Enhanced antibacterial activity of cotton via silver nanocapsules deposited by atmospheric pressure plasma jet
Xiaoman ZHANG (张潇漫), Xiaoping MA (马晓萍),Maoyang LI(李茂洋),Peiyu JI(季佩宇),Tianyuan HUANG(黄天源),Lanjian ZHUGE (诸葛兰剑) and Xuemei WU (吴雪梅),*
1 School of Physical Science and Technology, Soochow University, Suzhou 215006,People’s Republic of China
2 Collaborative Innovation Center of Suzhou Nano Science and Technology, Soochow University,Suzhou 215006, People’s Republic of China
3 The Key Laboratory of Thin Films of Jiangsu, Soochow University, Suzhou 215006,People’s Republic of China
4 Analysis and Testing Center, Soochow University, Suzhou 215123, People’s Republic of China
Abstract In this work, the antibacterial activity of cotton containing silver nanocapsules prepared by atmospheric pressure plasma (APP) deposition is investigated.The nanocapsules consist of a shell and a silver nanoparticle(AgNP)core,where the core is used to bring antibacterial activity,and the shell is utilized to suppress the potential toxicity of AgNPs.The surface morphology and the elements of the samples are analyzed by scanning electron microscopy (SEM), energy dispersive x-ray and x-ray photoelectron spectroscopy (XPS).The SEM results show that the skin of the cotton fibers will fall off gradually after APP treatment over 3 min, and the XPS results show that the Ag content will rise to 1.6%after APP deposition for 10 min.Furthermore,the antimicrobial activity tests show that the reduction rates of Escherichia coli and Staphylococcus aureus can achieve 100%when the sample is treated for 10 min,which exhibits excellent antibacterial activity.In addition, the UV absorption properties of the cotton will also be correspondingly improved, which brings a broader application prospect for antibacterial cotton.
Keywords: atmospheric pressure plasma (APP), nanocapsules, antimicrobial activity, cotton
1.Introduction
Silver nanoparticles (AgNPs) have become the most frequently used nano-engineering materials because of their ultrahigh surface-to-volume ratio and unique properties.In particular, in the biomedicine field, research on AgNPs has developed rapidly due to their broad-spectrum antibacterial properties against a variety of pathogenic microorganisms[1-3], and the primary reason is that AgNPs can initiatively release a large number of Ag ions when immersed in water[4], which can not only significantly reduce the activity of bacteria but also directly cause the distortion and death of bacterial cells [5].At present, AgNPs have proved to be effective biological fungicides that can kill bacteria (such as Escherichia coli,Staphylococcus aureus,etc)[6],fungi,(such as Candida albicans,etc)[7]and viruses(such as hepatitis B,HIV-1, etc) [8, 9].Therefore, embedding AgNPs on the surface of different materials will be a novel and effective method that can significantly reduce the risk of infection by harmful bacteria.
The methods of preparing AgNP-containing material are diverse, including chemical, physical and biological methods, and the advantages and disadvantages are extremely distinct [10-13].However, in this work, an atmospheric pressure plasma (APP)deposition method has been adopted,which is a low-cost, pollution-free and efficient solution.During the past few decades,research on APP has undergone diversified development, which is widely utilized in material surface modification[14-17],microbial inactivation and medical health [18-21], nano-coating deposition[22-24], sewage treatment [25], etc.In particular, APP deposition of nanomaterials can be applied to various substrates,e.g.silicon-based[26]and diamond-like carbon[27].Different types of nanomaterials can specifically improve the surface properties, such as wettability, corrosion resistance,hardness, friction, wear resistance and antibacterial properties.
