White light generation by regulating hydrogen bond-sensitive ESPT of naphthalimide dyes
Jin Li ,Qinglong Qio ,Ning Xu ,Wei Zhou ,Jingli Yun ,Zhocho Xu,c,∗
a State Key Laboratory of Fine Chemicals,School of Chemistry,Dalian University of Technology,Dalian 116024,China
b CAS Key Laboratory of Separation Science for Analytical Chemistry,Dalian Institute of Chemical Physics,Chinese Academy of Sciences,Dalian 116023,China
c University of Chinese Academy of Sciences,Beijing 100049,China
Keywords:White light ESPT Hydrogen bond Naphthalimide Double emission
ABSTRACT White light emitting systems of pure organic materials have attracted extensive research interest due to their better compatibility and functional scalability.The reported organic white light materials are mainly based on the multi-channel emission regulation of the compound itself or the mixing of multicolor luminescence materials,but studies on the dependence between multicolor luminescence and the external environment are lacking,which limits the application of these materials in areas such as identification and sensing.This paper reports that the 4-or 3–hydroxyl-substituted naphthalimides NapH1 and NapH2 form intermolecular hydrogen bonds with adjacent molecules in the environment,and undergo excitedstate intermolecular proton transfer under irradiation,resulting in blue-yellow or blue-red dual fluorescence emission,respectively.Since the two compounds have different two-color luminescence channels and the two-color intensity ratio is affected by the environment,and the intermolecular hydrogen bond is determined by the hydrogen bond receptor,polarity,and temperature in the environment,the full spectrum from blue to red light and white light emission can be obtained by adjusting the mixing ratio of the two dyes and the solvent polarity and the ambient temperature.This environmentally sensitive white emission is used to detect the alkalinity of different papers,and the dyed paper can be used as a test strip for acid-base vapor detection.
White light-emitting materials have attracted extensive attention due to their potential applications in optoelectronic devices,digital displays,lighting,and data encryption [1,2].Compared with inorganic materials,pure organic white light-emitting materials have many advantages,such as better compatibility,adjustability,functional extensibility,diversified molecular design,lower cost,toxicity,etc.[3].The preparation of organic white light-emitting materials usually adopts dye-crosslinked polymer materials [4],supramolecular assembly [5],and the formation of aggregates or deposition on the carriers [6].And the focus of the field research is mainly on the composition of illuminants,that is,the source of white light.Since the wavelength range of the white light spectrum is 400–700 nm,the most commonly used method to generate white light is to mix a variety of illuminants of different colors,among which the most representative is to mix red,green,and blue primary colors.Due to the obvious differences in emission brightness and stability of different color illuminants,the white light produced by mixing different types of dyes has poor stability in terms of light stability and material preparation repeatability.The white light emission of a single molecule has obvious advantages in material preparation,so it has become one of the goals pursued in the current research of organic white light materials[7].For a single molecule to emit white light,it needs to have multiple emission channels,the most common of which are blue and yellow dual fluorescence channels.However,there is still a large gap between the composite light emitted by the two channels and the white light with the chromaticity coordinate {CIE (0.33,0.33)}.Therefore,how to make a single molecule have three or more fluorescence emission wavelengths has become a strategy to obtain purer white light.However,dyes with blue,green,and red emissions have rarely been reported so far [8].
The reported organic white light materials are mainly based on the multi-channel emission regulation of the compound itself or the combination of multi-color materials,which are mostly used for display,and lack of studies on the dependence between multicolor luminescence and external environment,which limits the application expansion of these materials in fields such as recognition sense [9–11].
In this paper,we develop environmentally sensitive organic white light materials based on the isomers of hydroxy–naphthalimide,which are 4-substituted compoundNapH1and 3-substituted compoundNapH2,respectively (Scheme 1).Excited state proton transfer (ESPT) occurs inNapH1andNapH2,resulting in blue and yellow two-channel emission and blue and red twochannel emission,respectively.The change of polarity or temperature modulates the hydrogen bond between the hydroxyl group and the external environment,thereby changing the intensity ratio of the dual emission wavelengths ofNapH1andNapH2,in whichNapH1generates a luminescence close to white light.Since they are the same dye matrix and have similar chemical properties,the two dyes are mixed in a certain ratio to obtain white light with the CIE (0.30,0.33).This environmentally sensitive white emission can recognize the polarity,temperature,and surface alkalinity of different types of paper.A test strip capable of visually detecting acid-base vapors was prepared by spraying dyes onto paper.
