Development of necrotizing enterocolitis after blood transfusion in very premature neonates
Travis L. Odom · Jessica Eubanks · Nusiebeh Redpath · Erica Davenport · Dmitry Tumin ·Uduak S. Akpan
Abstract Background Prior studies report conflicting evidence on the association between packed red blood cell (PRBC) transfusions and necrotizing enterocolitis (NEC),especially in early weeks of life where transfusions are frequent and spontaneous intestinal perforation can mimic NEC.The primary objective of this study was to evaluate the association between PRBC transfusions and NEC after day of life (DOL) 14 in very premature neonates.Methods A retrospective cohort analysis of very premature neonates was conducted to investigate association between PRBC transfusions and NEC after DOL 14.Primary endpoints were PRBC transfusions after DOL 14 until the date of NEC diagnosis,discharge,or death.Wilcoxon ranked-sum and Fisher’s exact tests,Cox proportional hazards regression,and Kaplan–Meier curves were used to analyze data.Results Of 549 premature neonates,186 (34%) received transfusions after DOL 14 and nine (2%) developed NEC (median DOL=38;interquartile range=32–46).Of the nine with NEC after DOL 14,all were previously transfused (P <0.001);therefore,hazard of NEC could not be estimated.Post hoc analysis of patients from DOL 10 onward included five additional patients who developed NEC between DOL 10 and DOL 14,and the hazard of NEC increased by a factor of nearly six after PRBC transfusion (hazard ratio=5.76,95% confidence interval=1.02–32.7; P =0.048).Conclusions Transfusions were strongly associated with NEC after DOL 14.Prospective studies are needed to determine if restrictive transfusion practices can decrease incidence of NEC after DOL 14.
Keywords Necrotizing enterocolitis · Packed red blood cells · Transfusions · Very low birth weight infants
Introduction
Necrotizing enterocolitis (NEC) continues to be an important cause of mortality among premature infants.NEC occurs in 5%–7% of very low birth weight infants (VLBW,birth weight <1500 g) [1,2],with fatality rates of 20%–30% [1,3].The exact mechanism by which NEC occurs is unclear,but multiple predisposing factors have been identified,including genetics,immature intestinal barrier and function,abnormal microbial flora,and excessive immunoreactivity[3].Preventive strategies include feeding with human milk,standardized feeding practices and good antibiotic stewardship [4,5].Infants diagnosed with NEC are at risk for poor outcomes including gastrointestinal and neurodevelopmental problems [6,7].
With many infants receiving packed red blood cell(PRBC) transfusions in the neonatal intensive care unit(NICU) [8,9],the association between PRBC transfusions and NEC has been explored in several studies but remains controversial [10].Pathogenetic pathways that have been described include immunologic dysfunction [11],direct effects of PRBC storage [12],and reperfusion injury associated with severe anemia [10].Empirical evidence,however,has been mixed: some studies have indicated that PRBC transfusion is associated with increased likelihood of NEC [13–18];others have found no association between transfusions and NEC [19,20];and still others have suggested that transfusions may be protective against NEC[21–23].
Uncertain results on the association between PRBC transfusion and development of NEC may be related to confounding of transfusion receipt with the presence of severe anemia necessitating transfusion,or the early prodromal period of NEC mimicking symptomatic anemia for which the infant then receives a transfusion [15,24].This association may be confounded further by examining NEC occurring in the first two weeks of life,when transfusions are especially frequent[10,25],and when cases of spontaneous intestinal perforation (SIP) may be confused with NEC.Therefore,we sought to determine if PRBC transfusions after the first two weeks of life posed an independent risk factor for development of NEC among VLBW infants in a NICU with a relatively low transfusion rate.Secondarily,we examined the time from the first transfusion after the first two weeks of life to NEC diagnosis after the first two weeks of life,as well as the severity of anemia noted at the most recent transfusion before NEC was diagnosed.