For the APP deposition of AgNPs antibacterial material,Nikiforov et al [1] proposed a simple method for preparing AgNP-incorporated non-woven fabrics with high antibacterial efficiency.In this method, a stable high-speed N2direct current plasma jet was selected as the plasma deposition source, and three different types of nanoparticles (Ag,copper (Cu) and zinc (Zn) oxide nanoparticles) were employed as antimicrobial agents.The results showed that compared to ZnO nanoparticles, Ag and Cu nanoparticles were more resistant to Staphylococcus aureus (S.aureus).Beier et al [28] studied an APP chemical vapor deposition technique to create antibacterial active thin films.In the deposition step, the Ag nitrate solution was sprayed and converted into plasma to form AgNPs, and the results showed that the coating had a strong antibacterial effect on Escherichia coli(E.coli).In addition,Deng et al[4]adopted a novel three-step process to prepare AgNP-containing antimicrobial non-woven polyethylene terephthalate (PET)fabrics, and the antibacterial tests against E.coli and S.aureus showed an up to 99.7% reduction at AgNPs content of 2.1% and complete bacterial reduction at a higher Ag concentration of 7%.
Cotton plays an indispensable role in our daily life.In China, the Xinjiang Uyghur Autonomous Region is the largest cotton origin.Benefitting from the local unique geographical conditions,the cotton plant is abundant in yield and excellent in quality.Furthermore, all detection indicators of Xinjiang cotton exceed national standards,and it sells well at home and abroad.However,cotton or cotton products quickly breed bacteria when they are wet, so adding antibacterial activity to cotton is a highly effective and necessary solution.
In this paper, a novel method is proposed to produce nanocapsules with strong antibacterial activity on the surface of cotton cloth by APP.The article is structured as follows.In section 2,the experimental methods and the main principle as well as the APP discharge parameters are introduced.The specific method of the antibacterial test can be seen in section 2.5.In addition, the results of material testing are analyzed in section 3.Finally, the summary of the full test is shown in section 4, which discusses the significance and value of this study.
2.Experimental details
2.1.APP deposition system
The APP deposition system and the main principle diagram are shown in figure 1 below.
The system consists of two parts, namely, a plasma jet and an ultrasonic atomizer.The plasma jet is composed of a quartz tube with an outer diameter of 6 mm(thickness =1 mm), and two Cu rings (width=10 mm) are tightly attached to the outside of the quartz tube.The Cu ring above is used to connect the high-voltage supply, and the Cu ring below is utilized to connect the ground electrode.In the middle of them,an insulating medium with a width of 20 mm is placed to prevent arcing.Moreover, the distance between the nozzle of the plasma jet and the sample is 2 mm,as shown in figure 1(a), and high-purity Ar (99.999%) with a flow rate of 5 l min-1is used as the working gas.
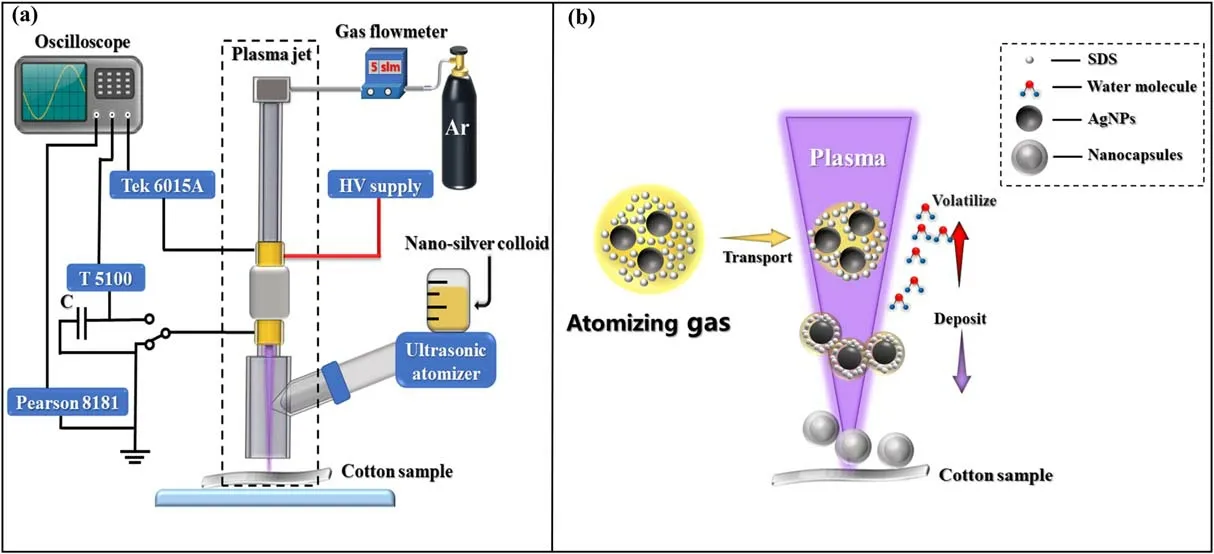
Figure 1.APP deposition system: (a) schematic diagram of the APP device and (b) main principle diagram.