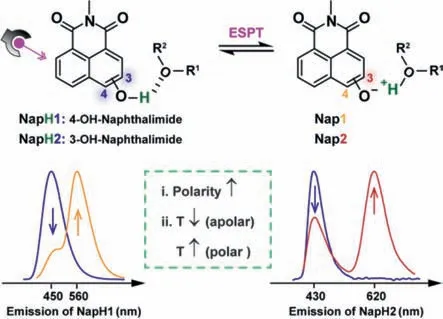
Scheme 1. Structure of NapH1 and NapH2,and their ESPT properties and influencing factors. Nap1 and Nap2 is the deprotonation form of NapH1 and NapH2.
Firstly,the absorption and fluorescence emission spectra ofNapH1andNapH2in different solvents were detected (Fig.1).The maximum absorption of the two in different solvents is around 370 nm and 380 nm,respectively (Figs.1a,c,and e).This wavelength is suitable for spraying portable displays or detection materials or instruments that use 365 nm as excitation light.In lowpolarity solvents,the emission wavelengths of the two are around 450 nm and 430 nm,respectively.Both two exhibited dual fluorescence emission with increasing polarity,with secondary fluorescence wavelengths around 560 nm and 620 nm,respectively(Figs.1b,d,and e).The absorption and fluorescence emission spectra ofNapH1andNapH2under alkaline conditions in different solvents indicate that the long wavelength emission peaks are from dehydrogenated productsNap1andNap2(Scheme 1,Fig.S1 in Supporting information).Compared withNapH1andNapH2,the absorption wavelength ofNap1andNap2in different polar solvents showed a large redshift and returned after adding acid to the solution.Under acidic conditions,NapH1andNapH2still produce long-wavelength fluorescence in strong polar solutions,which shows thatNapH1andNapH2form intermolecular hydrogen bonds with solvent molecules,and after being excited by light,ESPT occurs,andNap1andNap2are generated in the excited state thereby emitting long-wavelength fluorescence.The superposition of the two emission wavelengths makesNapH1andNapH2produce different colors in different solvents (Figs.1f and g).It can be seen that the emission ofNapH1in different solvents is closest to white light,and the CIE in ethanol is (0.27,0.35).No matter what kind of solventNapH2is in,there is no way to produce white light.As can be seen from Fig.1f,the CIE ofNapH1andNapH2are located on both sides of white.Therefore,we guessed that the mixture ofNapH1andNapH2would obtain ideal white light.It should also be pointed out that mostNapH1molecules deprotonate toNap1in the ground state in strongly alkaline solution DMF,and only a partial proportion of deprotonation occurs in acetonitrile,methanol,and water (Fig.1a).However,NapH2in the ground state hardly undergoes deprotonation reactions in different solvents (Fig.1c).
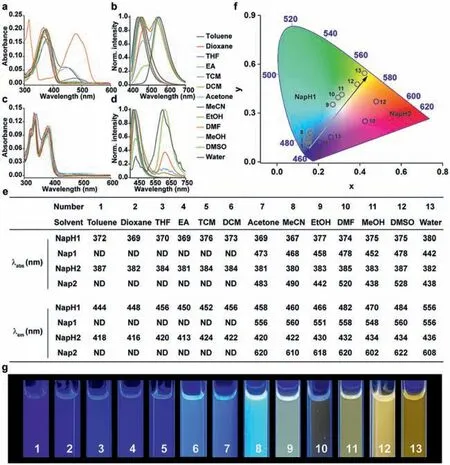
Fig.1. The ultraviolet–visible (UV–vis) absorption spectra of NapH1 (a);and NapH2 (c) in different solvents.The fluorescence emission spectra of NapH1 (b);and NapH2 (d)in different solvents.(e) The spectral data table of NapH1 and NapH2;(f) the CIE 1931 diagram of NapH1 and NapH2 in different solvents;(g) the luminescence photographs of NapH1 in different solvents.
