Distance-based α-amylase biosensor fabricated with amylopectin-coated mesoporous membrane
Binglu Zho ,Mshooq Khn ,Yulin Liu ,Wnjun Ti ,Chongyng Mu ,Wnli Wu ,Mi Zho,Yohong M,Li Yu,Jin-Ming Lin,Qiongzhng Hu,∗
a Qilu University of Technology (Shandong Academy of Sciences),Shandong Analysis and Test Center,Ji’nan 250014,China
b School of Pharmaceutical Sciences,Qilu University of Technology (Shandong Academy of Sciences),Ji’nan 250014,China
c Key Laboratory of Colloid and Interface Chemistry,Shandong University,Ministry of Education,Ji’nan 250100,China
d Key Laboratory for Biosensors of Shandong Province,Biology Institute,Qilu University of Technology (Shandong Academy of Sciences),Ji’nan 250353,China
e Beijing Key Laboratory of Microanalytical Methods and Instrumentation,MOE Key Laboratory of Bioorganic Phosphorus Chemistry & Chemical Biology,Department of Chemistry,Tsinghua University,Beijing 100084,China
Keywords:Paper biosensor α-Amylase Stimuli-responsive polymer Mesoporous membrane Distance Point-of-care testing
ABSTRACT Paper-based biosensors are widely employed in point-of-care testing (POCT) due to their convenience,portability,low cost,and ease of use.This study reports an integrated distance-based paper biosensor fabricated with a mesoporous membrane coated with stimuli-responsive polymer.The detection of αamylase (AMY) using amylopectin-coated mesoporous membrane is demonstrated as an example.After introducing the AMY solution,it is observed that the aqueous solution flows along the paper strip due to AMY-catalyzed hydrolysis of amylopectin.The flow distance is proportional to the concentration of AMY with a detection limit as low as 4 mU/mL.In addition,the detection of AMY is demonstrated in human serum.Furthermore,the inhibitory effect of acarbose on AMY is evaluated.This reagent-free and disposable biosensor allows single-step rapid detection of the analyte.This approach is very promising for the development of user-friendly,equipment-free,and cost-effective biosensors with remarkable sensitivity and excellent selectivity for disease diagnosis and hypoglycemic drug screening.
With the advent of COVID-19,the demand forin-vitrodiagnostics has increased exponentially [1].Point-of-care testing (POCT) is widely employed in diagnosing,treating,and prognosis diseases in home healthcare [2] and urgent situations [3].Paper is an ideal substrate for POCT development due to its universal availability,low cost,simple portability,high porosity,and low sample and reagent consumption [4].Therefore,paper-based biosensors have been extensively employed to monitor clinical and environmental samples [5,6].Currently,several representative paper-based devices are in practice for diagnosis,such as human chorionic gonadotropin (HCG) [7],acquired immunodeficiency syndrome (AIDS)[8],and SARS-CoV-2 [9].
Commercial paper-based biosensors mostly rely on color as the readout signal [10].The color change usually results from metal complexation,precipitate formation,or color dye’s pH change.Generally,the measurement of color change requires professional devices such as cameras,scanners,or color analyzers for quantification,which might hinder the potential applicability of paperbased devices [11].The distance-based signal readout is an appealing alternative for equipment-free naked-eye quantification of the analyte [12],because it only requires the measurement of length or diameter without the assistance of an external camera or scanner [13].In the distance-based microfluidic assay,the alteration in distance is always correlated with the analyte concentration [14].
Stimuli-responsive polymers (SRPs) have been extensively applied in the construction of biosensors [15].The SRP undergoes physical/chemical changes under external stimuli such as pH [8],small molecules [16],nucleic acids [17],proteins [18],and enzymes[19].However,most methods require chemical crosslinkers,encapsulation of biorecognition elements,and labeled nanoparticles,and encounter the disadvantages of complex preparation steps and low sensitivity.Recently,the development of distance-based biosensors on paperviamonitoring the viscosity change of SRPs in aqueous solution provides an effective means to address these problems[20,21].In the presence of the analyte,the viscosity of the polymer solution was changed due to enzymatic reaction,resulting in the change of the aqueous flow distance on the paper [22,23].Although these methods are simple,convenient,and label-free,the development of disposable and low-cost paper-based sensors that allow single-step detection is still very challenging.Therefore,it is highly demanded to construct versatile distance-based biosensors with minimal reagents and operation steps.