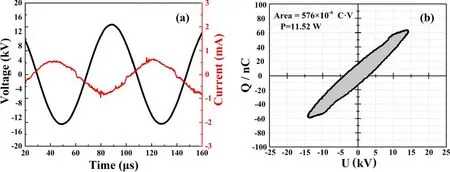
Figure 2.Electrical properties of the system: (a) current-voltage waveform and (b) Lissajous figures.
The plasma jet adopts a dielectric barrier discharge(DBD)structure,that is,the quartz tube exists in the middle of the Cu electrode and the plasma.This structure can rule out the possibility of electrode contamination or sputter.Meanwhile, the DBD structure can also limit the discharge current for the system and ensure that the plasma will not generate excessive temperature.In addition, the volatilization of water molecules can continuously reduce the plasma temperature,so that the sample will not be damaged by high temperature even in the long-term discharge process.
The main principle of the APP deposition nanocapsules is that the colloid, composed of sodium dodecyl sulfate(SDS), deionized water and nano-silver powder, is atomized and transported into APP, as shown in figure 1(b).After the aerosol droplets enter the plasma, due to the interaction with the APP,the surface will be negatively charged,which could prevent droplets from aggregating to a certain extent.Meanwhile, the water molecules are volatilized, which leads to continuous shrinkage of the droplets.Finally, the nanocapsules,consisting of SDS and AgNPs,are deposited on the cotton fibers.The SDS shell can prevent the AgNPs from being oxidized during the deposition process.
In this work, a power supply (CTP-2000K) with a maximum power of 500 W, frequency of 20 kHz, and adjustable voltage of 0-30 kV was adopted.In addition, an ultrasonic atomizer (Yuehua, WH-2000) was utilized to produce the atomized aerosol droplets 1-5 μm in size,and the atomization rate was about 2 ml min-1.
2.2.Electrical tests
The discharge voltage and current waveforms were recorded by an oscilloscope (R& RTO 1014.1 GHz.10 GSa/S),two high-voltage probes (Tektronix P6015A, P5100A) and a Pearson current monitor(Pearson 8181).In addition,a 140 pF non-polar capacitor and a 100:1 voltage probe (Tektronix P5100A) were used to calculate the transport charges.
The current-voltage waveform is shown in figure 2.From figure 2(a), it can be seen that the amplitude of the discharge voltage is approximately 14 kV, the frequency is 20 kHz and the current generated is 0.85 mA.The Lissajous figure is drawn according to discharge voltage and transport charges, as shown in figure 2(b), and the product of the area and discharge frequency is the active power [29], which is calculated to be approximately 11.52 W.
2.3.Experimental method
Before the APP deposition, nano-silver colloid needs to be prepared, and the specific methods are as follows:
(1) Nano-silver powder (80 nm, China Metallurgical Research Institute) and SDS (Sinopharm Chemical Reagent Co., Ltd) were added to the deionized water at a ratio of 1:20, and the mass fraction of nano-silver powder was 0.015%.
(2) The prepared solution was dispersed with an ultrasonic disperser for 4 h and then stirred with a magnetic stirrer for 4 h to obtain the dispersed solution.
(3) The dispersed solution was centrifuged (6000 r min-1)for 15 min, and a uniform nano-silver colloid was obtained.