Methods
This was a retrospective cohort study of premature neonates less than 32 weeks of gestation or less than 1500 g at birth,admitted to the NICU at ECU Health Medical Center(ECUHMC),between 2017 and 2019.We used a retrospective cohort design to represent all eligible infants potentially at risk of transfusion after day of life (DOL) 14 and subsequent development of NEC.ECUHMC is a tertiary-care regional referral hospital affiliated with ECU and its level 4 NICU serves a rural 29-county region in eastern North Carolina.Infants were eligible for this study if they were born at or transferred to ECUHMC before DOL 14,identified from our institution’s data entered in the Vermont Oxford Network database.During the study period,our hospital’s NICU transfusion practices during the first two weeks of life included transfusing neonates with PRBC to maintain a minimum hematocrit of 35% or to compensate for blood loss due to phlebotomy.For this study,we chose to focus on the period after the first two weeks of life to better examine the association of transfusions with NEC without the relationship being obscured by higher transfusion frequency and spontaneous intestinal perforation cases which frequently occur in the first 7–10 days of life.After the first two weeks of life,the decision to transfuse is provider-dependent and is based on hematocrit,reticulocyte count,patient acuity,and respiratory support requirements.All neonates are transfused at a volume of 15 mL/kg.The blood bank policy allows for leukoreduced,irradiated,sickle negative blood donated less than 15 days previously and preserved in adsol preservation solution or citrate phosphate dextrose adenine(Fenwal Laboratories,Lake Zurich,Illinois) to be used for neonatal transfusions.Each unit of blood is divided into smaller packs and used as needed for the same neonate until the expiration date,to limit the number of donors a neonate is exposed to.Neonates who have achieved full or partial enteral feeds have their feeds decreased to 40 mL/kg during the transfusion and for six hours post-transfusion,after which they are returned to the volume that they had previously been receiving.
We did not include transfusions occurring before DOL 14 in our primary analysis due to the frequency of transfusions during that period and to avoid the risk of mis-labeling spontaneous intestinal perforation cases which frequently occur in the first 7–10 days of life as NEC cases.We excluded infants that died,got discharged,developed any intestinal injury before DOL 14,required abdominal surgery at any time during the hospital stay other than for treatment of NEC,had other abdominal pathology,or had known congenital or chromosomal anomalies.We followed neonates in the study from DOL 14 until the date of NEC diagnosis,discharge,or death,whichever occurred first,censoring followup in June 2020.NEC diagnosis was retrospectively queried from the electronic medical record (EMR),determined by radiographic evidence of NEC stage IIa or greater using the modified Bell criteria [26].
The primary independent variable was receipt of PRBC transfusions after DOL 14.The occurrence and timing of all PRBC transfusions were determined from the EMR.We also queried the degree of anemia on the most recent laboratory study preceding each transfusion,and defined anemia as hematocrit less than 35%,and severe anemia as hematocrit less than 24%.In our survival analysis,we modeled the hazard of NEC separately before and after the earliest transfusion that occurred after DOL 14.Data on all subsequent transfusions were used to calculate the total number of transfusions after DOL 14.Among infants who developed NEC,we also examined the latest transfusion before the NEC diagnosis,to determine the severity of anemia at that time,and the time elapsed between the latest transfusion and diagnosis of NEC.
We assessed additional covariates on DOL 14 for all patients,including type of feeds received,mode of respiratory support,infant demographics (gestational age,birth weight,sex,race/ethnicity),maternal age,and infant comorbidities including respiratory distress syndrome (RDS;radiographic or clinical diagnosis of surfactant deficiency),patent ductus arteriosus (any size,diagnosed on echocardiography),intraventricular hemorrhage (any grade,diagnosed on head sonography),anemia,hyperbilirubinemia (elevation in serum bilirubin level requiring phototherapy).We also collected data on any significant events or procedures including full courses of antibiotics (at least seven days of therapy) received at any point before development of NEC.We defined transfusion-associated NEC as an episode of NEC occurring within two days of the preceding transfusion [16,27].In a post hoc analysis,we examined NEC and transfusion data from DOL 10 onwards,among patients otherwise eligible for our study (i.e.,including patients who developed NEC on DOL 10–13).Diagnoses before DOL 10 were not considered because SIP cases generally occur within the first ten days of life [28].
