Mono-functionalized pillar[n]arenes: Syntheses,host–guest properties and applications✰
Wenzhi Yang,Wenjie Zhang,Jingyu Chen,Jiong Zhou
Department of Chemistry,College of Sciences,Northeastern University,Shenyang 110819,China
Keywords:Pillar[n]arenes Host–guest properties Supramolecular polymer Molecular machines Biological application
ABSTRACT Pillar[n]arenes are a novel class of macrocyclic hosts reported by Ogoshi and co-workers in 2008.Because of their rigid pillar structures,interesting host–guest properties and ease of modifications,pillar[n]arenes have been developed rapidly in the field of functional materials and biomedicine.The modifications of pillar[n]arenes at different positions can give them varied characteristics.Functional groups can be introduced into one position of pillar[n]arenes without changing host–guest properties of pillar[n]arenes.A series of pillar[n]arene dimers,trimers,tetramers and metallacycles can be constructed by mono-functionalized pillar[n]arenes.In this review,two synthetic methods of mono-functionalized pillar[n]arenes are summarized and structures containing monofunctionalized pillar[n]arenes are described.Furthermore,the applications of mono-functionalized pillar[n]arenes in different fields (e.g.,supramolecular polymers,sensors,molecular machines,catalysis,biological applications and light-harvesting systems) are also introduced.Hopefully,this article will be useful for researchers studying pillar[n]arenes,especially the mono-functionalized pillar[n]arenes.
1.Introduction
The syntheses and applications of supramolecular macrocycles(e.g.,crown ethers,cyclodextrins,calixarenes and cucurbiturils)with well-defined cavities are of great significance for the development of supramolecular chemistry [1–8].Pillar[n]arenes are a new class of macrocyclic hosts first reported by Ogoshi and co-workers in 2008 [9].Pillar[n]arenes are composed of hydroquinone ether units connected by methylene bridges in theparapositions,which have symmetrical pillar structures and modifiable rims.Compared with other traditional supramolecular macrocycles,the cavities of pillar[n]arenes are more easily controlled and the modifications of their rims are more convenient,which enable pillar[n]arenes to have more unique properties and functions [10–19].The electron-rich cavities of pillar[n]arenes can also accommodate different guests by host–guest interactions to form interesting supramolecular systems [20–24].Due to these advantages,pillar[n]arenes are widely used in various fields,such as functional materials,fluorescent sensing,catalysis and biological applications[25–34].
The functionalization of pillar[n]arenes can endow them with new physical or chemical properties to prepare functional materials.Functionalized pillar[n]arenes can be divided into six categories according to their substitution positions,that is,mono-functionalized,di-functionalized,rim-differentiated,perfunctionalized,bridge-functionalized and phenyl functionalized pillar[n]arenes [35–40].Each type of functionalized pillar[n]arenes has different functions and characteristics,which expands the application of pillar[n]arenes [41–46].Compared to other functionalized pillar[n]arenes,the mono-functionalized pillar[n]arenes are convenient to synthesize,and new functional groups can be introduced without changing the host–guest properties of the cavity [47–50].For example,Stoddart and co-workers prepared a mono-functionalized pillar[5]arene with a viologen group,which formed self-complexation at low concentrations [51].The interaction forces of self-complexation are comparable to host–guest interactions of pillar[5]arenes.Mono-functionalized pillar[n]arenes can also be used as building blocks of pillar[n]arenes dimers,trimers and tetramers to construct novel materials with more complex topologies.Due to the generation of unique topological structures,these materials usually have chemical and mechanical properties different from discrete pillar[n]arenes [52,53].In addition,mono-functionalized pillar[n]arenes have been used to prepare pillar[n]arenes-based supramolecular polymer materials,which puts forward higher requirements for the structural design of mono-functionalized pillar[n]arenes [54,55].
In this review,we systematically summarize the synthetic strategies of mono-functionalization pillar[n]arenes.Moreover,the structures of mono-functionalized pillar[n]arenes-based dimers,trimers,and tetramers are described.We also discuss the host–guest properties of mono-functionalization pillar[n]arenes and the kinds of recognized guests.Furthermore,the applications of monofunctionalized pillar[n]arenes in different fields are elucidated,such as supramolecular polymers,sensors,molecular machines,catalysis,biological applications and light-harvesting systems (Table S1 in Supporting information).
2.Syntheses and host–guest properties of mono-functionalized pillar[n]arenes
There are two main approaches for the syntheses of mono-functionalized pillar[n]arenes.One is preparing monofunctionalized pillar[n]arenes by the co-cyclization of two different monomers [56–58].The other is deprotecting an alkoxy group on the pillar[n]arenes and then functionalizing it to obtain monofunctionalized pillar[n]arenes [59–61].Stoddart and co-workers prepared a mono-functionalized pillar[5]arene and achieved the detection of alkylamines [62].Then Cao and co-workers obtained a mono-functionalized pillar[5]arene with high yield by optimizing reaction conditions,and found that the ratio of monomers had an effect on the yield [56].Mono-functionalized pillar[5]arenes are also important building blocks to synthesize pillar[5]arenes-based dimers,trimers and tetramers [63–66].The cavity structures of pillar[n]arenes also give them unique host–guest properties [67–70].This section will briefly introduce the syntheses and host–guest properties of mono-functionalized pillar[n]arenes.
2.1. Co-cyclization
Ogoshi and co-workers reported the reaction of 1,4-dimethoxybenzene with paraformaldehyde under the catalysis of Lewis acid to form 1,4-dimethoxy pillar[5]arene (DMP[5]A) in 2008 [9].The solvent has a template effect on the synthesis of pillar[n]arenes (n=5,6).For example,DMP[5]Acan be prepared in high yield with 1,2-dichloroethane as a template solvent,and 1,4-bis(methylcyclohexyl) pillar[6]arene (MCP[6]A) can be prepared with chlorocyclohexane as a template solvent (Fig.1a) [71,72].The synthesis of high-level pillar[n]arenes (n≥7) is difficult because the template effect cannot be used to prepare these homologues.
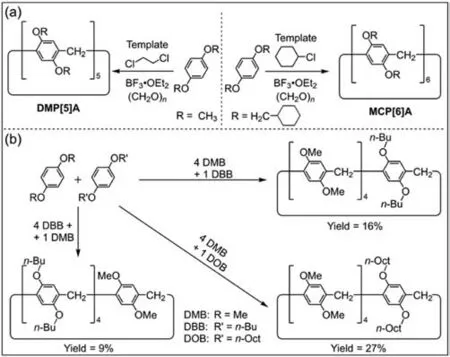
Fig.1. (a) Syntheses of 1,4-dimethoxy pillar[5]arene (DMP[5]A) and 1,4-bis(methylcyclohexyl) pillar[6]arene (MCP[6]A) by template methods [71,72].(b)Preparation of co-pillar[5]arenes by co-cyclization of different monomers [75].
The synthesis of specifically functionalized pillar[n]arenes can greatly expand their applications [73,74].The physicochemical properties and host–guest behaviors of pillar[n]arenes can be regulated by introducing different repeating units.Huang and coworkers prepared co-pillar[5]arenes by co-cyclization in 2010 (Fig.1b) [75].They obtained a series of co-pillar[5]arenes by condensation of 1,4-dimethoxybenzene (DMB),1,4-dibutoxybenzene (DBB)and 1,4-dioctyloxybenzene (DOB).It was found that the yield of copillar[5]arenes could be increased using hydroquinone diether with longer aliphatic chains.
Subsequently,many mono-functionalized pillar[5]arenes were synthesized from two different monomers by co-cyclization (Fig.2).The bromoethane-modified pillar[5]areneH1was synthesizedviaco-cyclization by Stoddart and co-workers in 2011 [62].CompoundH1was prepared by the condensation of 5.0 equiv.of DMB with 1.0 equiv.of 1-(2-bromoethoxy)-4-methoxybenzene in the presence of BF3·OEt2and paraformaldehyde.The Br group onH1can be replaced by an azide group to obtain azide-modified pillar[5]areneH2.Pyrene-modified pillar[5]areneH3was prepared fromH2viacopper(Ⅰ)-catalyzed azide-alkyne cycloaddition (Fig.2a).However,the difficult purification and low yield of the cocyclization product limited the applications of mono-functionalized pillar[5]arenes.