In addition, the remaining nano-silver powder at the bottom of the centrifuge tube was collected and weighed to calculate the content of nano-silver powder in the colloid by difference, which was about 90 ppm.
High-quality Xinjiang cotton cloth (100% cotton, and thickness=0.15 mm)was selected as the sample,which was cut into rounds 10 cm2in size,and the sample was immersed in the nano-silver colloid for 24 h, and finally put into the device for deposition of nanocapsules (atomization rate =2 sccm, and Ar flow rate=5 l min-1).The deposition time can be divided into eight groups: 0, 0.5, 1, 2, 3, 4, 5 and 10 min.After deposition, the sample was dried by the Ar plasma jet for 1-2 min.The drying time depends on the degree of dampness of the sample.
2.4.Characterization and measurements
The size of AgNPs in the prepared nano-silver colloid was accurately tested using a transmission electron microscope(TEM, Tecani-G20, acceleration voltage of 200 kV).The surface morphology of the samples before and after treatment was observed by scanning electron microscopy (SEM, Regulus Su8100 microscope),and the elements of the nanoparticles were identified by energy dispersive x-ray spectroscopy (EDX, Regulus Su8100 microscope).The surface elements were measured by x-ray photoelectron spectroscopy(XPS,Thermo ESCALAB 250 XI), which employs an Al Kα monochromatic excitation x-ray source with an energy of 1486.6 eV at 150 W.The pass energy was set at 20 eV,and the spectra were calibrated by a C 1s peak at 284.8 eV.
The UV resistance of the cotton samples before and after treatment was tested by measuring the absorption values in the wavelength range of 200-800 nm using UV absorption spectroscopy (UV3600, Shimadzu, Resolution: 0.1 nm), and the cotton samples were inserted into the sample stand and placed in front of the integrating sphere for measurement.In addition,the nano-silver colloid was tested by this instrument.The colloid was poured into the quartz cuvette and put into the instrument for the direct test.
2.5.Antibacterial assessment
The antibacterial activity of the samples was tested against E.coli and S.aureus.The two strains were prepared into bacterial suspensions with a concentration of 1 × 108CFU ml-1by phosphate buffers(PBS,0.03 mol l-1,pH=7.2)for antibacterial testing.Moreover, the antibacterial activity of the samples was assessed using the improved AATCC 100 Method, and the specific operation steps are as follows:
(1) The sample (size = 10 cm2) colonized with 1 ml of bacterial suspension was placed in a conical flask at room temperature for 24 h.
(2) 100 ml of PBS was added into the conical flask and left for 5 min, then put into the shaking table for full oscillation to ensure that the bacteria were completely washed out of the samples.
(3) 0.1 ml of the 1:10 dilution was colonized on Luria-Bertani(LB)solid medium and cultured at 37°C for 24 h so that the plate counting technique(PCT)could be used to further evaluate the antibacterial activity of the sample.
3.Results and discussions
3.1.Nano-silver colloid
The results of the UV absorption spectra of the nano-silver colloid are shown in figure 3(a).The transparent bright yellow nano-silver colloid has a peak around 415 nm, which is a typical surface plasma resonance (SPR) peak for AgNPs in the region of 400-450 nm, and the result indicates that the nano-powder has successfully dispersed and the colloid has finally formed [30, 31].Moreover, from the TEM image is illustrated in figure 3(b), it can be observed that the particle diameter is about 80 nm.
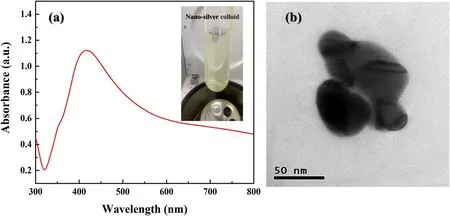
Figure 3.Nano-silver colloid: (a) UV absorption spectra and a photo of the nano-silver colloid and (b) TEM image of a single Ag particle.
