Efficient water disinfection with ball milled Mg-biochar: The key roleof trace Cu
Yanhao Jin ,Suixiaohen Chen ,Peiwen Huang ,Xiongjian Chen,∗ ,Chun-Yan Lin ,Li-Ping Li ,Xiao Chen,Rui Ding,Jianxi Liu,Riyao Chen,∗
a College of Environmental and Resource Sciences,Fujian Normal University,Fuzhou 350117,China
b School of Materials and Chemical Engineering,Minjiang University,Fuzhou 350108,China
c Research and Development Center for Watershed Environmental Eco-Engineering,Beijing Normal University,Zhuhai 519087,China
d Fujian Key Laboratory of Pollution Control & Resource Reuse,Fuzhou 350007,China
Keywords:Disinfection Magnesium Cu Hydrogen peroxide Biochar
ABSTRACT In this study,magnesium and coconut shell carbon (CSC) were prepared by a ball milled process and used for water disinfection with adsorbing tiny amounts of copper(II).Dissolved oxygen (DO) was reduced to hydrogen peroxide (H2O2) via a two-electron pathway by Mg corrosion.Cu(II) in the wastewater will be enriched on the CSC surface and efficiently catalyzes H2O2 for inactivating E. coli.The results show that E. coli with an initial concentration of approximately 106 CFU/mL was under the detection limit(<4 CFU/mL) within 15 min.All of the Cu(II) could be adsorbed by the composite and catalyzed H2O2 to different active species.The quenching experiments,electron spin resonance (ESR) capture measurements and the UV-vis spectroscopy detection confirmed the present of the hydroxyl radicals (•OH),superoxide radicals (•O2−) and Cu(III).Different with tradition Fenton like process,Cu(III),rather than radicals,played the major role during the Mg-CSC/Cu(II) process.In addition to the cellular membrane damage,most of the bacterial genomic DNA was also be degraded and the bacterial reactivation was avoided.The Mg-CSC/Cu(II) process also showed a satisfied disinfection performance in real wastewater treatment.Overall,this study provides a new strategy for water disinfection.
Millions of people die annually from diarrhea caused by waterborne bacteria and enteric viruses,which are transmitted through polluted water and human excreta [1].Advanced oxidation processes (AOPs),such as O3[2],H2O2and persulfate [3] have become a very active research field of water disinfection due to their high efficiency.Particularly hydrogen peroxide (H2O2),which is considered to be a more promising sterilant,because of its advantage of no chemical residues [1,4].However,the sporicidal activity of H2O2is not very efficient without catalysts,which limits its application in disinfection [5,6].
Activating H2O2to more strongly oxidizing reactive oxygen species (ROS) to realize efficient water treatment is developed rapidly,including Fenton,Fenton-like and photo-Fenton processes[7–9].However,most studies were based on adding catalysts or consuming a large amount of energy,such as doped metal catalysts[10] or co-UV [11],which may lead to secondary contamination or a high cost [12,13].Trace metal ions such as Fe(III) or Cu(II) is commonly present in natural water or wastewater [14].Compared with Fe(III),Cu(II) is more soluble in a wide pH range (3∼7) [15,16],causing great research interests recently [17].On the other hand,copper is toxic to humans even in trace amounts.Its accumulation in human body can cause abdominal pain,vomiting,and even movement and neurological dysfunction [18].Removing trace copper from wastewater and enriching it to efficiently catalyze H2O2for disinfection is very meaningful.Fenton like mechanisms,such as generating•OH and•O2−as H2O2catalyzed by Cu(I)/Cu(II) is commonly used to explain the Cu(II)/H2O2system [19].While,it had been reported that a higher oxidation state of copper (Cu(III))might be generated in a certain condition [20].Thus,the disinfection mechanisms of Cu(II)/H2O2should be further clarified.