α-Amylase (AMY) is a significant biomarker in the human body,which widely exists in blood,urine,lotion,semen,and saliva.The concentration of AMY is related to many diseases,such as pancreatic cancer,acute pancreatitis,acute alcoholism,hepatitis,and cholecystitis [24].Anti-diabetic drugs like acarbose are an effective inhibitor of AMY that can be used to control postprandial blood glucose [25].Currently,the AMY detection methods mainly include electrochemistry [26],immunoassay [27],fluorometry [28],colorimetry [29],and others.These methods are usually limited to detection in the central laboratory and are difficult to be applied in on-site real-time detection,which may affect the timely diagnosis and treatment of acute diseases.Therefore,developing a simple,portable,rapid,sensitive,low-cost,and user-friendly AMY biosensor is critical.
Herein,we demonstrate a novel strategy for developing a distance-based biosensor assisted with the SRP-coated mesoporous membrane.The detection of AMY and its inhibitor is illustrated as an example (Fig.1).The components of the distance-based biosensor are integratedviaa paper lamination method (Fig.S1 in Supporting information).Highly crosslinked amylopectin,an AMY substrate,is coated onto the mesoporous membrane to block the membrane micropores.The paper strip and amylopectin-coated membrane are sequentially placed in a 3D-printed flow channel with a sample port on the top.The sample port is designed to load the sample on the amylopectin-coated mesoporous membrane.
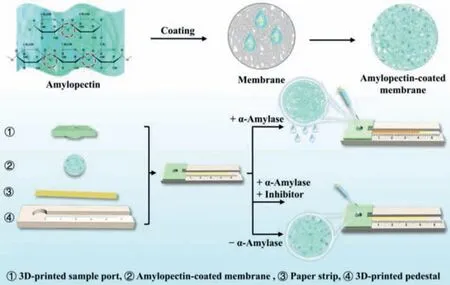
Fig.1. Schematic diagram of the distance-based α-amylase biosensor fabricated with amylopectin-coated mesoporous membrane to detect α-amylase and its inhibitor.
When the target is introduced into the sample zone,it hydrolyzes the amylopectin and changes the mesoporous membrane’s permeability.The solution passes through the mesoporous membrane from the sample zone and streams along the paper strip.The amount of AMY is positively correlated to the aqueous flow distance.The detection of AMY is successfully achieved in human serum.Furthermore,the screening of the AMY inhibitor is also accomplished,showing the potential of the method in screening hypoglycemic drugs.This biosensor is disposable,inexpensive,and user-friendly,allowing reagent-free and single-step detection with considerable convenience,high portability,and more practicality.Therefore,developing commercial POCT devices with various potential applications is very promising.
The experimental details are provided in the Supporting information.The seepage flow distance is directly obtained from the scale,and the aqueous coverage ratio (CR) is defined for further data analysis as illustrated in Eq.1:
where,PflowandPtotalare the pixel values of the seepage flow and the paper strip’s total area,respectively.
First,the feasibility of the distance-based biosensor for AMY detection was evaluated.On the uncoated poly(tetrafluoroethylene)(PTFE) mesoporous membrane,the aqueous solution passed through the membrane and flowed along the paper strip with a CR value of 93.3% (Fig.2A).Contrarily,the aqueous solution was retained in the sample zone comprised of 5 wt% amylopectincoated PTFE membrane (CR=0).However,with the introduction of AMY (10 U/mL),the aqueous solution flowed on the paper strip with a CR value of 80.2%.The aqueous flow was attributed to the AMY-catalyzed hydrolysis ofα-1,4-glycosidic bonds of amylopectin(Fig.S2 in Supporting information),which reduces the amylopectin attachment to the membrane,thereby inducing the increase of the permeability of the amylopectin-coated PTFE membrane.While adding a mixture solution of 10 mg/mL acarbose and 10 U/mL AMY solution,the mixture was retained in the sample zone without seepage,suggesting the acarbose-induced inhibition of AMY.These results validate the feasibility of the paper-based biosensor to detect AMY and acarboseviadistance readout.
The experimental conditions play an essential role in determining the performance of the biosensor.Optimization of membrane materials,pore sizes (Fig.S3 in Supporting information),the drying temperature of amylopectin-coated PTFE membranes (Fig.S4 in Supporting information),and the amylopectin concentrations to coat the membranes (Figs.S5-S7 in Supporting information) are provided in the Supporting Information.In light of the optimal performance of the AMY biosensor,the PFTE membrane with a pore size of 0.45 μm at the drying temperature of 37°C was finally selected and an amylopectin concentration of 5 wt% was used for further experiments.
