancers did not require surgery after preoperative immunotherapy,and this treatment strategy deserves further investigation.
CONCLUSlON
Our study demonstrated that preoperative PD-1 blockade immunotherapy with or without chemotherapy achieved significant results and acceptable adverse events in dMMR/MSI-H gastrointestinal malignancies.Some low cases of low rectal cancer or duodenal cancer can achieve cCR and avoid surgery and achieve organ preservation.Larger clinical trials are needed to conform our results.
ARTlCLE HlGHLlGHTS
Research background
Neoadjuvant programmed death protein (PD)-1 blockade immunotheapy has been sufficiently applied in a variety of cancers,but was rare in metastatic mismatch repair-deficient (dMMR)/microsatellite instability-high (MSI-H) gastrointestinal malignancies.Since the NICHE Study have showed their inspiring results that neoadjuvant immunotherapy was an efficient and safe method to improve colon cancer patients’ outcome,NCCN guideline then recommend immune checkpoint inhibitors to cT4b gastric or colorectal cancer.However,whether preoperative immunotherapy can expand to other stage gastrointestinal malignancies is still unknown.
Research motivation
We performed this study among 36 initially surgical resected difficult dMMR/MSI-H gastrointestinal malignancies such gastric cancer,duodenal cancer and colorectal cancer patients who received preoperative PD-1 blockade immunotherapy followed by surgery in order to investigate if the indication of preoperative immunotherapy can expand to initially surgical resected difficult dMMR/MSI-H gastrointestinal malignancies and evaluate the safety and efficacy.
Research objectives
To the best of our knowledge,this retrospective study is one of the few to summarize dMMR/MSI-H gastric,duodenal,and colorectal cancers for preoperative immunotherapy.The cohort was a sequential case analysis that only one patient was excluded from the cohort because symptoms disappeared after PD1 therapy and she refused to examination and further treatment.
Research methods
The limitations of this study included its small sample size,retrospective design,short follow-up time,and different neoadjuvant regimens and cycles.However,our study was a real-world clinical study in patients who required preoperative immunotherapy,and the group was a continuous case cohort,with the exception of one patient who declined to be enrolled because of resolution of symptoms.
Research results
Our study demonstrated that preoperative PD-1 blockade immunotherapy with or without chemotherapy could achieve significant effect and acceptable adverse events in dMMR/MSI-H gastrointestinal malignancies.
Research conclusions
Our study demonstrated that preoperative PD-1 blockade immunotherapy with or without chemotherapy could achieve significant effect and acceptable adverse events in dMMR/MSI-H gastrointestinal malignancies.Some low rectal cancer or duodenal cancer can achieve clinical complete response and avoid surgery to achieve organ preservation.Large sample clinical trials are needed.
Research perspectives
This study compared preoperative immunotherapy for dMMR/MSI-H in different gastrointestinal tumors,and showed that specific treatment strategies could be used for different tumor sites.It was encouraging to find that a high proportion of dMMR/MSI-H duodenal and low rectal cancers did not require surgery after preoperative immunotherapy,and this treatment strategy deserves further investigation.
FOOTNOTES
Author contributions:Wu AW contributed to conception and design of the study,draft and final approval of the manuscript;Li YJ contributed to collection of the data,draft the manuscript,study design and statistical analysis;Wu AW,Li YJ,Liu XZ and Yao YF,Li ZW,Zhang XY,Lin XF contributed to quality control of the study especially the surgery part,acquisition of data;All authors approved the final manuscript.
Supported bythe National Natural Science Foundation of China,No.82173156;Beijing Hospitals Authority Clinical Medicine Development of Special Funding,No.ZYLX202116.
lnstitutional review board statement:This study was approved by the ethics committee of Peking University cancer hospital (approval no.2022YJZ39).
lnformed consent statement:All study participants,or their legal guardian,provided informed written consent prior to study enrollment.
Conflict-of-interest statement:All the authors declare that they have no conflict of interest.
Data sharing statement:Technical appendix,statistical code,and dataset available from the corresponding author at drwuaw@sina.com.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license,which permits others to distribute,remix,adapt,build upon this work non-commercially,and license their derivative works on different terms,provided the original work is properly cited and the use is noncommercial.See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:China
ORClD number:Ying-Jie Li 0000-0001-6091-8911;Yun-Feng Yao 0000-0003-3660-3412;Nan Chen 0000-0002-0085-7472;Zhong-Wu Li 0000-0003-3440-9077;Ai-Wen Wu 0000-0003-1877-7005.
S-Editor:Liu JH
L-Editor:A
P-Editor:Liu JH
 World Journal of Gastrointestinal Surgery2023年2期
World Journal of Gastrointestinal Surgery2023年2期
- World Journal of Gastrointestinal Surgery的其它文章
- Does size matter for resection of giant versus non-giant hepatocellular carcinoma? A meta-analysis
- Prognostic value of preoperative immune-nutritional scoring systems in remnant gastric cancer patients undergoing surgery
- Hepatobiliary manifestations following two-stages elective laparoscopic restorative proctocolectomy for patients with ulcerative colitis: A prospective observational study
- Hypophosphatemia as a prognostic tool for post-hepatectomy liver failure: A systematic review
- Analysis of the impact of ERAS-based respiratory function training on older patients’ ability to prevent pulmonary complications after abdominal surgery
- Primary malignant melanoma of the esophagus combined with squamous cell carcinoma: A case report