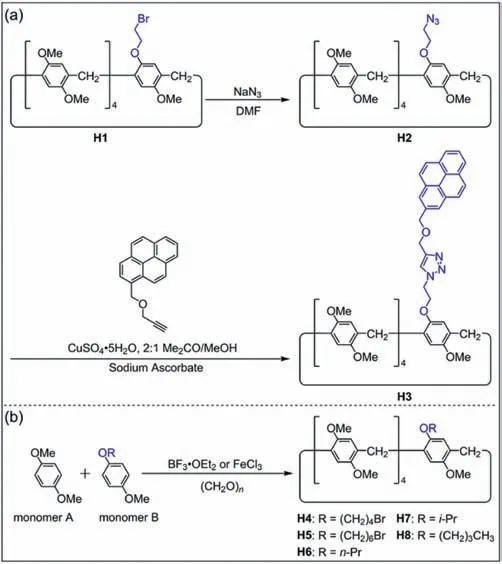
Fig.2. (a) Synthesis of pyrene-modified pillar[5]arene H3 [62].(b) Synthesis of mono-functionalized pillar[5]arenes H4-H8 by co-cyclization [56].
In order to improve the yield of mono-functionalized pillar[5]arene,Cao and co-workers optimized the reaction conditions [56].They found that the solvent,catalyst and the ratio of two monomers in the co-cyclization process affected the yield of mono-functionalized pillar[5]arenes.After screening various catalysts and solvents,it was found that FeCl3was more efficient than BF3·OEt2as the catalyst in the presence of paraformaldehyde and CH2Cl2.More importantly,the ratio of two monomers also had a great impact on the yield.When the ratios of two monomers were 8:1 and 16:1,yields of mono-functionalized pillar[5]arenes reached 64% and 85%,respectively.A series of mono-functionalized pillar[5]arenes were synthesized with high yields by this method(Fig.2b,H4-H8).It is noteworthy that co-cyclization is not suitable for the synthesis of high-level pillar[n]arenes (n≥6) because functional groups will hinder the co-cyclization of monomers [76,77].
2.2. Deprotection
In addition to the direct synthesis of mono-functionalized pillar[5]arenes through co-cyclization,functional groups can also be introduced into pillar[5]arenes by deprotection.Demethoxylation is an effective strategy to obtain post-functionalized pillar[n]arenes,because the hydroxyl groups obtained by demethoxylation are favorable reactive groups for the introduction of various functional groups [78–82].Ogoshi and co-workers synthesizedDMP[5]Aand then deprotected all methoxy groups ofDMP[5]Awith excess BBr3(20 equiv.ofDMP[5]A) to obtain a per-hydroxylated pillar[5]arene[9].In order to obtain mono-hydroxylated pillar[5]arene,they demethylatedDMP[5]Ain the presence of 0.9 equiv.of BBr3to afford mono-hydroxylated pillar[5]areneH9in moderate yield (Fig.3a) [61].The mono-functionalized pillar[5]areneH10was produced by etherification betweenH9and octyltrimethyl ammonium hexafluorophosphate.
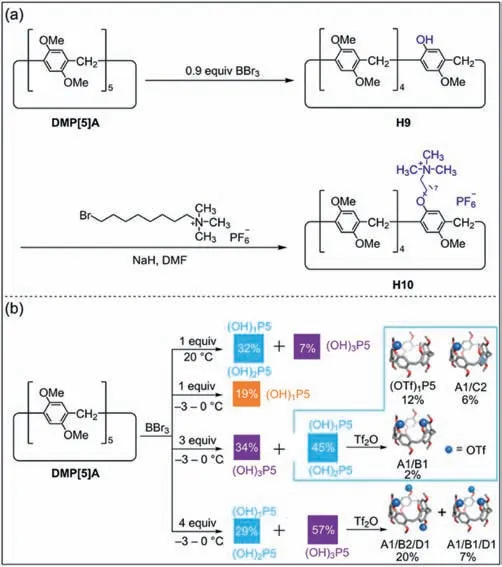
Fig.3. (a) Synthesis of mono-functionalized pillar[5]arenes H10 by deprotection[61].(b) Synthesis of mono-,di-,and tri-functionalized pillar[5]arenes.Copied with permission [83].Copyright 2015,American Chemical Society.
The reaction temperature and time are also important factors that affect the yield ofH9.Cao and co-workers obtained the monohydroxylated pillar[5]areneH9with a yield of 60% by increasing the amount of BBr3,decreasing the reaction temperature and prolonging the reaction time [60].Stoddart and co-workers further investigated the effects of feed ratio,reaction temperature and reaction time on the formation ofH9by demethoxylation (Fig.3b) [83].They found that demethoxylation ofDMP[5]Aoccurred continuously under kinetic control to sequentially generate monohydroxylated pillar[5]arenes,di-hydroxylated pillar[5]arenes,until per-hydroxylated pillar[5]arenes.Therefore,the desired hydroxyl–substituted pillar[5]arenes can be obtained by controlling the feed ratio,reaction temperature and reaction time.However,regioisomers of hydroxyl–substituted pillar[5]arenes are unavoidably formed in practice,which makes it challenging to separate them.The introduction of trifluoromethanesulfonic anhydride groups into the hydroxyl moieties of the hydroxyl–substituted mixtures can enhance their polar differences,allowing triflate derivatives of pillar[5]arenes to be separated by silica gel chromatography.Deprotection of pillar[n]arenes can not only be used to prepare monofunctionalized pillar[5]arenes but also mono-functionalized pillar[6]arenes [84].However,there are few examples of high-level mono-functionalized pillar[n]arenes (n≥7) due to the low yield.
Mono-functionalized pillar[5]arenes are efficient building blocks for producing pillar[5]arene dimers,trimers and tetramers [85–88].Ogoshi and co-workers synthesized a pillar[5]arene dimerH11via1,4-bis(bromomethyl)benzene andH9(Fig.4a) [89].Li and co-workers also obtained a pillar[5]arene dimerH12by 1,2-bis(bromomethyl)-benzene andH9[90].Yang and coworkers used 9,10-distyrylanthracene to react withH4to produce a 9,10-distyrylanthracene bridged pillar[5]arene dimer [91].The bromobutane-modified pillar[5]areneH4can also react with Na2Se2to obtain selenium-containing pillar[5]arene dimers [92].The Se functional group can serve as a redox block to generate responsive systems.However,the yield of pillar[5]arene dimers was not ideal.To improve the yield of pillar[5]arene dimers,Yang and co-workers designed a sulfhydryl-modified pillar[5]arene,and obtained a high yield (94%) of disulfide-bridged pillar[5]areneviaI2oxidation [93].Zhao and co-workers synthesized pillar[5]arene trimerH13by copper(Ⅰ)-catalyzed azide-alkyne cycloaddition between azide-modified pillar[5]areneH2and tripropargylamine(Fig.4b) [94].Wang and co-workers also prepared pillar[5]arene tetramerH14with tetraphenylethene (TPE) as the core throughH2and a TPE derivative [95].
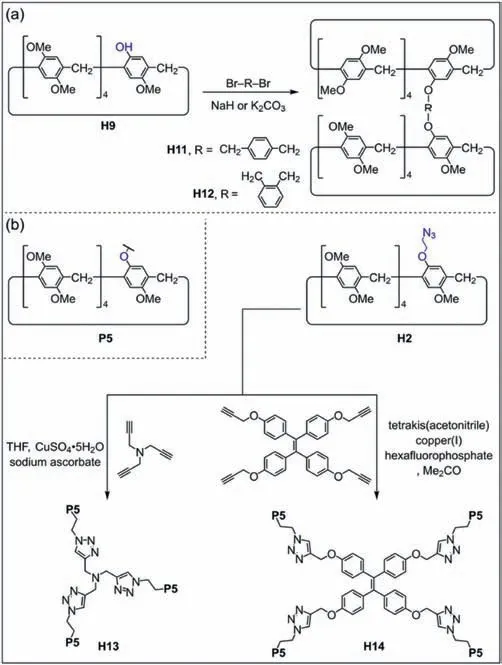
Fig.4. (a) Synthesis of pillar[5]arene dimers H11 and H12 by mono-hydroxylated pillar[5]arene H9 [89,90].(b) Synthesis of pillar[5]arene trimer H13 and tetramer H14 by copper(Ⅰ)-catalyzed azide-alkyne cycloaddition [94,95].
The two methods of synthesizing mono-functionalized pillar[n]arenes have their advantages and limitations.The cocyclization can directly obtain mono-functionalized pillar[5]arenes through two different monomers,but it is difficult to prepare highlevel mono-functionalized pillar[n]arenes (n≥6).The deprotection method can obtain high-level mono-functionalized pillar[n]arenes.However,the regioisomers of hydroxyl–substituted pillar[5]arenes can lead to difficulties in the purification of mono-hydroxylated pillar[5]arene.