In addition to the catalysts,the H2O2resource is also particularly important.In 2018,the global H2O2consumption was approximate 6.5 million tons [21].Currently,the H2O2production still heavily relies on the anthraquinone process,which required large amount of organic reagents [22].Besides that,H2O2is unstable and dangerous during transportation and storage process [23].Then,synthesizing H2O2insituby electrochemical methods has attracted attention due to its mild reaction conditions,simple operation,and green reactants.Dissolved oxygen (DO) could be reduced to H2O2through the two-electron pathway or H2O through the four-electron pathway on the cathode surface (Eqs.1 and 2) [24].Carbon materials prefer the two-electron reduction process and are commonly used for H2O2electrochemical production [25].Jinet al.[26] used reticulated vitreous carbon (RVC) cathode to generate H2O2for water disinfection.About 106CFU/mLE.colibacteria were inactivated within 240 min.While,except the stable electricity supply,complex and expensive electrolysis instruments were required for H2O2electrosynthesis.
The standard electrode potential of the two-electron oxygen reduction reaction (ORR) is −0.146 V [27].If metals with more lower electrode potential,such as Mg (−2.372 V) contact with appropriate carbon materials,they will form a galvanic-type corrosion cell under certain conditions [28].Metal corrosion may drive the ORR process spontaneously.Compared with other carbon materials,such as CNTs which are very expensive and threaten human healthy [29],biochar,such as coconut shell carbon (CSC) which is a soil conditioner has the advantages of low cost and wide availability,has a wide application prospect for water treatment [30].When CSC and Mg are combined together,dissolved oxygen on the CSC surface can be reduced to H2O2driven by Mg corrosion.In addition,trace amounts of copper in the wastewater could be adsorbed by CSC due to its excellent adsorption performance [31].The enriched copper may active theinsitugenerated H2O2to provide a promising application strategy for water treatment.
This study aims to develop a novel water disinfection strategy that can produce H2O2insituand enrich tiny amounts of copper ions.For this purpose,the Mg-CSC composite was prepared and characterized.The disinfection performance of the Mg-CSC/Cu(II)was investigated.The active species in the disinfection process were analyzed by quenching experiments,electron spin resonance(ESR) capture measurements and the UV-vis spectrum scanning analysis.TheE.colireactivation was also analyzed.Finally,the influences of operating factors and the feasibility for real wastewater treatment had been investigated.
The Mg-CSC composites were prepared by a ball milling method.Specifically,a certain mass of Mg and CSC powder was added to a nylon jar,and ball milling was carried out at a rate of 250 rpm for 3 h with polyethylene glycol as the binder.After cooling to room temperature,the obtained composites were named as Mg-CSC.Argon was used to provide the inert atmosphere to avoid the zero-valent magnesium oxidation.Details of reagents can be found in Text S1 (Supporting information).
The surface morphology of the Mg-CSC composite was studied by scanning electron microscopy (SEM,Regulus 8100,Hitachi,Japan).Energy-dispersive spectrometry (EDS,Octane Elect Plus,EDAX,USA) was performed to investigate the elemental distribution.The crystallographic structure of the sample was established by X-ray diffraction (XRD,BrukerD8 Adv.,Germany).Reactive oxygen species (ROS) were detected by electron paramagnetic resonance (EPR,MS 5000X,Freiberg,Germany).Text S2 (Supporting information) shows detailed test methods and conditions.Bacterial enumeration and experimental procedures were recorded in Text S3 and Text S4 (Supporting information),respectively.In addition,Text S3 shows the procedures of DNA extraction and agarose gel electrophoresis.
The surface morphology of the Mg-CSC composites (prepared by ball milling) was investigated by SEM.As shown in Fig.S1(Supporting information),the surfaces of Mg and CSC before ball milling are smooth and free of impurities.The surface morphology of the Mg-CSC composite after ball milling is showed in Fig.S2(Supporting information),which clearly reveals that Mg and CSC are in direct contact and tightly bonded.The size of CSC is slightly smaller than that of Mg,which adheres to the Mg surface.Thus,ball milling promotes the effective combination of Mg and CSC.It is noteworthy that the tight combination of Mg and CSC is essential for electron transfer to generate H2O2.In addition,EDS was performed to determine the elemental distribution and relative element content of the Mg-CSC composite surface.As shown in Fig.1a,Mg showed the strongest signal,followed by carbon.It is because the mass ratio of Mg to CSC is 5 to 1 for the Mg-CSC composite preparation.A small amount of oxygen was also observed,due to a small part of surface Mg oxidation.The EDS results were also powerful evidence for the tight bonding of Mg and CSC in the Mg-CSC composite.