We summarized continuous data using medians with interquartile ranges (IQR),and categorical data were summarized using counts with percentages.On bivariate analysis,we compared receipt of PRBC transfusions and other patient characteristics according to whether patients developed NEC after DOL 14,using Wilcoxon rank sum tests or Fisher’s exact tests,as appropriate.In further analysis,we evaluated the onset of NEC using survival analysis techniques,including Kaplan–Meier curves and Cox proportional hazards regression.To characterize the timedependent impact of PRBC transfusions on the hazard of developing NEC,we included the earliest PRBC transfusion as a piecewise-constant,time-varying covariate in the multivariable Cox model.The hazard ratio (HR) associated with this covariate represented the instantaneous change in the hazard of developing NEC after receiving PRBC transfusion.Due to the low event rate of NEC,only statistically significant covariates from bivariate analysis were included as control variables in the Cox model.Variables leading to perfect prediction (i.e.,where one or more cell counts in the NEC group was zero) were not included in the multivariable analysis.We performed all analyses using Stata/SE 16.1 (College Station,TX: StataCorp,LP),and consideredP<0.05 statistically significant.
The study protocol was reviewed and approved by the ECU Institutional Review Board with a waiver of consent(University and Medical Center Institutional Review Board approval number: UMCIRB 20-000643).No identifying information was included in this retrospective study.This research was conducted in accordance with the ethical standards of all applicable national and institutional committees and the World Medical Association’s Helsinki Declaration.
Results
We identified 718 patients meeting birth weight or gestational age criteria admitted to our NICU between 2017 and 2019.We excluded 18 patients admitted after DOL 14,72 patients discharged before DOL 14,55 patients with congenital or chromosomal anomalies,and 24 patients who developed intestinal injury,had abdominal surgery,or had other abdominal pathology by DOL 14.Of the remaining 549 patients,186 (34%) received PRBC transfusions after DOL 14,with a median of three transfusions after DOL 14 (IQR=1–5).Nine of 549 (1.6%) patients in the primary analysis developed NEC after DOL 14 (median DOL of NEC diagnosis: 38 days;IQR=32–46 days).There were 616 total transfusions in the control group and 27 transfusions in the group developing NEC after DOL 14.There was no statistically significant difference in the median pre-transfusion hematocrit values between groups.Median hematocrit values were 30% (IQR=27–32) in the control group vs.32% (IQR=26–35) in the group developing NEC after DOL 14 (P=0.194).
The timing of NEC diagnosis is summarized in the Kaplan–Meier curve shown in Fig. 1,showing that nearly all cases were diagnosed by two months of life.All nine patients who were diagnosed with NEC after DOL 14 had also received PRBC transfusions after DOL 14 but before the NEC diagnosis (median of two transfusions;IQR=1–5),seven (78%) were transfused within 48 hours of NEC diagnosis,seven (78%) received transfusions prior to DOL 14,and four (44%) died before discharge.Most patients developing NEC shortly after transfusion were severely anemic.Of seven patients in this group,four had hematocrit <24% (as low as 20.4%),and a fifth had hematocrit of 24.1%.Three of the patients who developed NEC after DOL 14 (including one developing NEC shortly after PRBC transfusion)had had a previous course of antibiotics lasting seven days or longer.Among patients who developed NEC after PRBC transfusion,prior significant events and procedures (each referring to a different patient) included pulmonary hemorrhage,generalized cutaneous fungal infection,code event and removal of a retained umbilical artery catheter in the operating room,and sepsis.