2.3. Host–guest properties
The pillar structure and electron-rich cavity of pillar[n]arenes give their unique host–guest properties [96–100].Pillar[5]arenes typically possess electron-rich cavities with a diameter of approximately 4.7 ˚A,suggesting their suitability for complexing linear molecules (Fig.5) [101–103].The host–guest complexation between pillar[n]arenes and guests is mainly driven by van der Waals,hydrogen bond,π-stacking and electrostatic interactions.
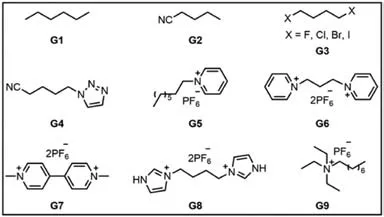
Fig.5. Typical structures of guests can interact with pillar[5]arenes by host–guest interactions.
Pillar[5]arene can form host–guest complexes with linear alkane (G1) through C–H…πinteractions.While the pillar[5]arene dimerH11can provide two binding sites and thus it has strong complexation withG1.But the bulky cyclohexane and branched alkanes barely interact with pillar[5]arene dimers [89].The introduction of electron-withdrawing groups (e.g.,cyano and halogen atoms) at the end of straight-chain alkanes can also enhance the stability of the host–guest complex [104,105].In general,the association constant of pillar[5]arenes with mononitrile (G2) is much lower than that of dinitrile,due to the synergistic effect of dinitrile on the multiple hydrogen bonds and dipole-dipole interactions of pillar[5]arenes [104].As the alkane chain increases,the association constant of pillar[5]arenes with dinitrile decreases obviously,because longer alkane chain generates weaker C–H…π,hydrogen bonds and dipole-dipole interactions (van der Waals forces)with pillar[5]arenes.Neutral dihaloalkane (G3) can also be used as a guest molecule to complex with pillar[5]arenes [105].The association constant increases with the increase of the polarizability ofG3(F<Cl<Br<I),which indicates that the interaction of dihaloalkane is greatly affected by the dispersion force.Triazole is a common unit in “click” chemistry,and the guest containing triazole (G4) can strongly complex with pillar[5]arenes [106].In order to improve the stability of host–guest complexes,electrondeficient cationic groups (such as pyridinium,G5) are often introduced at the end of linear alkanes [9].Li and co-workers investigated the complexation behavior of a series of bis(pyridinium)derivatives (G6) and paraquats (G7) with pillar[5]arenes,and found that solvent polarity had a significant effect on the complexation strength [107].When the solvent was acetone/DMSO (1:1),the association constant of pillar[5]arenes withG7increased by 7.3 times compared with that in pure acetone.Imidazole (G8) and amine(G9) can also interact with pillar[5]arenes to form stable host–guest complexes [108,109].The pillar[6]arene has a large cavity(6.7 ˚A),and thus can be complexed with larger alkanes such as 1,4-diazabicyclo[2.2.2]octane [84].
3.Applications of mono-functionalized pillar[n]arenes
Based on the above two synthetic methods,monofunctionalized pillar[n]arenes can be prepared more easily.Mono-functionalized pillar[5]arenes have specific functions through their electron-rich cavities and functional groups.The functionality and host–guest properties of mono-functionalized pillar[n]arenes make them widely used in many fields,such as supramolecular polymers,sensors,molecular machines,catalysis,biological applications and light-harvesting systems.
3.1. Supramolecular polymers
Supramolecular polymers are assembled from low-molecularweight monomers through non-covalent interactions,such as hydrogen bonds,π-πinteractions,hydrophobic interactions and metal-ligand bonds.Due to the dynamic properties of non-covalent bonds,supramolecular polymers have certain reversibility and stimuli responsiveness [110–115].The host–guest chemistry and special functional groups of mono-functionalized pillar[5]arenes make them widely used in supramolecular polymers [116].
Although C–H…πinteractions are weak hydrogen bonds,they play important roles in both chemical and biological systems[117,118].In 2011,Huang and co-workers reported octyl-modified pillar[5]areneH15based linear supramolecular polymers driven by C–H…πinteractions (Fig.6a) [119].The introduction of the octyl group enhances the solubility ofH15in most organic solvents and can act as a guest moiety to interact with anotherH15to form a linear supramolecular polymer (Fig.6b).As the concentration ofH15increased,its diffusion coefficient decreased gradually,which indicated that the supramolecular polymer was concentration dependence (Fig.6c).In addition,rod-shaped fibers were extracted from high-concentration monomer solutions and observed by scanning electron microscopy (SEM),which also provided a direct basis for the formation of supramolecular polymer (Fig.6d).This work provides a facile method for preparing supramolecular polymers based on C–H…π.
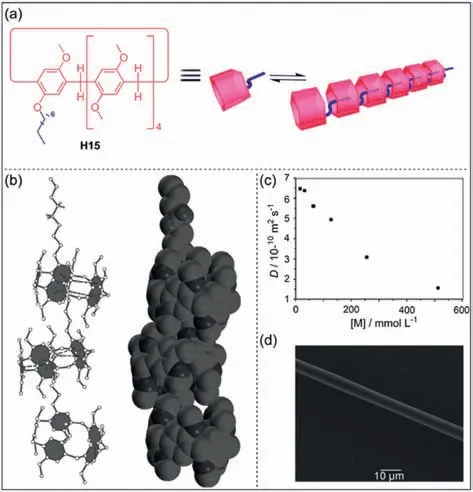
Fig.6. (a) Cartoon representation of octyl-modified pillar[5]arene H15 and the formation of supramolecular polymer.(b) Single crystal structures of supramolecular polymer.(c) Concentration dependence of diffusion coefficient D of H15.(d) SEM image of supramolecular polymer.Copied with permission [119].Copyright 2011,John Wiley and Sons,Inc.
Subsequently,Yang and co-workers synthesized doubledynamic polymers by anthracene-modified pillar[5]areneH16and imidazoleG10(Fig.7a) [120].Anthracene functional groups can dimerize at wavelengths greater than 350 nm,and dissociate when exposed to wavelengths less than 300 nm or by heating.Thus,dynamic polymers were obtained through the multiple hydrogen bonds betweenH16andG10.The depolymerization and polymerization of supramolecular polymers can be controlled by temperature and photoirradiation (Fig.7b).In addition,they reported a stiff stilbene-modified pillar[5]arene,which underwentZ-Eisomerization under light,leading to various aggregation behaviors [121].Due to the subtle correlation between monomer structures and aggregation behaviors,it is possible to design new photo-responsive supramolecular self-assemblies.
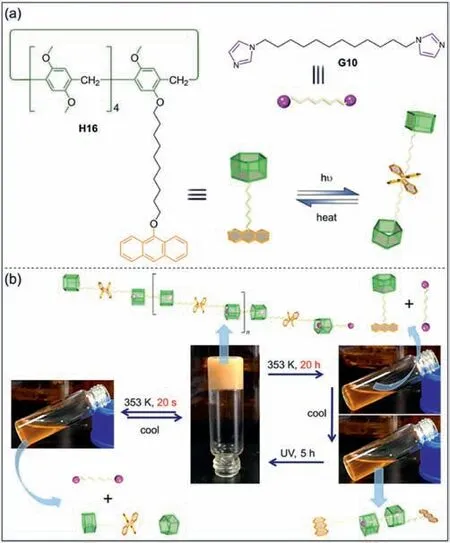
Fig.7. (a) Chemical structures of H16 and G10 and dimerization of H16.(b) Transition between gels and sols of supramolecular polymers composed of H16 and G10.Copied with permission [120].Copyright 2011,American Chemical Society.
The introduction of fluorescent groups in supramolecular polymers can construct stimuli-responsive supramolecular gel systems,which expands the application of pillararenes [122,123].In 2014,Yang and co-workers reported a TPE-functionalized pillar[5]arene tetramer with fluorescence enhancement [124].A supramolecular gel with strong blue fluorescence was formed after the interaction of the pillar[5]arene tetramer with the triazole-based neutral linker.The strong blue fluorescence was due to the restriction of internal rotation of the TPE after the formation of the supramolecular gel.This work provided new ideas for the design of fluorescent supramolecular gels based on pillar[5]arenes.