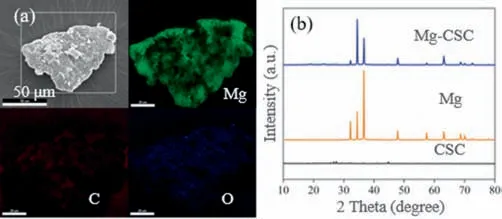
Fig.1. Elements mapping (a) and XRD spectra (b) of Mg-CSC composites.
The crystal structure of the obtained Mg-CSC composite was analyzed by XRD.As shown in Fig.1b,the main diffraction peaks of the Mg-CSC composite at 32.17°,34.39°,36.60°,47.81°,57.36°,63.06°,68.65°,and 69.99°correspond to the (100),(002),(101),(102),(110),(103),(112),and (201) crystal planes of Mg (JCPDS Card No.00-004-0770).The results suggested that Mg in the Mg-CSC composite was mainly present as the simple substance.In addition,the faint diffraction peaks of CSC at 26.74°,27.54°,and 44.82° might be caused by the impurities or byproducts generated during the preparation process.Fortunately,these were not the dominant species.The results of the XRD analysis also demonstrate that zero-valent Mg is the major component in the composites,indicating that the Mg-CSC composite was successfully synthesized.
The disinfection performance of the Mg-CSC/Cu(II) was clarified and showed in Fig.2a.E.colicould not be effectively inactivated by CSC powder or Mg powder alone (only 0.7 or 1.1 log removal).The slight decrease was ascribed to the surface adsorption of the CSC or Mg powder.The disinfection effect of Cu(II) alone was also virtually negligible (about 0.4 log removal).Similar to silver ion,the bacteriostatic ability of Cu(II) is thought to be the result of the action of Cu(I) generated by intracellular reducing agents with thiol groups,leading to protein denaturation [32].However,the Cu(II)concentration was too low to pose a serious threaten toE.coliin this study.Meanwhile,the disinfection efficiency was significantly enhanced,when the bacteria were treated by the CSC/Cu(II) process (3.8 log removal).No Cu(II) could be detected after several minutes (not present).It is because Cu(II) can be adsorbed by the CSC,resulting in a local high concentration of Cu(II) on the CSC surface and thus enhancing the antibacterial effect [33].Compared with CSC or Mg alone,the Mg-CSC composites demonstrated a better antibacterial activity (2.5 log removal within 30 min).As Mg was supported by CSC,oxygen could be reduced to H2O2driven by Mg corrosion.Its concentration was about 21.6 mg/L (Fig.2b).H2O2can destroy the cell membrane and freely diffuse into the cell.Then,it reacts with the internal irons to destroy the microorganisms by an inner Fenton process.As the damage accumulate,the lethal dose is reached,and a proportion of theE.coliis killed [34].On the other hand,E.colican protect against damage by expressing more catalases,which dissociates H2O2into H2O and O2[35].Thus,the disinfection rate was still very slow treated by the Mg-CSC composite.Once 2 μmol/L of Cu(II) was added to the water,the disinfection rate was significantly increased.Only 20 min was required to achieve the detection limit (about 5.6 log removal).Interestingly,only 4.3 log removal was achieved when the water was treated by a higher concentration of additional H2O2and Cu(II).It was because low concentrations of Cu(II) were difficult to trigger an efficient catalytic reaction to generate reactive species for disinfection.When the water was treated by the Mg-CSC composite,almost no Cu(II) was detected after 10 min,indicating that Cu(II)was adsorbed by the composite.Thus,the local Cu(II) concentration on the Mg-CSC surface was higher than that in the aqueous phase,which in turn led to an efficient catalytic reaction to inactiveE.coli.