Fig.1 Kaplan–Meier plot of necrotizing enterocolitis (NEC) diagnosis after day of life (DOL) 14
Patient characteristics are compared according to development of NEC after DOL 14 in Table 1.Patients who developed NEC were more likely to have received PRBC transfusions than patients who did not develop NEC (100 vs.33%,P<0.001 on Fisher’s exact test).Additionally,all patients who developed NEC had been diagnosed with RDS and were still receiving continuous positive airway pressure or mechanical ventilation on DOL 14.Patients who developed NEC were more likely to have been diagnosed with anemia (67% vs.31%,P=0.031)and tended to have been born at a lower gestational age(GA) than patients who did not develop NEC (median: 26 vs.30 weeks,P<0.001).Similarly,patients who developed NEC had lower birthweight than those who did not develop NEC (median: 787 vs.1220 g,P=0.001).Because all patients who developed NEC after DOL 14 had also previously received PRBC transfusions,the HR associated with PRBC transfusion could not be estimated.

Table 1 Patient characteristics according to development of necrotizing enterocolitis after day of life 14 (N =549)
In additional post hoc analysis,we expanded the sample to include five patients diagnosed with NEC on DOL 10–14,and to account for PRBC transfusions received on or after DOL 10.Of the 554 patients in this secondary analysis,193 (35%) received PRBC transfusions after DOL 10 and 14 (2.5%) developed NEC after DOL 10.On bivariate analysis,79% of patients diagnosed with NEC on or after DOL 10 had previously received PRBC transfusions,compared to 34% of patients who were not diagnosed with NEC (Fisher’s exact test,P=0.001).On multivariable Cox proportional hazards analysis adjusted for GA,and diagnosis of anemia (Table 2),the hazard of developing NEC on or after DOL 10 increased by a factor of nearly six after receiving PRBC transfusion (HR=5.76,95% confidence interval=1.02–32.7;P=0.048).The multivariable analysisdid not control for birthweight due to high collinearity with GA (r=0.74),while results were similar if controlling only for birthweight and not GA.
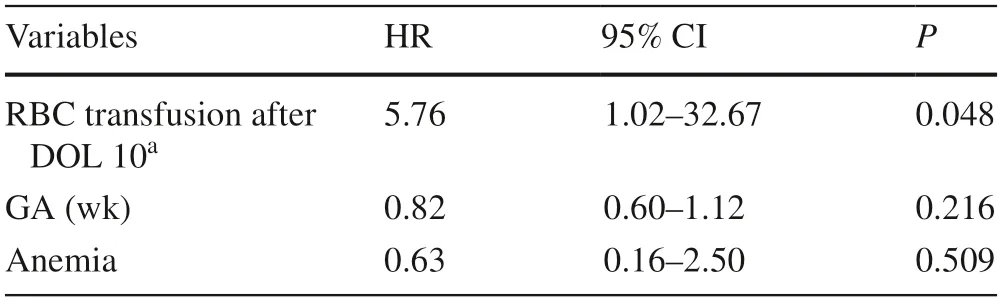
Table 2 Multivariable Cox proportional hazards model of developing necrotizing enterocolitis after day of life 10 (N =554)
Discussion
We conducted this study to examine the association of PRBC transfusion with NEC in VLBW infants in our NICU with a relatively low transfusion rate of PRBC transfusion after the early neonatal period.We hypothesized that even with the low rate of transfusion,there would still be a correlation of NEC with PRBC transfusions.We found a low incidence of NEC in the overall study sample after DOL 14.However,in this age range,all patients who were diagnosed with NEC had previously received PRBC transfusions,and 78% (7/9)were diagnosed with NEC within 48 hours of receiving a transfusion.On a post hoc analysis,we confirmed the strong association between PRBC transfusions and hazard of developing NEC after decreasing the postnatal age cutoffto DOL 10,while controlling for factors associated with NEC development on univariate analysis.However,we did not find a significant relationship between severe anemia and NEC.The low incidence of NEC in our population highlighted the association of PRBCs with NEC especially outside the early neonatal period,suggesting that NEC occurring in the first few weeks of a neonate’s life may have a different pathogenesis from transfusion-associated NEC.To our knowledge,this is the first report focusing on the association of PRBC transfusions with NEC after DOL 14.