Compared with the network information,paper information has higher security and confidentiality,and the use of invisible ink can increase the security of paper information [125–127].Recently,Wang,Page,Wu,Sessler,Huang and co-workers prepared time-dependent invisible inks based on supramolecular networks(SN) (Fig.8a) [128].The mono-functionalized pillar[5]arene grafted polymerH17and the pentanenitrile grafted polymerH18formed photochromicSNthrough the host–guest interaction.The colorless spiropyran supramolecular network (SP-SN) was transformed into the corresponding violet merocyanine supramolecular network(MC-SN) under UV light.WhenG11was added,the polymer network was more dispersed,so the fading rate was accelerated after visible light irradiation (Fig.8b).Merocyanine is a kind of molecule affected by aggregation-induced emission (AIE),and fluorescence spectroscopic characterization was performed to investigate the effect ofG11on SN.Films of SP-SNand MC-SNwere prepared by solvent evaporation.Compared with the original MC-SNfilm,the emission intensity of MC-SN+G11film decreased sharply at 677 nm and blue-shifted (Fig.8c).The MC-SNfilm gradually transforms into SP-SNunder 460 nm UV light irradiation (Fig.8d).This demonstrated that the aggregation degree ofSNcould be regulated by adding the competitive guestG11.The spiropyran changed from the colorless state to the violet merocyanine under UV irradiation,resulting in purpleSNregion.The region ofSNcombined withG11faded fast under visible light,forming a Tai Chi pattern (Fig.8e).The mixture ofH17andH18could also be used as invisible ink to secure encrypted text and inkjet printing.This work may provide a method for preparing new anti-counterfeiting materials.
Both disulfide and diselenide bonds are valuable functional groups for their fast reactivity and potential bioactivity [92].Disulfide-bridged pillar[5]arene dimer and diselenide-bridged pillar[5]arene dimer have been successfully synthesized and used to prepare supramolecular polymers [92,93].Interestingly,these polymers exhibit fluorescent properties,which may expand applications in bioimaging and erasable paper.
The formation of sophisticated structures through hierarchical self-assembly has always been an attractive research topic in supramolecular chemistry [129].Supramolecular metallacycles can be constructed by coordination-driven self-assembly [130,131].An interesting example is the multiple stimuli-responsive supramolecular gels constructed by discrete hexakis-pillar[5]arene metallacycles (Fig.9a) [132].Yang and co-workers synthesized hexakis-pillar[5]arene metallacyclesH20andH21by six monofunctionalized pillar[5]arenesH19viametal coordination.MetallacyclesH20orH21can interact with the neutral dinitrile guestG14to form supramolecular gels through host–guest interactions(Fig.9b).It is one of the few stimuli-responsive supramolecular gels based on multi-pillar[5]arene derivatives,where macroscopic changes can be visualized under the stimuli of temperature,halide and competitive guest.This study provides a new method for the efficient preparation of smart materials and broadens the application of hierarchical self-assembly.
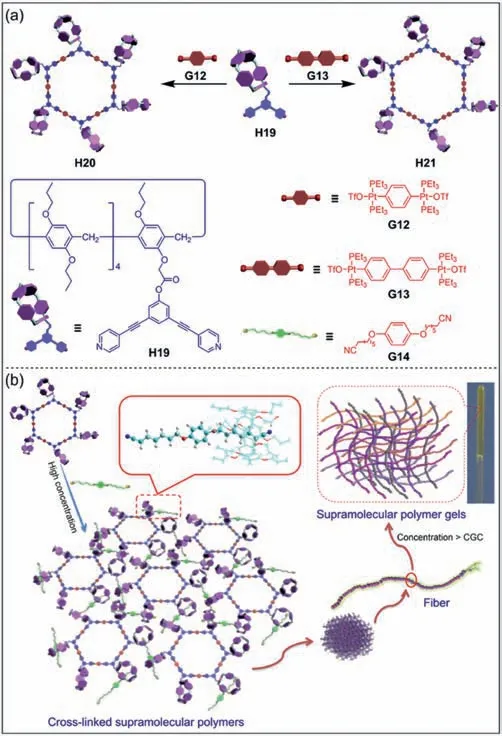
Fig.9. (a) Schematic illustration of the formation of self-assembled hexakispillar[5]arene metallacycles H20 and H21.(b) Cartoon representation of the formation of supramolecular polymer gels.Copied with permission [132].Copyright 2014,American Chemical Society.
Apart from pillar[5]arenes,pillar[6]arenes have also been used to prepare supramolecular polymers [133].Wang and co-workers prepared mono-functionalized pillar[6]arene-based supramolecular polymers and further constructed stimuli-responsive materials with redox properties [134].A supramolecular network can be produced by mixing the pillar[6]arene-based polymer with the ferrocene functionalized polymer based on host–guest interactions.Furthermore,the supramolecular network can be controlled by external stimuli such as redox and competitive guests.Wang and co-workers prepared pillar[6]arene-terminal-modified poly(N-isopropylacrylamide)H22and ferrocene-terminal-modified methoxy–poly(ethylene glycol)G15(Fig.10a) [135].The host–guest complexH22⊃G15exhibits hydrophilicity at 25 °C.When the temperature is raised to 37 °C,theH22⊃G15will show amphiphilicity.Moreover,the amphiphilicH22⊃G15can further assemble into supramolecular polymer vesicles in water.The size of the vesicles increased from 104 nm to 207 nm after loading DOX·HCl(Fig.10b).The DOX·HCl-loaded vesicles can control the release by cooling down to 25 °C or adding oxidizing agent AgNO3(Fig.10c).This supramolecular vesicle system is expected to be used in drug delivery system.
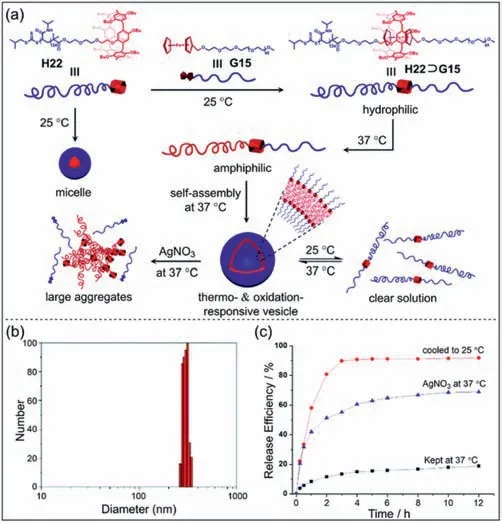
Fig.10. (a) Chemical structures of H22, G15,and schematic illustration of thermaland oxidation-responsive of supramolecular vesicle H22⊃G15.(b) Particle size distribution curve of DOX·HCl-loaded vesicles.(c) The cumulative release curves of DOX·HCl-loaded vesicles in different conditions.Copied with permission [135].Copyright 2017,Royal Society of Chemistry.
The dynamic responsiveness and reversibility of supramolecular polymers based on mono-functionalized pillar[n]arenes have been widely studied.With the further understanding of the properties of supramolecular polymers based on mono-functionalized pillar[n]arenes,they are expected to play crucial roles in degradable plastics,controllable drug release and anti-counterfeiting materials.
3.2. Sensors
Materials with sensing potential can be constructed by modifying photo-responsive units or fluorescent units on pillar[n]arenes.Due to the reversibility of non-covalent bonds,the aggregation behavior and energy transfer of fluorescent systems can often be tuned through host–guest interactions of pillar[n]arenes [136–138].
Azobenzene and its derivatives can undergo reversiblecis-transisomer changes under alternating UV and visible light irradiation[139].Stoddart and co-workers synthesized azobenzene-modified pillar[5]areneH23by the copper(Ⅰ)-catalyzed azide-alkyne cycloaddition between 4–hydroxy-4'-ethynyl-azobenzene and azidemodified pillar[5]arene (Fig.11a) [140].The azobenzene ofH23was in thecis-structure under visible light,thus forming vesicles.WhenH23was irradiated with UV light,the azobenzene changed from thecis-totrans-isomerizationH24,shortening the molecular length,which caused vesicles to reorganize into nanoparticles.UV–vis spectra was performed to demonstrate the occurrence of isomerization ofH23.The intensity of theπ-π∗absorption band decreased,while the intensity of then-π∗absorption band increased,indicatingtrans-tocis-isomerization (Figs.11b and c).They also synthesized pyrene-modified pillar[5]arene for the detection of low-concentrations of alkylamines and alkanediamines[62].The fluorescence of pyrene-modified pillar[5]arene could be quenched through photoinduced electron transfer when alkanediamines were added.However,the single-terminal amine group had little effect on fluorescence,which may be caused by the orientation of the amine terminal (toward or away from pyrene).This indicates the potential application of mono-functionalized pillar[5]arenes in sensors.