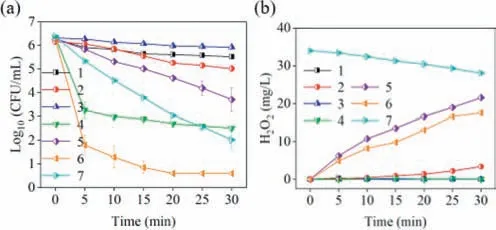
Fig.2. E. coli inactivation (a) and H2O2 concentration (b) in different system.(1 CSC=0.0834 g/L;2 Mg=0.334 g/L;3 Cu(II)=2 μmol/L;4 CSC=0.0834 g/L,Cu(II)=2 μmol/L;5 Mg-CSC=0.5 g/L;6 Mg-CSC=0.5 g/L,Cu(II)=2 μmol/L;7 H2O2=1 mmol/L,Cu(II)=2 μmol/L.Other parameters were set as follows: T=25°C,O2=400 mL/min,initial E. coli=∼106 CFU/mL,initial pH 5.8).
Hydroxyl radicals or superoxide radicals is generally considered as the active oxygen species in copper ion-mediated Fenton-like reactions (Eqs.3 and 4) [36].The contributions of different ROS in the Mg-CSC/Cu(II) disinfection process were investigated and the results were showed in Fig.3.Increasing the IPA concentration (scavenger of•OH) could gradually prevent theE.coliinactivation.Moreover,the characteristic peak of•OH (typically 1:2:2:1 peak in Fig.3d) was also detected.Although the influent of IPA was not significant,it revealed that•OH played a role for disinfection.As shown in Fig.3b,the inactivation rate ofE.coliwas also inhibited in the presence of TEMPOL (scavenger of•O2−) and the signal of•O2−existed in the EPR detection (Fig.3e).It has been confirmed that•O2−can block the iron-sulfur clusters of the intracellular biosynthetic enzymes through electrostatic interactions to killE.coli[37].In addition,•O2−could induce the cycling of Cu(II)/Cu(I) (Eqs.5 and 6) [38].Therefore,•O2−also played a role forE.coliinactivation.
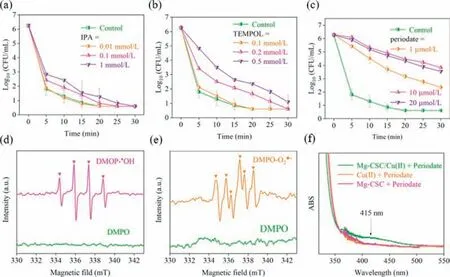
Fig.3. Influence of IPA (a),TEMPOL (b) and periodate (c) on the E. coli inactivation.EPR signals of DMPO-•OH (d) in water and DMPO-O2•−(e) in methanol solution.UV-vis spectra of Cu(III) (f).T=25 °C,O2=400 mL/min,initial E. coli=∼106 CFU/mL,initial pH 5.8,Mg-CSC=0.5 g/L,Cu(II)=4 μmol/L.
Except excited by light,previous studies have found that1O2can be generated by radical conversion [39].As L-histidine (scavenger of1O2) added to the water,E.coliinactivation rate decreased(Fig.S3a in Supporting information).However,the characteristic spectra of1O2were not detected by EPR (Fig.S3b in Supporting information).Previous study [40] found that L-histidine could form complexes with Cu(II).The concentration of free copper ions would be decreased and theE.coliinactivation would be inhibited.In addition,Luetal.[41] reported that the role of1O2may be overestimated due to the rapid quenching of1O2in water and the false positive results.Therefore,the role of1O2played forE.coliinactivation was negligible in this study.