The rate of NEC in our unit was lower than the 5%–7%that has been previously reported [1,2].Only 1.6% of our sample developed NEC after DOL 14,and 2.5% after DOL 10.This may be due to a high prevalence of human milk feeding in our NICU and the presence of a standardized feeding protocol,both of which have been shown to be protective against NEC [5,29].Up to 90% of the neonates in our unit had achieved full enteral feeds with human milk (maternal or donor) by DOL 14.Collecting data after the first two weeks of life was intended to reduce confounding from SIP and early cases of NEC which may have a different pathogenesis from transfusion-related injury.Prior research has suggested that NEC includes two or more distinct entities with different multifactorial triggers [30],with the pathogenesis of early-onset NEC being related to the early period of hemodynamic and respiratory instability,whereas NEC after DOL 14 appears to occur in neonates with lower GA and birth weight,and is associated with complications of prolonged hospitalization,such as nosocomial infections [31].In our study,the median age at diagnosis for NEC cases diagnosed after DOL 14 was 38 days,agreeing with the previous studies reporting delayed onset of NEC in cases associated with transfusions [17,27].In contrast to other studies that have reported that transfusion-related NEC occurs in about a third of NEC cases [16,17,27],78% of the cases of NEC in our unit had received a PRBC transfusion within the preceding 48 hours.The observed temporal proximity between RBC transfusions and development of NEC in this study is a finding not attributable to overuse of PRBC transfusions,since the 34% transfusion rate in our study was well below previously cited rates of 53%–58% [10,17].The association between PRBC transfusion and subsequent development of NEC remains controversial [10].A review of three randomized trials showed that more frequent transfusions due to higher transfusion thresholds led to a lower incidence of NEC,inferring that there is no association of blood transfusions with NEC [21].A few more studies also show no association between PRBC transfusions and NEC [19,20].This contrasts with a prospective multicenter observational study that found a lower incidence of NEC in lower frequency transfusion NICUs [14],suggesting that fewer transfusions may decrease the risk of NEC,and the many observational studies that show an association between PRBC transfusions and NEC [13,15–19].Some studies report that blood transfusions may be protective against the development of NEC[22,23,32],and still others suggest that it is the severity of anemia that increases the risk of NEC,rather than the transfusions themselves [33,34].Anemia may predispose the gut to tissue hypoxia and ischemia,increasing the risk for NEC [33,35].The neonates at highest risk for NEC are also frequently anemic [8–10,33,34].In addition,the early signs of NEC may be confused for symptomatic anemia,triggering a PRBC transfusion [35].These factors may contribute to the conflicting evidence regarding severe anemia,transfusions,and NEC.
In this study,despite a relatively low transfusion rate,we found a significant association of NEC following PRBC transfusions with most NEC cases occurring within 48 hours after transfusion.The neonates in this study were fed nearly exclusively with human milk during the time where susceptibility to NEC is highest,which may have prevented traditional NEC,leaving only transfusion-related cases.Approximately half the cases of NEC in this study also had severe anemia;therefore,like many other studies,we could not conclude that anemia is an independent risk factor for NEC.
Transfusion with PRBC is safe for majority of infants who receive it,but in rare cases it may contribute to developing NEC.Other infrequent complications in neonates include circulatory overload,infection,hemolytic and graft vs.host disease [36].Proposed mechanisms of transfusionassociated NEC include immaturity of compensatory microvascular mechanisms in the extremely premature neonate,leaving the intestines vulnerable to hypoxia and anemia[35],and exposure to pro-inflammatory molecules in stored blood,such as cytokines and fetal calprotectin,which could potentially injure the intestinal mucosa [37,38].Strategies to prevent anemia,such as delayed cord clamping and erythropoietin stimulating agents,have been proposed;however,evidence of their effectiveness in preventing anemia and NEC is lacking [39,40].Transfusions,rather than anemia,have been more reliably linked to the development of NEC,and restrictive PRBC transfusion policies have not been associated with poorer short-or long-term outcomes[41–43].Perhaps then,the focus should be on utilizing more restrictive transfusion policies instead of the prevention of anemia,at least for the purposes of decreasing cases of NEC.