3.2.Surface morphology
A single fiber is selected from each cotton cloth sample for SEM tests.The results are shown in figure 4.It can be seen from the SEM images at 5k times magnification that there are many wrinkles on the surface of the untreated cotton fibers.After treatment, the surface of the cotton fibers is destroyed by APP, especially with the treatment time over 3 min.Furthermore, from figure 4(g), after 5 min of treatment, the skin gradually falls off,and the inside of the cotton fiber,which is relatively smooth,is further exposed.In addition,after 10 min of deposition,a large number of nanoparticles can be seen on the surface of the sample, as shown in figure 4(h).The process of the skin gradually falling off will inevitably affect the deposition rate,and the same result will be reflected in the subsequent tests,so the treatment time needed to be extended to more than 5 min to solve this problem.

Figure 4.SEM images of cotton fibers: (a) untreated, (b)-(h) AgNP deposition time at 30 s, 1, 2, 3, 4, 5 and 10 min, respectively.

Figure 5.Morphology and composition of the Ag nanocapsules:(a)SEM image at 5k times magnification,(b),(c)SEM image at 120k times magnification and (d) EDX image of figure 5(c).
However, the cotton fiber cannot withstand a high accelerating voltage (10 kV), so it is impossible to obtain a high-magnification image.Therefore, it is necessary to replace the cotton fiber with high-voltage resistant carbon fiber to complete further tests.The APP treatment time is selected as 10 min,and the results are represented in figure 5.Figure 5(a)shows the carbon fiber at 5k times magnification,and a large number of particles can be discovered, indicating that the method can be used on the carbon fiber cloth.Although the particles are not as dense as those in figure 4(h),the carbon fiber can be a good substitute for cotton fiber to take high-magnification SEM images.Figures 5(b)and(c)are the photos of the nanocapsules at 120k magnification, from which the core and shell of the nanocapsule can be distinguished and its size is about dozens of nanometers.In addition, the component of the nanocapsule core is tested by EDX,which is shown in figure 5(d),where a large bulk of Ag signals are generated in the center of the nanocapsule,and the results fully prove that the core is composed of AgNPs.
3.3.Chemical composition

Table 1.The surface atomic percentage of the untreated sample and 10 min deposition sample.