In general,ROS could be generated by a Fenton like process in the Cu(II)/H2O2system.Cu(II) and Cu(I) react with H2O2to form•O2−and•OH (Eqs.3 and 4),respectively.However,recent studies suggest that a higher oxidation state of copper (Cu(III)) might be generated during the advanced oxidation processes [14].Cu(III) has strong oxidation ability and can degrade pollutants efficiently [42].As shown in Fig.3c,in the present of 1 μmol/L periodate (scavenger of Cu(III)),only 3.9 log removal was achieved after 30 min.As the dose of periodate raised to 10 μmol/L,the disinfection performance was further inhibited.Cu(III) can form complexes with periodate,and the Cu(III)-periodate complexes has a characteristic peak at 415 nm [43].As shown in Fig.3f,adding periodate showed a clear characteristic peak at 415 nm,indicating the presence of Cu(III) in the water.In fact,•OH was more likely to be generated under acidic conditions [44].However,the pH value increased from 5.8 to 8.9 within 30 min due to Mg corrosion (Fig.S4 in Supporting information).Cu(II) could be adsorbed by CSC and H2O2was also generated on the Mg-CSC surface,which was benefit to form Cu(I)and further form Cu(III) (Eqs.4 and 7).Compare with quenching ROS,quenching Cu(III) showed a more significant inhibition.Thus,Cu(III) rather than•OH,was the main active species for disinfection in the Mg-CSC/Cu(II) process.
In order to further investigated theE.colideath mechanisms,SEM,agarose gel electrophoresis and bacterial regrowth experiments were performed.As shown in Fig.4a,the cells before treatment were full.The microgrooves on the surface were evenly distributed,and the cell membrane was not damaged.However,bacterial shrinkage and cell membrane rupture were severe after treatment (Fig.4b).The cell membrane is an important protective barrier that ensures the stability of the intracellular environment.Phospholipids and proteins on the cell membrane can selectively prevent extracellular substances from entering the intracellular through permeation function [4].While,fatty acids on cell membranes,such as phospholipid bilayers and peptidoglycans,are extremely sensitive to peroxidation [4].
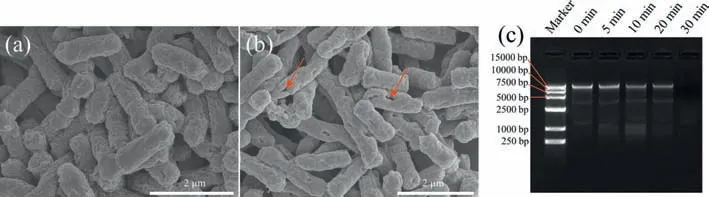
Fig.4. SEM images of E. coli before (a) and after (b) treating and DNA abundance (c) of E. coli inactivated by Mg-CSC/Cu(II).T=25 °C,O2=400 mL/min,initial E. coli=∼106 CFU/mL,initial pH 5.8,Mg-CSC=0.5 g/L,Cu(II)=4 μmol/L.
Active species,such as ROS and Cu(III) would preferentially attack the cell membrane.While,the microorganisms may become reactivated,if only the cell structure was destroyed [45,46].Fortunately,H2O2could freely enter the cells,and further attack the intracellular substances [36].It had been confirmed that DNA damage was more critical for complete disinfection [47].As shown in Fig.4c,the DNA degradation was becoming more serious as the treatment time increasing.The genomic DNA was completely degraded after 30 min.As the treatment time increased,more ROS entered the cells and broken the phosphodiester bonds in the DNA base pairs [48,49].The reactivation test was also conducted.As shown in Fig.S5 (Supporting information),no colony was found when the water was stored for 24 or 48 h.Thus,complete disinfection was achieved in this study.