There were several limitations to our study.As it is an observational study,we are unable to imply causation between the examined factors and findings.The study is also limited by its retrospective nature,preventing the ability to control factors surrounding PRBC transfusions.Since we only looked at NEC cases occurring after DOL 14,we observed a low incidence of NEC in the overall sample,which may limit the strength of the findings.However,this limitation is mitigated by the much stronger association between NEC and PRBC transfusion found in this study compared to previous reports.We did not address the question of possible confounding from transfusions being administered to infants with severe anemia who may be exhibiting early signs of NEC,who then go on to develop overt NEC within 48 hours of receiving a transfusion.However,we do not think that this affected our results since this situation would stem from NEC being confused for symptomatic anemia,in which case we would have found a significant association of NEC with severe anemia.Although we collected data on antibiotic use and several common comorbidities,we did not include culture-proven sepsis or line infections in our secondary analysis and we did not examine relationships regarding specific infectious organisms,which could potentially impact need for transfusion and development of NEC.
In our NICU,the combination of mostly human milk feeding as the initial feeding choice for VLBW infants,the use of a standardized feeding protocol,and the relatively low frequency of PRBC transfusions outside the critical period of the first two weeks has resulted in a low overall incidence of NEC.In this study,NEC occurring after DOL 14 was strongly associated with PRBC transfusion,and occurred in smaller,more premature neonates,as seen in previous reports [35].Large,multicenter studies are needed to fully define risk factors that increase susceptibility to NEC after PRBC transfusion.Additionally,future studies may need to focus on NEC with delayed onset,which is more likely to be transfusion-associated than early onset cases.Until there is more data,the present findings support that NEC burden may be reduced by applying proven preventative measures against NEC and restrictive transfusion practices.
AcknowledgementsWe thank Sherry Moseley,RN for assistance with data acquisition for this study.We thank the James and Connie Maynard Children's Hospital at ECU Health Medical Center for sharing data used in this study.
Author contributionsOT participated in study conception and design,data acquisition,interpretation of results,and drafting of the manuscript.EJ,RN and DE participated in data acquisition,interpretation of results,and drafting of the manuscript.TD participated in study conception and design,statistical analysis,interpretation of results,and drafting of the manuscript.AU participated in study conception and design,data acquisition,interpretation of results,and critical revision of the manuscript and supervised the study.All authors reviewed the final version of the manuscript and approved its submission for publication.
FundingThere was no financial or materials support for this study.
Data availabilityIdentified study data cannot be provided due to the need to protect patient confidentiality.Deidentified data may be provided by the authors upon request.
Declarations
Ethical approvalThe study protocol was reviewed and approved by the ECU University and Medical Center Institutional Review Board with a waiver of consent (approval number: UMCIRB 20–000643).No identifying information was included in this retrospective study.This research was conducted in accordance with the ethical standards of all applicable national and institutional committees and the World Medical Association’s Helsinki Declaration.
Conflict of interestNo financial or non-financial benefits have been received or will be received from any party related directly or indirectly to the subject of this article.The authors have no conflict of interest to declare.
 World Journal of Pediatrics2023年1期
World Journal of Pediatrics2023年1期
- World Journal of Pediatrics的其它文章
- Editors
- Information for Readers
- Instructions for Authors
- Comparison of updated birth weight,length and head circumference charts by gestational age in China with the INTERGROWTH-21st NCSS charts: a population-based study
- Clinical characteristics of pediatric cases infected with the SARS-CoV-2 Omicron variant in a tertiary children’s medical center in Shanghai,China
- Pediatric body mass index trajectories and the risk of hypertension among adolescents in China: a retrospective cohort study