Cotton cloth samples are selected for the XPS test, which is utilized to analyze the component changes before and after treatment.The relative content of Ag and XPS spectra of Ag 3d and C 1s can be seen in figure 6, and the surface atomic percentage of the untreated sample and 10 min deposition sample are listed in table 1.From figure 6(a), it can be illustrated that the relative content of Ag increased gradually with the APP treatment time, but decreased slightly at the 3 min deposition.The reason is that the skin of the cotton fibers will gradually fall off, which affects the deposition rate.However, ultimately, the Ag content will rise significantly, which can reach 1.6% after 10 min of treatment.Furthermore, figures 6(b) and (c) show the C 1s and Ag 3d spectra of the untreated sample and 10 min deposition sample, respectively.
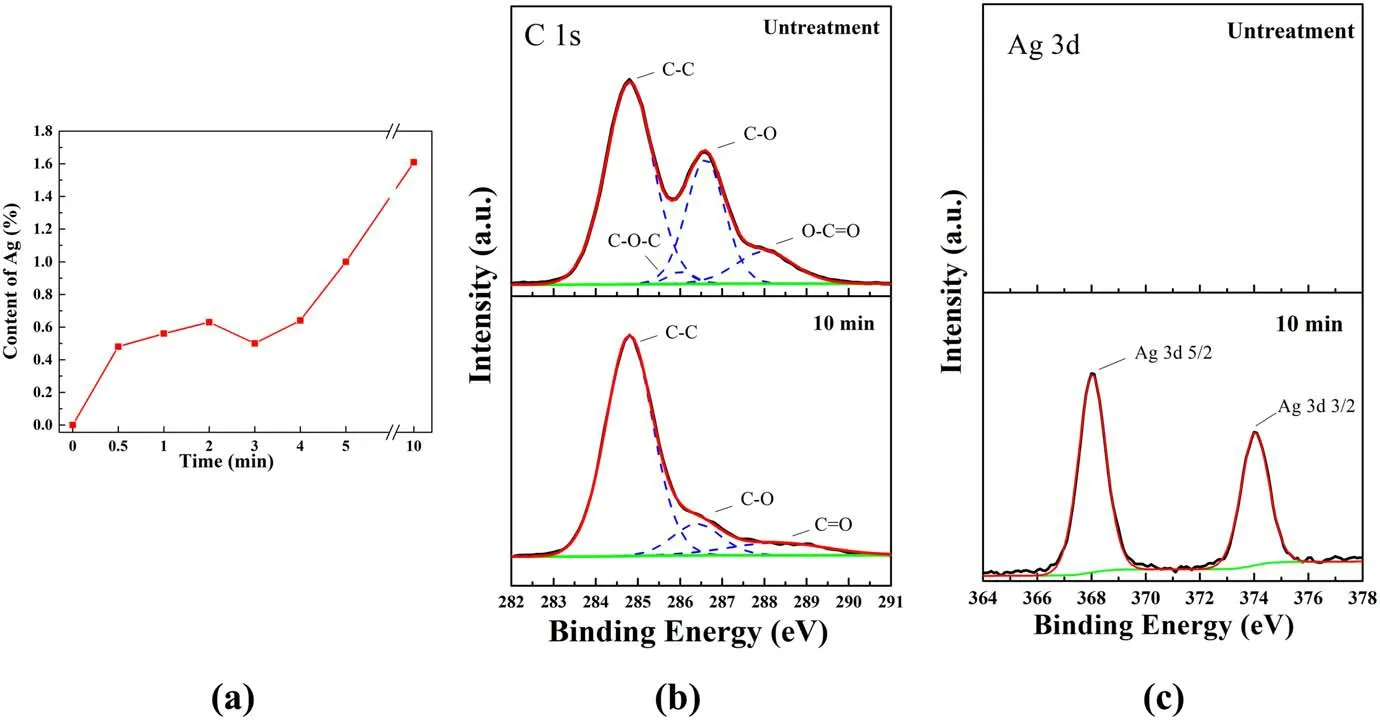
Figure 6.Change in the relative content of Ag and XPS spectra: (a) change in the relative content of Ag, (b) C 1s spectra of the untreated sample and 10 min deposition sample and (c) Ag 3d spectra of the untreated sample and 10 min deposition sample.
In figure 6(b), the C 1s spectra of the untreated sample can be decomposed into three components [32, 33]: (1) a component at a binding energy of 284.8 eV corresponding to C-C with a content of 48.1%; (2) a component at a binding energy of 286.3 eV corresponding to C-O with a content of 18.6%; (3) a component at a binding energy of 288.3 eV corresponding to C=O with a content of 7.3%.The change in the C 1s spectra after treatment is shown below.The content of C-C drops to 28%, the content of C-O increases to 41.98%, and the C=O peak at 288.3 eV changes to O-C=O at 288.7 eV, with a content of 6.03%.In figure 6(c), the untreated sample has no signal in the Ag 3d spectra.However, after 10 min of deposition, two components of Ag 3d5/2 at 368.2 eV and Ag 3d3/2 at 374.2 eV can be found in the spectra, from which the spin-orbit separation is 6.0 eV,and the energies are in good agreement with the reported values for the binding energies of AgNPs [5, 34], which also evidences the presence of AgNPs in the cotton fibers.Moreover,the untreated sample is mainly composed of C and O, the content of which is approximately 78.4% and 19.6%,respectively.After 10 min of deposition, the oxygen content increases from 19.6% to 42.2%.In addition, due to the addition of SDS, the content of Na and S in the 10 min deposition sample is 6.4% and 8%, respectively.