The influences of different factors on disinfection had been investigated.When 0.1 g/L of Mg-CSC was added to the water,only about 5.3 log removal was achieved after 30 min (Fig.5a).Adding more Mg-CSC composite could generate more H2O2,which was benefit for active species generation (Fig.5b).As the dosage increased to 0.2 g/L,complete disinfection was achieved within 25 min.However,the disinfection rate was not obviously improved when the dosage increased to 0.5 or 1 g/L.It was because the damage accumulating to the lethal dose also required a certain time,due to the self-defense mechanisms of the bacteria.Cu(II) concentrations were also important for disinfection.As can be seen from Fig.5c,only approximately 4.5-log removal was achieved,when the Cu(II) dosage was 1 μmol/L.As it increased to 2 and 4 μmol/L,complete disinfection was achieved within 20 and 10 min,respectively.The influence of Cu(II) on H2O2was not significant (Fig.5d).More copper ions would be adsorbed by CSC,when the Cu(II) concentration was increased.It is favorable to form ROS and Cu(III).Moreover,the influence of DO on the disinfection process cannot be neglected.The disinfection rate was slow at the non-gas budding or air budding conditions (Fig.5e).It was because DO severely affect the H2O2production (Fig.5f),which in turn influenced theE.coliinactivation.
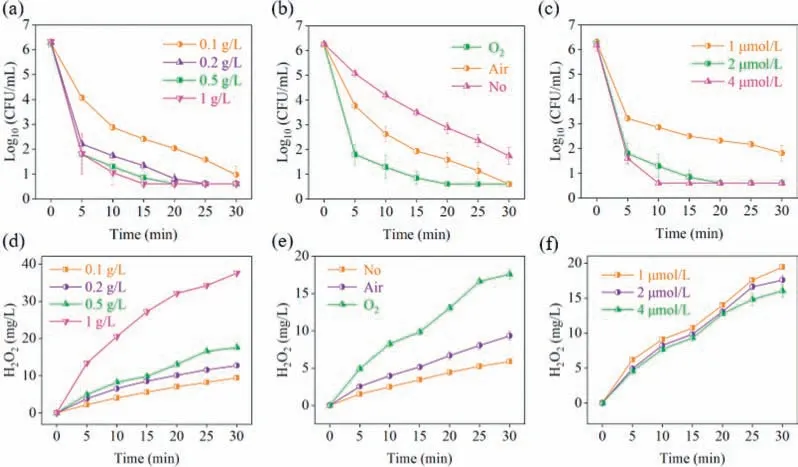
Fig.5. The E. coli inactivation and H2O2 generation during Mg-CSC/Cu(II) process with different Mg-CSC composites dosages (a,b),Cu(II) dosages (c,d) and budding conditions (e,f).T=25 °C,O2=400 mL/min,initial E. coli=∼106 CFU/mL,initial pH 5.8,Mg-CSC composite=0.5 g/L,Cu(II)=2 μmol/L.
Organic matters (e.g.,HA) are widely distributed in natural water.The effect of HA on theE.coliinactivation was investigated.As can be seen from Fig.6a,the disinfection rate had no obvious change,when 0.1 mg/L of HA was added to the water.However,E.coliinactivation was inhibited as the amount of HA was increased.When the HA concentration was increased from 1 mg/L to 2 mg/L,the inhibiting effort was more significant.As a kind of organic matters,HA could scavenge ROS [50].In addition,it also could quench Cu(III).Then,the disinfection efficiency was decreased.
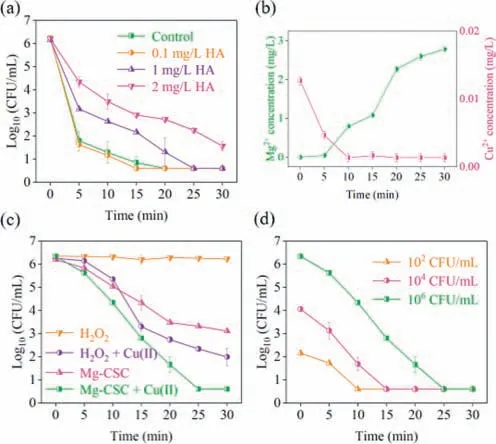
Fig.6. Influence of HA on E. coli inactivation (a) and the variation of Cu2+ and Mg2+ during the disinfection process (b).Disinfection performance in treating real wastewater (c,d).T=25 °C,O2=400 mL/min,initial E. coli=∼106 CFU/mL,initial pH 5.8,Mg-CSC composite=0.5 g/L,Cu(II)=2 μmol/L.