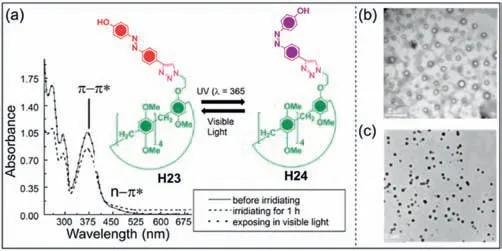
Fig.11. UV–vis spectra of H23 in mixed solvents and its variable conformation regulated by UV and visible lights.(b) TEM image of H23 in visible light (c) TEM image of H24 in UV light.Copied with permission [140].Copyright 2011,the Royal Society of Chemistry.
Rhodamine B is widely used in the preparation of fluorescent probes and supramolecular host–guest systems [141–143].Sun,Zhou,Yang and co-workers prepared a novel supramolecular Föster resonance energy transfer (FRET) system by rhodamine B functionalized pillar[5]areneH25and cyano-modified boron dipyrrometheneG16(Fig.12a) [144].The FRET systemH25⊃G16formed by the multiple hydrogen bonds ofH25withG16can be controlled by adding trifluoroacetic acid (TFA) and triethylamine(TEA).In order to further understand the acid-base responsiveness,the UV–vis spectra ofH25andG16under different conditions were studied.When TFA was added,H25was protonated toH26.Therefore,a new absorption peak appeared at 554 nm and theH25of solution changed from colorless to red (Fig.12b).A similar phenomenon was observed forG16(Fig.12c).Additionally,H25⊃G16can self-assemble into nanoparticles in acetone,resulting in fluorescence quenching of host–guest complexes.This research is significant to the design of molecular sensors based on pillar[n]arene and fluorophores.
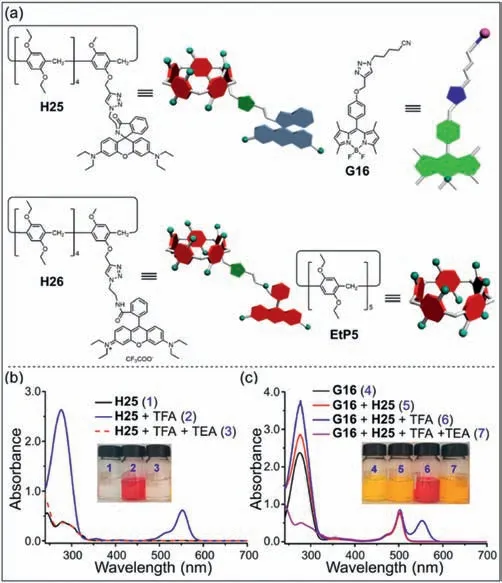
Fig.12. (a) Structures and cartoon representations of H25, H26, G16 and EtP5.UV–vis absorption spectra of (b) H25 and (c) G16 under different conditions.Copied with permission [144].Copyright 2018,American Chemical Society.
Paraquat and its derivatives are easily soluble in water and highly toxic,while most fluorescent sensors can only detect paraquat but are difficult to removal paraquat [145,146].The instrumental separation method faces the problems of expensive equipment and high personnel cost,it is necessary to develop a simple and efficient method for the detection and removal of paraquat.Lin and co-workers prepared a novel tripodalpillar[5]arene [147].Two adjacent pillar[5]arenes bound to a paraquat guest,thus creating a “synergistic effect” to achieve fluorescence sensing.Moreover,a test paper based on tripodalpillar[5]arene was prepared.The detection range for paraquat was 10−6∼10−1mol/L,which could be used as a simple and efficient test kit for the detection of paraquat in water.It is worth mentioning that due to the “synergistic effect”,the tripodal-pillar[5]arene had a significant adsorption capacity for paraquat in water,with the adsorption efficiency reaching 98.40%.The “synergistic effect”offers a new idea to design more sensitive paraquat sensors.
3.3. Molecular machines
On the basis of mono-functional pillar[5]arenes,molecular machines with stimuli-responsiveness have been developed [148].Recently,Wang,Wen,Yang and co-workers synthesized daisy chain dendrimers,which contained 21 [c2]daisy chain rotaxanes and were distributed within the dendrimer skeleton (Fig.13a) [149].The [c2]daisy chain rotaxane was composed of two urea-modified pillar[5]arenesH27through N–H…O hydrogen bonds.When polar solvents were added,hydrogen bond acceptors could interact with the urea group to change the [c2]daisy chain rotaxane from a contracted state to a stretched state.Therefore,the sizes of daisy chain dendrimers could be adjusted under solvent stimulation.A dynamic polymer film was constructed by adding daisy chain dendrimers into the polyvinylidene difluoride.The film folded rapidly in DMSO and returned to its original shape after being re-immersed in water (Fig.13b).This work provides a strategy for preparing new molecular machines based on daisy chain dendrimers.
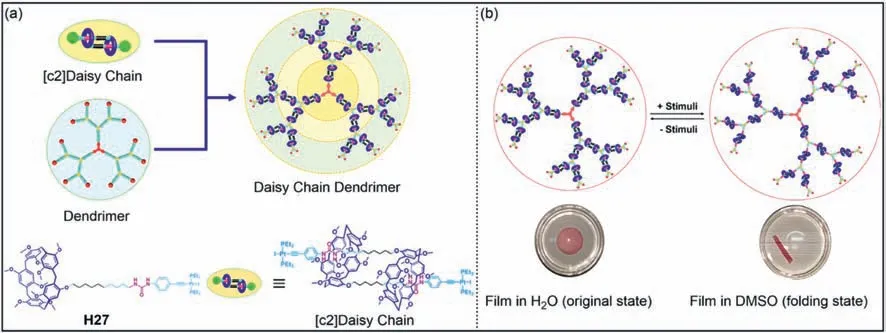
Fig.13. (a) Cartoon representation of a daisy chain dendrimer with [c2]daisy chain rotaxanes incorporated on the branches.(b) Schematic illustration of the proposed mechanism of the controllable shape transformations of daisy chain dendrimer-based polymer composite films.Copied with permission [149].Copyright 2020,American Chemical Society.
Mechanical bonds play important roles in building molecular machines [150].In 2019,Champness and co-workers synthesized dimers of rylene diimide dyes using mechanically interlocked handcuffs strategy (Fig.14a) [151].The design of the handcuff structure enabled perylene diimides (PDI,G17) and naphthalene diimides (NDI,G18) to interact without being constrained by rigid covalent bonds.In cyclic voltammetry (CV) experiments,the redox behavior ofH28⊃G17was significantly different from that ofmodel 1(Fig.14b).This suggests that theH28⊃G17dimer has unique electrical properties.The CV ofH28⊃G18was obviously more complicated thanH28⊃G17(Fig.14c).The reduction reaction ofH28⊃G18occurred in a more negative potential than that ofH28andmodel 2,indicating that there was molecular interaction between PDI and NDI.These results further demonstrated that the interactions inH28⊃G17andH28⊃G18,which were regulated by the redox state of the molecular handcuffs and were weaker than rigid covalent bonds.The design method of molecular handcuffs allows molecules to exhibit different optical and electrical properties without being constrained by rigid covalent bonds.
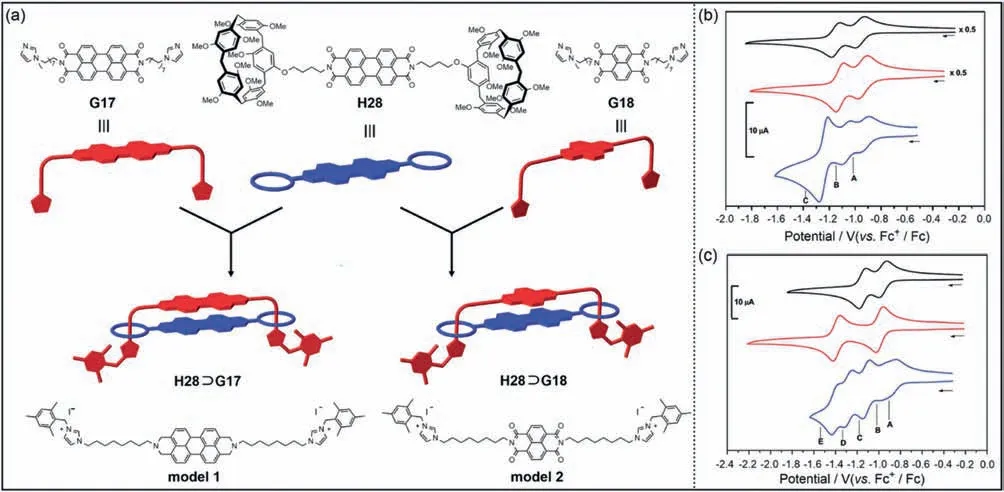
Fig.14. (a) Cartoon representation of synthetic molecular handcuffs by H28, G17 and G18.(b) Cyclic voltammetry of H28⊃G17 (blue) together with H28 (black) and model 1(red).(c) Cyclic voltammetry of H28⊃G18 (blue) together with H28 (black) and model 2 (red).Copied with permission [151].Copyright 2019,the Royal Society of Chemistry.