To further verify that the AMY-catalyzed hydrolysis of amylopectin coating,fluorescence,paper diffusion,iodine colorimetry,water contact angle (WCA),and scanning electron microscopy(SEM) studies were conducted.A viscosity-sensitive fluorescent probe,thioflavin T,was added to the amylopectin solution,which shows high fluorescence.However,the fluorescence intensity of the solution decreased after adding AMY to the solution,suggesting the decrease of viscosity due to amylopectin degradation in the presence of AMY (Fig.2B).Iodine colorimetry was employed to examine the AMY-catalyzed cleavage of amylopectin on the PTFE membranes.Distinctive color changes were unambiguously observed due to the iodine-amylopectin reaction on the PTFE membrane (Fig.2B inset).The WCA tests show that the wetting speed of the water droplet (4 μL) significantly decreased on amylopectincoated PTFE membrane compared to bare PTFE membrane.Subsequently,the wetting speed of the water droplet was enhanced after exposure to AMY (Fig.2C).Also,the SEM images show that amylopectin attached to the fiber and diminished the pore sizes in the PTFE membrane and the porosity increased after AMY hydrolysis(Fig.2D).In addition,the paper diffusion method was also carried out.5 μL amylopectin (5 wt%) solutions were hydrolyzed through different concentrations of AMY and dropped onto the filter paper,respectively.Due to the viscosity variance of the AMY-catalyzed solutions,the diffusion area raised with increasing concentrations of AMY (Fig.3).The above experiments validated the feasibility of the method to detect AMY by coating amylopectin on the PTFE membrane.
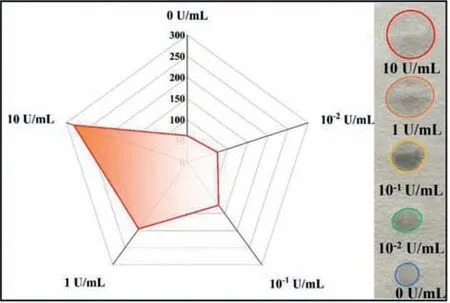
Fig.3. Diffusion areas of 5 wt% amylopectins hydrolyzed by different concentrations of AMY on filter paper.The inset image shows the photograph of these solutions diffusing on filter paper.The mixtures of 5 wt% amylopectin and different concentrations of AMY were individually prepared,incubated at 37°C for 20 min,and then added onto the filter paper.The volume of each mixture is 5 μL.
Under optimal conditions,the responses of the distance-based biosensor were evaluated at different AMY concentrations from 1 U/mL to 10 U/mL (Fig.4A).Fig.4B and Fig.S8 (Supporting information) show the CR values measured at different seepage flow lengths along the paper strips.The seepage flow distance raised with the increase of AMY concentration.A linear relationship between the flow distance and the AMY concentration was obtained.The detection limit of AMY was determined to be 4 mU/mL (based on 3σ/slope).Table S1 (Supporting information) shows that the biosensor’s performance is very competitive among reported methods.
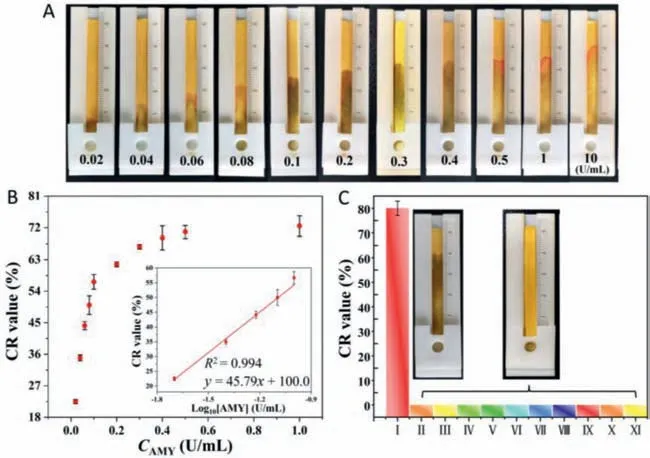
Fig.4. The sensitivity and selectivity of the distance-based AMY biosensor.(A) Seepage flow distances at different AMY concentrations (0–10 U/mL).(B) Plot of CR values against AMY concentrations and a linear plot of CR values as a logarithmic function of AMY concentrations.(C) CR values and images of seepage on paper in (I) 10 U/mL AMY,(II) heparin sodium,(III) uric acid,(IV) KCl,(V) MgSO4,(VI) lysozyme,(VII) urease,(VIII) trypsin,(IX) lipase,(X) pepsin,and (XI) hyaluronidase,respectively.The concentrations of all enzymes were 10 U/mL,and the concentrations of all small molecules and salts were 0.1 mg/mL.
The specificity of the distance-based biosensor for AMY detection was also tested compared to other enzymes,small molecules,and salts.Fig.4C and Fig.S9 (Supporting information) show the representative seepage flow along the paper strip in the presence of AMY.However,the seepage flow distances were negligible in the presence of other substances (e.g.,heparin sodium,uric acid,KCl,MgSO4,lysozyme,urease,trypsin,lipase,pepsin,and hyaluronidase).These results suggest the high specificity of the distance-based biosensor for AMY detection.