3.4.Antibacterial analysis
The capability of the cotton cloth samples to prevent viable bacteria colonization is assessed using the improved AATCC 100 Method.Figure 7 shows the consequence of E.coli and S.aureus culture in the control group, untreated sample,5 min deposition sample and 10 min deposition sample,respectively.The specific methods are shown in part 2.5.It can be seen from figure 7 that untreated samples do not have any antibacterial activity,but the 5 min deposition sample and 10 min deposition sample both exhibit strong antibacterial activity against E.coli and S.aureus,which indicates that the growth of microorganisms is severely affected by the Ag nanocapsules.More importantly, the antibacterial activity of the 5 min deposition sample and 10 min deposition sample against both bacteria can reach 99% and 100%, respectively.Although the Ag content of the 10 min deposition sample is less than 2%, the antibacterial activity was strong enough, so it was not necessary to further increase the deposition time to increase the Ag content,and 10 min was the most appropriate deposition time.

Figure 7.Photographs of E.coli and S.aureus colonies on LB solid medium incubated with different samples.
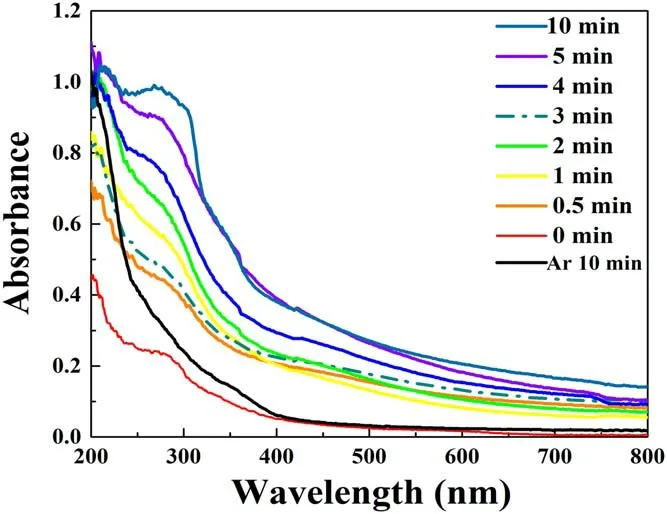
Figure 8.UV absorption spectroscopy of samples at different deposition times.
3.5.UV absorption properties
The UV (wavelength less than 400 nm) resistance of the fabric is influenced by many factors, such as fiber type,structure, color, etc.Significantly, the UV resistance of untreated cotton cloth is very poor because it does not contain any functional groups that can absorb UV, so it is extremely important to improve the UV resistance.The test results of the UV absorption spectrum are shown in figure 8.The UV absorption capability of the cotton increases with APP treatment time.Meanwhile,the 3 min deposition sample shows an obvious decline, which is also related to the damage to the skin of the cotton fibers.However, the SPR peak for AgNPs[35,36]between 400-450 nm cannot be observed in figure 8,because the Ag content on the surface of the samples is too low.In addition,the black curve in figure 8 is the result of the pure Ar plasma jet treatment for 10 min without adding nanosilver colloid.Compared to the 10 min deposition sample,there is no absorption peak near 300 nm.Therefore, the addition of SDS is the main reason for the improvement in UV absorption properties.
3.6.Assessment
Recent research has proved that[37]in the process of aerosolassisted plasma deposition if the solute has a surfactant nature, it tends to accumulate at the interface and form nanocapsules.In this study, the added SDS not only becomes a dispersant for nano-silver colloids to prevent the agglomeration of AgNPs but also has surfactant properties.This can also promote the formation of the nanocapsule shell during the deposition process, and the shell can also protect the AgNPs from oxidation of O3produced by APP.Moreover, after plasma treatment of cotton fibers, the skin will gradually fall off, and the interior of cotton fibers is also activated by APP,which is more conducive to the deposition of nanocapsules.To further prolong their service lifetime, the antibacterial cotton can be put in the middle to form a multi-layer cotton cloth, as shown in figure 9.When encountering water, the SDS shell will dissolve rapidly, and AgNPs particles can release a large number of Ag ions to realize an antibacterial function.However, the application of the antibacterial cotton produced by this method has certain limitations.For instance,the nanocapsules cannot release Ag ions without water, and once all the Ag ions are released,the cotton cloth will lose its antibacterial activity.