In addition,light can be employed as an energy source to power molecular machines without byproducts [152,153].Yang and coworkers designed a rotaxane-like structure containing stiff stilbene and pillar[5]arene [154].Photoisomerization of stiff stilbene induced the directional movement of rotaxane.The stiff stilbene can serve as a stopper to prevent the shedding of pillar[5]arene and as a chromophore to trigger the movement of pillar[5]arene.This work may be useful for understanding the correlation between molecular machines and conformational transitions.Huang and coworkers prepared a rotaxane dimer from an amino-modified pillar[5]arene,and then used bis(trifluoromethyl)phenyl isocyanate as a stopper to obtain a solvent-driven molecular spring [155].They discovered that the rotaxane dimer was contracted in CDCl3with a length of 31 ˚A and extended in DMSO–d6with a length of 37 ˚A.The length of the rotaxane dimer can change as a spring according to the polarity of the solvent.This study provides a method for simulating biological stretching of environmentally responsive supramolecular polymers.
3.4. Catalysis
Pillar[n]arenes have important applications in the field of supramolecular catalysis due to their unique cavity structure and functionality [156,157].Mono-functionalized pillar[n]arenes can be selectively introduced into catalytic units,providing highly efficient catalytic sites.The cavity of pillar[n]arenes can provide a good catalytic environment for catalytic reactions to improve catalytic effi-ciency.
Gold nanoparticles play important roles in nanomaterials due to their high chemical stability and excellent functional properties [158–160].The combination of gold nanoparticles with supramolecular macrocycles can enhance the overall catalytic performance and molecular recognition performance [161,162].Zhou,Chen,Jiang and co-workers modified pillar[5]arene onto gold nanoparticles to play a catalytic role [163].Gold nanoparticles can be modified by pillar[5]arene and anthracene derivative to form gold assemblies.The reduction reaction of 2,6-dichloro-4-nitrophenol can be catalyzed by gold assemblies,and the depolymerization of gold assemblies make it easier to separate from the catalytic system.
Collisional diffusion between excited photoredox catalysts (PRC)and the substrate limits the photo-induced electron transfer efficiency [164,165].Esser and co-worker reported that PRCpillar[5]arenes could pre-associate with the substrate,significantly shortening the reaction time (Fig.15a) [166].In order to further study the catalytic efficiency of PRC-pillar[5]arenes,three catalysts with different linkers,H29,H30andH31,were prepared (Fig.15b).4-Bromobutyrate (G19) and 5-bromovaleronitrile (G20) were used as substrates for the catalytic reaction,and the catalytic efficiencies of the three catalysts were explored (Fig.15c).The results showed that the addition of methyl phenothiazine (MePT) andDMP[5]Adid not accelerate the reaction (entry 3 in Fig.15c).Compared with phenyl phenothiazine (PhPT) and MePT,the catalytic yields ofH29,H30andH31increased by 1.6∼3.8 times (entries 5–7 in Fig.15c).This study proposes the concept of substrate pre-association in the photocatalytic system.The construction of this photocatalytic system can be applied to different types of catalysts to achieve more effective photocatalysis.
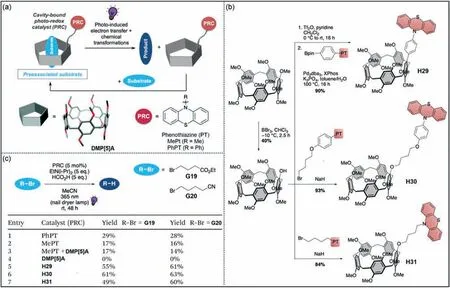
Fig.15. (a) Schematic representation of photoredox-catalyzed reductive dehalogenation.(b) Synthesis of three photocatalysts H29, H30 and H31.(c) Comparison of catalytic reactions with different catalysts.Copied with permission [166].Copyright 2021,the Royal Society of Chemistry.
Recently,Zhao,Wang,Yao and co-workers prepared a hybrid supramolecular polymer based on acyldrazone-functionalized pillar[5]arene [167].The porous structure of the hybrid supramolecular polymer was observed by SEM and TEM.In practical applications,the efficiency and recyclability of catalysts are important indexes for evaluating their performances.Using hybrid supramolecular polymer as catalyst to reduce nitrobenzene,the catalytic conversion rate of 93% was still reached after 5 cycles,and the morphology remained stable.This provides a new approach for the application and development of supramolecular polymers based on mono-functionalized pillar[5]arenes.
With high extinction coefficient and quantum yield,perovskite quantum dots are potential photocatalytic materials [168,169].The cavity structure and host–guest properties of pillar[n]arenes provide good opportunities for preparing photocatalytic perovskite materials.Lu,Wang and co-workers designed supramolecular assemblies composed ofH32withG21through host–guest interactions (Fig.16a) [170].TheH32⊃G21had thymine unit,which could further coordinate with Pb2+to formPb2+@H32⊃G21,and then add Cs-oleate to generateCsPbBr3@H32⊃G21.CompoundCsPbBr3@H32⊃G21has a multilayer nanosheet structure.It was noteworthy that eosin Y had been used as a photocatalyst in crosscoupling hydrogen evolution reactions,but its absorption in the UV region was very small [171].TheCsPbBr3@H32⊃G21had a strong absorption in the UV–vis region.Therefore,compared with the eosin Y alone,the use ofCsPbBr3@H32⊃G21significantly increased the yield of the catalytic product (Fig.16b).Gas chromatography analysis showed thatCsPbBr3@H32⊃G21had good photocatalytic activity,and its yield was more than twice than that of eosin Y alone.This work provides the possibility of using perovskite quantum dots to prepare supramolecular photocatalysts.
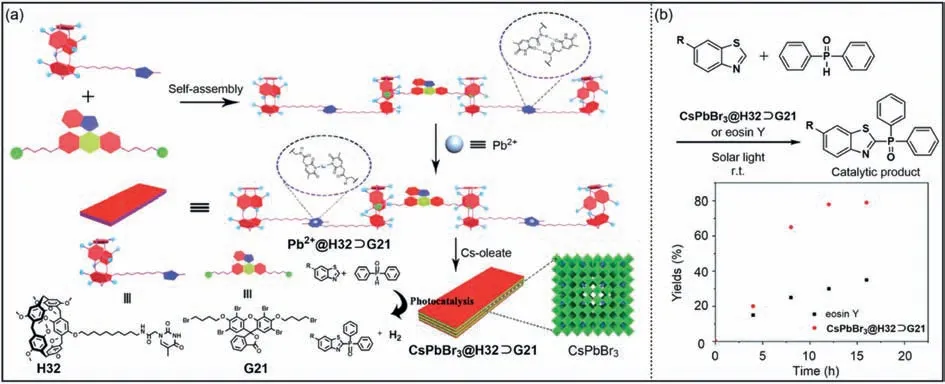
Fig.16. (a) Chemical structures of H32 and G21 and cartoon representation of the self-assembly and photocatalytic hydrogen evolution reaction processes of CsPbBr3@H32⊃G21.(b) Yield of catalytic product generated by cross-coupling hydrogen evolution reaction using eosin Y and CsPbBr3@H32⊃G21.Copied with permission[170].Copyright 2019,the Royal Society of Chemistry.