The Mg-CSC composite could absorb Cu2+.At the same time,Mg2+might be accumulated with Mg corrosion.The concentrations of Mg and Cu ions had been detected during the disinfection process.As shown in Fig.6b,no Cu ion could be detected after 10 min,indicating that Cu was adsorbed by the composite.The concentration of Mg ions increased as the water treatment proceeded.Pure Mg corrosion driven the dissolved oxygen reduction due to its low standard electrode potential (E°Mg2+/Mg=−2.372 Vvs.SHE)[34].Therefore,the Mg2+concentration increased.However,part of the Mg ions could be adsorbed on the CSC.Besides that,the pH value was increased (Fig.S4).The solubility of Mg2+is very small at alkaline condition.Thus,the concentration of residual Mg2+was lower than 3 mg/L,which met the water treatment standards.
The disinfection performance of Mg-CSC/Cu(II) for secondary effluent was also investigated.Although theE.coliconcentration decreased 4.2 orders of magnitude treated by the H2O2/Cu(II),complete disinfection could not be achieved (Fig.6c).Similarly,the disinfection with Mg-CSC alone was not complete.However,in the present of trace amounts of Cu(II),theE.coliconcentration was under the detection (5.6 log removal) within 25 min.Compared with simulated wastewater,the components of the real wastewater were complex (Table S1).The inorganic salts and organic matters in the wastewater could influence the generation of the active species [26].Complete disinfection was still achieved in a short time.The influence of the initialE.coliconcentration on real wastewater disinfection was also investigated (Fig.6d).The lower bacteria concentration,the less treatment time.In conclusion,the above studies clearly demonstrated that the Mg-CSC/Cu(II) system has a satisfied disinfection performance even under complex conditions.
In summary,a novel water disinfection was achieved by generating H2O2insituand adsorbing trace copper using the Mg-CSC composite.Driven by the spontaneous Mg corrosion,DO was reduced to H2O2on the CSC surface.In the presence of Cu(II),H2O2was decomposed to ROS (•OH and•O2−).In addition,Cu(I) was converted to Cu(III) due to ROS transformation and electron transfer.Cu(III) played the main role for the disinfection.Both the cell structure and genomic DNA were destroyed,andE.coliwas completely inactivated.The treatment time was reduced from 25 min to 15 min,when the Mg-CSC dosage increased from 0.2 mg/L to 1 mg/L.More Cu(II) in the water also enhanced the disinfection performance.The treatment time needed to reach the DL (approximately 5.6-log removal) decreased to 10 min with 4 μmol/L Cu(II).All of the copper was adsorbed by the composite,and the dissolved Mg2+met the water treatment standard.Satisfied disinfection was also achieved for treating the secondary effluent of the urban wastewater treatment plants.In conclusion,it is a sustainable strategy for wastewater disinfection.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We gratefully acknowledge the financial support of this research by the National Natural Science Foundation of China (No.22006016),the Key Project of Fujian Provincial Department of Science and Technology (Nos.2021Y0009 and 2019Y0010),the Natural Science Foundation of Fujian Province,China (No.2021J011026).
Supplementary material
Supplementary material related to this article can be found in the online version,at doi:10.1016/j.cclet.2023.108444.
 Chinese Chemical Letters2023年12期
Chinese Chemical Letters2023年12期
- Chinese Chemical Letters的其它文章
- Spin switching in corrole radical complex
- Benzothiadiazole-based materials for organic solar cells
- Mono-functionalized pillar[n]arenes: Syntheses,host–guest properties and applications✰
- Recent advances in two-step energy transfer light-harvesting systems driven by non-covalent self-assembly✩
- From oxygenated monomers to well-defined low-carbon polymers
- Doping-induced charge transfer in conductive polymers