The distance-based biosensor was also used to study the inhibitory effect of acarbose on AMY.The inhibitory effects of acarbose (Fig.5A) at different concentrations on AMY were measured.Fig.5B demonstrates that the increasing acarbose concentration inhibits the AMY activity,reducing the seepage flow distance on the paper strip.And the half maximal inhibitory concentration (IC50)was determined and calculated to be 10.76±0.66 μg/mL from the sigmoid curve (Fig.5C).Therefore,this method works well for studying the inhibitory effect of acarbose on AMY.
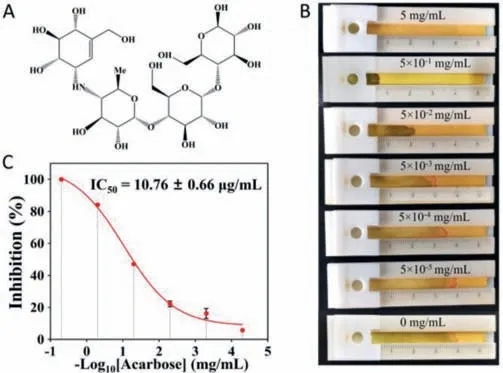
Fig.5. Evaluation of the inhibitory effect of acarbose on AMY using the distancebased biosensor.(A) The chemical structure of acarbose.(B) Image of seepage flow on paper at different acarbose concentrations.(C) The inhibition of AMY at different acarbose concentrations.
The human serum contains about 50 mU/mL AMY in healthy people compared to above 200 mU/mL in pancreatitis patients[30].The performance of the distance-based biosensor for AMY detection in human serum was also evaluated (Fig.S10 in Supporting information).The human serum samples were diluted 10 folds,and then different concentrations of AMY were added,resulting in final AMY concentrations of 50,200,and 300 mU/mL,respectively.
The recoveries of different concentrations of AMY in human serum were examined by a standard addition method.Table 1 summarizes the recoveries of 107.4%,93.2%,and 99.7%,respectively.Therefore,the results indicate that the distance-based biosensor works well in human serum samples.
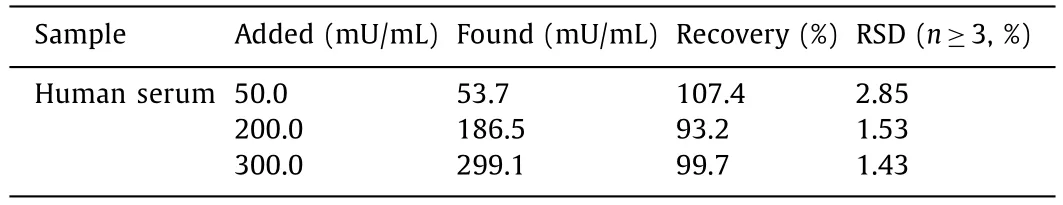
Table 1 Recovery of AMY detection in human serum.
In summary,this work presents a disposable,low-cost,and reagent-free paper-based biosensor with distance readout for single-step detection of AMY and its inhibitor.The principle is based on the AMY hydrolysis of the amylopectin-coated mesoporous membrane,enhancing the membrane’s permeability and allowing the seepage to flow along the paper strip.The detection of AMY in human serum and the analysis of the AMY inhibitor are successfully demonstrated.As a user-friendly and lowcost method,the distance-based biosensor fabricated with an amylopectin-coated mesoporous membrane is competitive for the rapid,quantitative,and high-throughput detection of AMY in different applications.Furthermore,this robust strategy is also very promising for the detection of various analytesviacoating the mesoporous membranes with different stimuli-responsive polymers.
Declaration of competing interest
The authors declare that they have no competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the National Key R&D Program of China (Nos.2021YFB3201200,2021YFB3201202),the Taishan Scholar Program (No.tsqn201812088),the Natural Science Foundation of Shandong Province (No.ZR2022YQ12),the Shandong Scientific and Technical Small and Medium-sized Enterprises Innovation Capacity Improvement Project (No.2022TSGC2533),the Science,Education and Industry Integration Innovation Pilot Project from Qilu University of Technology (Shandong Academy of Sciences) (No.2022JBZ02–04).
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2023.108462.
 Chinese Chemical Letters2023年12期
Chinese Chemical Letters2023年12期
- Chinese Chemical Letters的其它文章
- Spin switching in corrole radical complex
- Benzothiadiazole-based materials for organic solar cells
- Mono-functionalized pillar[n]arenes: Syntheses,host–guest properties and applications✰
- Recent advances in two-step energy transfer light-harvesting systems driven by non-covalent self-assembly✩
- From oxygenated monomers to well-defined low-carbon polymers
- Doping-induced charge transfer in conductive polymers