3.5. Biological application
The anticancer effect of oxygen (O2)-dependent therapy is significantly limited by tumor hypoxia [172,173].Organometallic drug complexes have stable and efficientinsituO2delivery [174,175].Fan,Tian and co-workers synthesized a ruthenium (II)-coordinated supramolecular metallodrug complex (RuSMDC) through the host–guest interaction ofH33withG22(Fig.17a) [176].The guestG22has two functional units,one is the Ru center that catalyzes the decomposition of H2O2into O2,and the other is the curcumin(Cur) unit that controls cell proliferation and apoptosis.The hostH33has a camptothecin (CPT) functional unit that can inhibit DNA synthesis.Furthermore,RuSMDCcan self-assemble into Rucontaining supramolecular metallodrug micelles (RuSMDMs).To explore the catalytic activity ofRuSMDMson the decomposition of H2O2into O2,Ru-doped supramolecular drug micelles (Ru-doped SDMs) were prepared as the control group.The cumulative release of O2increased rapidly after addingRuSMDMsto the H2O2solution,while the H2O2solution withoutRuSMDMsproduced almost no O2(Fig.17b).The antitumor effect ofRuSMDMswas then evaluated by tail vein injection in B16 tumor-bearing mice.Treatment ofRuSMDMs+light showed the best inhibitory effect on tumor growth (Fig.17c).This may be becauseRuSMDMscan effectively provide O2inside cancer cells to alleviate the hypoxic environment of tumor tissues.A large amount of O2produced byRuSMDMscan provide cytotoxic1O2to inhibit tumor growth.This study provides a new approach to circumvent the self-limitation of O2-dependent therapy due to tumor hypoxia.
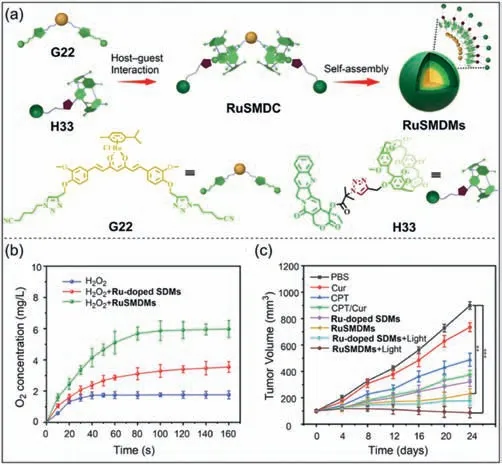
Fig.17. (a) Schematic illustration of the formation of RuSMDC and its self-assembly into RuSMDMs.(b) Time-dependent O2 production of RuSMDMs.(c) Relationship between time and tumor volume after different conditions.Copied with permission[176].Copyright 2021,John Wiley and Sons,Inc.
Supramolecular block copolymers are formed by linking different polymer chains through non-covalent interactions and have tunable and reversible properties [177].They show good potential for PDT [178].Xu,Cao,Zhang and co-workers encapsulated the photosensitizer pyropheophorbide-a (Pha) in a copolymer composed of a pillar[5]arene-modified polymer and a viologen salt[179].In cell experiments,nanoparticles formed by Pha-loaded copolymer showed excellent PDT efficacy and low dark toxicity.
Pt-based metallacycles can be combined with AIEgens to improve optical properties [180–182].Yang,Stang,Sun and coworkers obtained the supramolecular photosensitizerH36⊃G23through the host–guest interaction betweenH36andG23(Fig.18a)[183].PhotosensitizerH36⊃G23had a significant enhancement of AIE and a better capacity of cytotoxic ROS generation thanG23alone.For improving the stability and targeting of the photosensitizer,H36⊃G23was incorporated into DSPE-PEG5000 to prepareH36⊃G23nanoparticles (H36⊃G23NPs).BothS.aureusandE.colifluoresced after incubation withH36⊃G23NPs for 2 h,indicating that they may be used as fluorescent labels and photodynamic agents for bacteria.The changes of fluorescence intensity showed thatH36⊃G23NPs was accumulated at the infection site and had good vascular permeability (Fig.18b).TheH36⊃G23NPs were then intravenously injected into mice bearingS.aureus.TheS.aureusinfected wound area of the control group did not change significantly within 4 d after injection,while the wound area was significantly reduced in the presence ofH36⊃G23NPs and light (Fig.18c).Notably,the wound inS.aureus-infected mice was nearly healed under 6 d of light andH36⊃G23NPs treatment,while the wound in mice without light showed no significant change.These results provide new ideas for developing effective photosensitizers based on Pt metallacycles for biological applications.
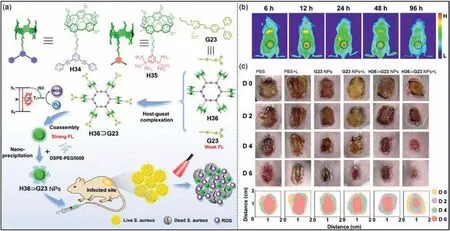
Fig.18. (a) Chemical structures of H34, H35, H36, G23,and supramolecular photosensitizer H36⊃G23 for effective photodynamic inactivation.(b) Fluorescence imaging of mice infected with S. aureus after the injection of H36⊃G23 for 6 h,12 h,24 h,48 h and 96 h.(c) Photographs of wounds infected with S. aureus after different treatments at 0,2,4,and 6 d.Copied with permission [183].Copyright 2022,John Wiley and Sons,Inc.
Fluorescence imaging is an important biotechnology,however,the aggregation-caused quenching effect of traditional organic fluorophores often limits the further application of fluorescent substances [184].TPE unit is a typical group with AIE phenomenon,which has been widely studied in fluorescent materials [185,186].Yu and co-workers constructed a luminescent agent based on mono-functionalized pillar[5]areneH37and TPE derivativeG24(Fig.19a) [187].Due to the high sensitivity and stability,H37⊃G24was further used to cell imaging.When HeLa cells were cultured withH37⊃G24for 4 h,it was observed strong fluorescence in the cells (Fig.19b).Fluorescence images confirmed thatH37⊃G24could penetrate cell membranes.This study demonstrates that the combination of AIEgens and host–guest chemistry holds great potential in drug delivery and cell imaging.
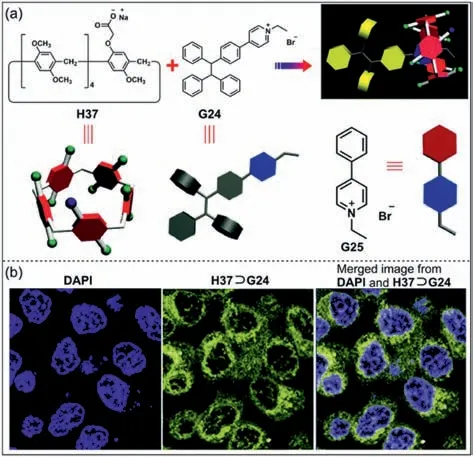
Fig.19. (a) Chemical structures of H37, G24 and model compound G25.(b) Confocal laser scanning microscope images of HeLa stained with DAPI and H37⊃G24.Copied with permission [187].Copyright 2016,the Royal Society of Chemistry.
Combination therapy is an effective strategy to solve the limitations of single-drug chemotherapy [188–191].Since the efficacy of different drug components varies,it is very important to realize the controlled release of drugs in combination therapy.Fan,Liu,Tian and co-workers reported a novel supramolecular drugdrug complexes (SDDC) that enabled the sequential release of two drugs in tumor cells (Fig.20a) [192].TheSDDCwere composed of cisplatin-modified pillar[5]arene dimers (H38) and cyanomethylpropionyl-camptothecin (G26) through host–guest interactions.Furthermore,SDDCcould form supramolecular vesicles (SVs)in water,showing a high carrying capacity.Following the delivery ofSVsto tumor cells,the anticancer effects ofH38andG26were significantly improved due to the host–guest dissociation and degradation of ester bonds.In addition,it can be clearly observed thatSVshas the strongest inhibitory effect on tumor with different drugs (Fig.20b),andSVsalso have the strongest tumor inhibition rate (Fig.20c).This study shows that two different drug molecules can significantly improve the efficiency of combination therapy through supramolecular systems.
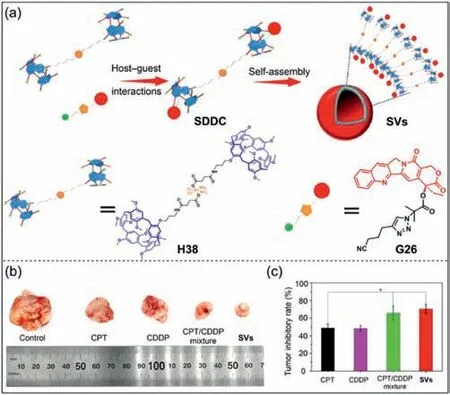
Fig.20. (a) Schematic illustration of the formation of supramolecular drug-drug complexes (SDDC) and their self-assembly into supramolecular vesicles (SVs) in water.(b) Photos of tumors treated in different ways.(c) Tumor inhibition rate under different conditions.Copied with permission [192].Copyright 2020,American Chemical Society.