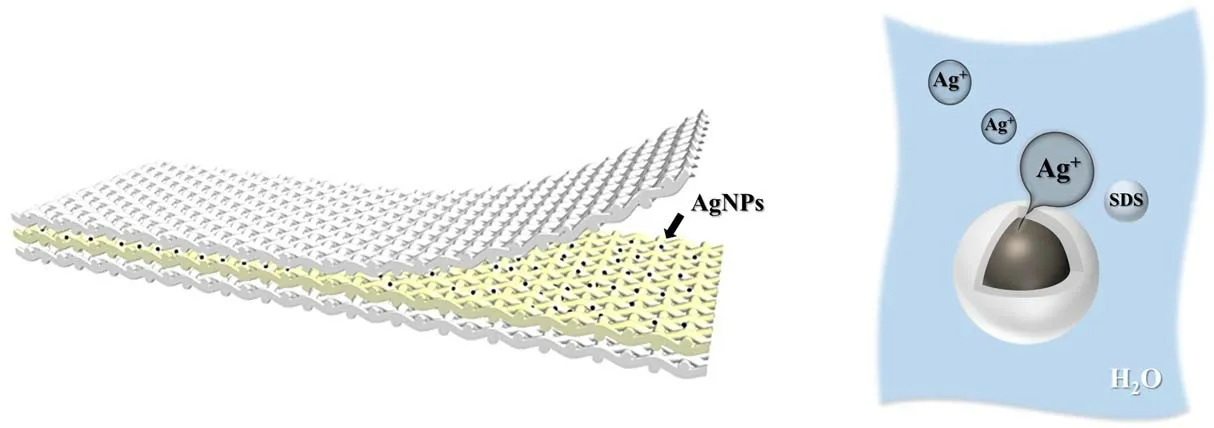
Figure 9.Multi-layer antibacterial cotton cloth.
4.Conclusion
In this work, a novel method of preparing AgNPs-containing cotton cloth was proposed,and the difference is that the shell was added outside AgNPs to form Ag nanocapsules through the APP method.The AgNP core was used to bring antibacterial activity,and the shell composed of SDS was utilized to prevent the excessive release of Ag ions to suppress the potential toxicity of AgNPs as well as prevent oxidation of AgNPs.The specific method was to atomize the nano-silver colloid to form aerosol and transport it into the self-designed device for nanocapsule deposition.Under the action of plasma, the water molecules in the aerosol droplets were volatilized continually,which led to the continuous shrinkage of the droplets, while Ag nanocapsules with an SDS shell were formed and deposited on the cotton fibers.The surface characteristics of cotton were detected by SEM, EDX and XPS.The results indicated that under APP treatment,the skin of the cotton fibers would gradually fall off.After 10 min of deposition, the Ag content can rise to 1.6%.Meanwhile, the antimicrobial activity of the cotton was assessed using the improved AATCC 100 Method against E.coil and S.aureus.The results demonstrated that the antibacterial cotton cloth exhibited the strongest antimicrobial activity for both bacteria,where the reduction rates of the 5 min deposition sample and 10 min deposition sample can reach 99% and 100% for two bacteria, respectively.In addition, the UV absorption capability of the cotton drastically increased with the treatment time.
In summary, the antibacterial cotton cloth shows strong antibacterial activity and can be utilized in many situations,which has a great amount of economic value and broad application prospects.
Acknowledgments
This work is supported by National Natural Science Foundation of China (Nos.11975163 and 12175160), together with a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
 Plasma Science and Technology2023年3期
Plasma Science and Technology2023年3期
- Plasma Science and Technology的其它文章
- Relativistic toroidal light solitons in plasma
- Valley-dependent topological edge states in plasma photonic crystals
- Bulk moduli of two-dimensional Yukawa solids and liquids obtained from periodic compressions
- Modeling of magnetized collisional plasma sheath with nonextensive electron distribution and ionization source
- Observation of the poloidally asymmetrical density perturbation of sawtooth collapse on J-TEXT
- Alfvén continuum in the presence of a magnetic island in a cylinder configuration