Nanoclusters have been widely used in cell imaging and drug delivery because of their good biocompatibility [193,194].However,most nanoclusters have the problems of weak luminescence and low quantum yield.Khashab and co-workers prepared a class of silver nanoclusters (NCs) decorated with dithiolate lipoic acid terminated pillar[5]arenes (LA-P5) [195].TheLA-P5can interact with cetrimonium bromide surfactant (CTAB) through host–guest interaction,resulting in a nearly 2000-fold luminescence enhancement.After the interaction of the positively chargedCTABwith pillar[5]arenes,it can redistribute the charge on the surface ofNCsand alter their optical characteristics.TheNCshave good stability,which can be stable at room temperature for 4 months,so they have potential applications in biological imaging.This method provides a direction for preparing a new generation of metal nanoclusters for biological applications.
3.6. Light-harvesting systems
The light-harvesting system in nature is an effective method to solve energy problems by utilizing solar energy [196–199].Tang,Yang and co-workers reported the pillar[5]arene polymerH39containing methyl methacrylate,methacrylamide and vinyl-modified pillar[5]arene (Fig.21a) [200].PolymerH39formed supramolecular polymer networks (SPNs) with AIEgen TPE-(CN)4G27through host–guest interactions (Fig.21b).Due to the tunable host–guest interactions,the fluorescence intensity of SPNs can be regulated by a variety of factors,including different guest binding sites,solvents,competitive reagents and pillar[5]arene unit density.More interestingly,light-harvesting systems were produced when two distinct AIEgens TPE-(TA-CN)4G28and DSA-(TA-CN)2G29were incorporated intoH39concurrently.These light-harvesting systems can vary from cool to warm tones depending on the molar ratios of the two AIEgens (Fig 21c).Compared with supramolecular complexes constructed by small molecules,the strategy of combining AIEgens and polymers improves the stability and processability of fluorescent materials.
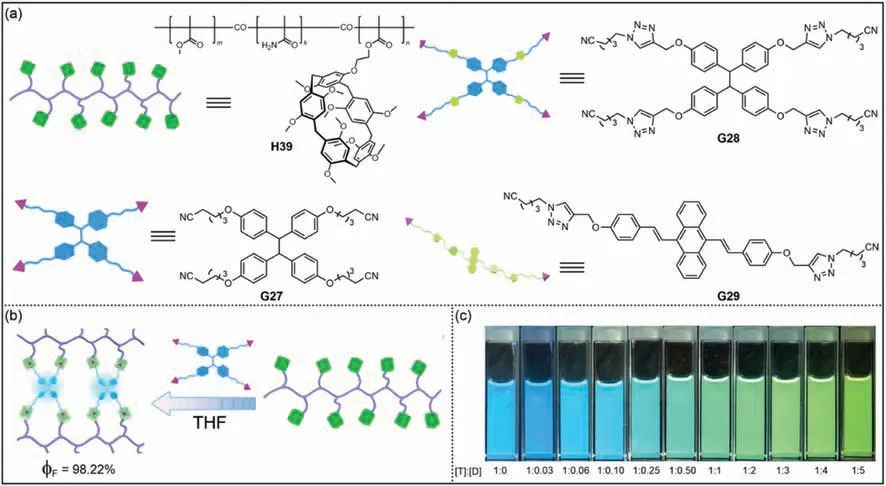
Fig.21. (a) Schematic representation of H39, G27, G28 and G29.(b) Schematic illustration of the construction of supramolecular polymer networks (SPNs) based on polymer H39 and AIEgen G27.(c) Color changes of SPNs with different ratios of TPE-(TA-CN)4 G28 and DSA-(TA-CN)2 G29,T and D denote as G28 and G29.Copied with permission[200].Copyright 2019,John Wiley and Sons,Inc.
Tang,Cao and co-workers constructed an artificial lightharvesting system composed of pillar[5]arene dimer [201].Pillar[5]arene dimer combined with two guests to form nanoparticles with enhanced energy transfer.Wang and co-workers prepared a light-harvesting system based on BODIPY bridged pillar[5]arene dimerH40,BODIPY guestsG30andG31(Fig.22a) [202].CompoundH40⊃G30andH40⊃G31both exhibited strong absorption from 300 nm to 700 nm,and showed slightly different FRET effects.To further study the energy transfer process,fluorescence spectra ofG30andG31were studied (Figs.22b and c).The emission peak ofH40at 522 nm gradually weakened with the addition ofG30,while the peak ofG30at 585 nm increased significantly.A similar phenomenon was observed forG31,and the change of fluorescent color after adding the guest could be directly observed by naked eyes.This work provided a new polymer model for the preparation of light-harvesting systems,and expanded the applications of pillar[n]arenes in optoelectronic materials.
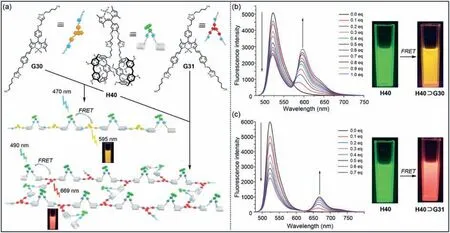
Fig.22. (a) Chemical structures of H40, G30 and G31 and cartoon representations of FRET systems of two functional supramolecular polymers.Fluorescence spectra of H40 with the addition of (b) G30;(c) G31.Copied with permission [202].Copyright 2015,the Royal Society of Chemistry.
4.Summary and outlook
In summary,the syntheses,host–guest properties and applications of mono-functionalized pillar[n]arenes are described.Cocyclization and deprotection of pillar[n]arenes are two methods that can be used to synthesize mono-functionalized pillar[n]arenes.Although the co-cyclization method is more convenient for the synthesis of mono-functionalized pillar[5]arenes,the functional group in the monomer will hinder the possibility of cocyclization.Thus,the high-level mono-pillar[n]arenes (n≥6) are synthesized mainly by the deprotection of pillar[n]arenes.Monofunctionalized pillar[n]arenes can be modified with new functional groups without changing the host–guest properties,and can also be used as building blocks to construct a series of pillar[n]arene dimers,trimers and tetramers.The unique properties of mono-functionalized pillar[n]arenes make them widely used in supramolecular polymers,sensors,molecular machines,catalysis,biological applications and light-harvesting systems.Although mono-functionalized pillar[n]arenes have shown great application prospects in many fields,there are still some challenges to be overcome in this rapid development process.
(Ⅰ) Despite the fact that there are two methods for the syntheses of mono-functionalized pillar[n]arenes,the products are mainly mixtures containing homologs of mono-functionalized pillar[n]arenes,and it is difficult to purify the products.Therefore,it is necessary to develop a more efficient method for large-scale synthesis of mono-functionalized pillar[n]arenes.
(Ⅱ) So far,mono-functionalized pillar[n]arenes are mainly focused on pillar[5]arenes,while there are few examples of pillar[6]arenes and high-level pillar[n]arenes.It is required to design and synthesize large cavity pillar[n]arenes (n≥6) and explore their applications since the large cavity structure will give pillar[n]arenes new properties.
(Ⅲ) Mono-functionalized pillar[n]arenes can be used to construct a variety of pillar[n]arenes-based polymer materials with good mechanical properties and stability.Topology plays an important role in many polymer materials,which can enhance the physical and chemical properties of polymers.It is also a challenging work to synthesize pillar[n]arenes-based polymer materials with topological structures (especially high-order topological structures)through simple and efficient methods.
(Ⅳ) Mono-functionalized pillar[n]arenes have specific functional groups and host–guest properties,which make them are useful in catalysis,biological applications and light-harvesting systems.In addition,the rigid pillar structure and functionality of mono-functionalized pillar[n]arenes allow them may be used as liquid crystal units to construct supramolecular liquid crystals.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank the National Natural Science Foundation of China (No.22101043),the Fundamental Research Funds for the Central Universities (No.N2205013) and Northeastern University for financial support.
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2023.108740.
 Chinese Chemical Letters2023年12期
Chinese Chemical Letters2023年12期
- Chinese Chemical Letters的其它文章
- Spin switching in corrole radical complex
- Benzothiadiazole-based materials for organic solar cells
- Recent advances in two-step energy transfer light-harvesting systems driven by non-covalent self-assembly✩
- From oxygenated monomers to well-defined low-carbon polymers
- Doping-induced charge transfer in conductive polymers
- Quinoline-based anti-MRSA agents: Current development,structure-activity relationships,and mechanisms