To clarify the conditions forNapH1andNapH2to mix and emit white light,we first choose two solvents with low polarity and high polarity to mix in different ratios and detect the fluorescence emission spectra of the two in these solvents (Fig.2 and Fig.S2 in Supporting information).The tested systems include dioxane-DMSO,MeCN-DMSO,and dioxane-water.Both compounds do not undergo ESPT in dioxane and acetonitrile,but in DMSO and water,most of the molecules undergo ESPT after being excited by light.With the increase of the proportion of DMSO or water content,the intermolecular hydrogen bond strengthens,leading to the increase of long-wavelength fluorescence intensity,accompanied by the gradual weakening of short-wavelength fluorescence intensity.The luminescence ofNapH1gradually changed from blue to yellow in different proportions of mixed solutions.
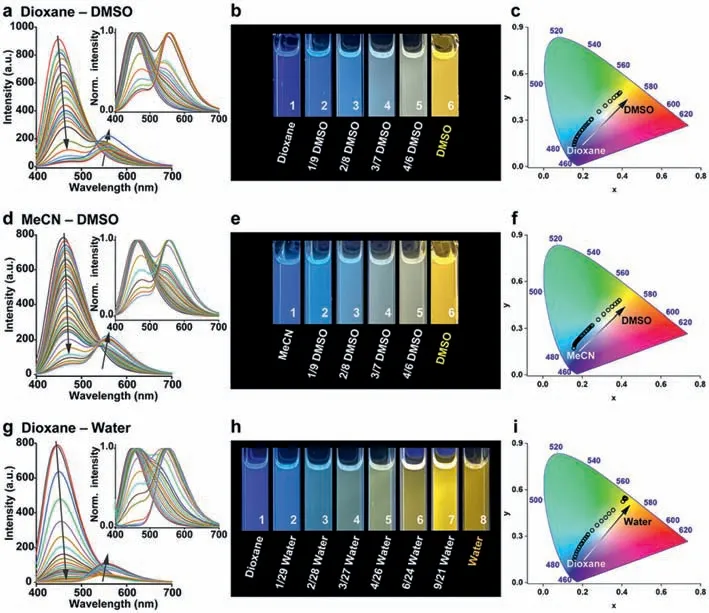
Fig.2. The fluorescence emission spectra of NapH1 in (a) dioxane-DMSO;(d) MeCN-DMSO;and (g) dioxane-water system.The corresponding luminescence photographs of NapH1 in (b) dioxane-DMSO;(e) MeCN-DMSO;and (h) dioxane-water system.The CIE 1931 diagram of NapH1 in (c) dioxane-DMSO,(f) MeCN-DMSO,and (i) dioxane-water system.
Next,we chose dioxane and DMSO as the mixed solution and detected the emission of the two solvents at different mixing ratios with different mixing ratios ofNapH1andNapH2(Fig.3,Figs.S3 and S4 in Supporting information).When dioxane and DMSO are mixed in a 5/5 ratio,andNapH1andNapH2are mixed in a 5/5 ratio,the color coordinate is (0.30,0.33),closest to white light CIE(0.33,0.33).
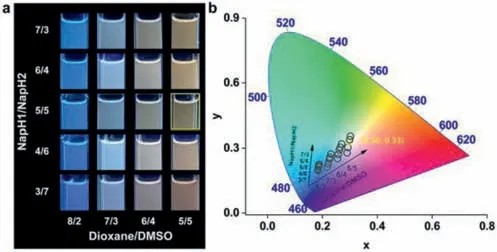
Fig.3. (a) Luminescence photograph;and (b) CIE 1931 diagram of different mixing ratios of NapH1 and NapH2 and different ratios of dioxane and DMSO.
The deprotonation reaction of conjugated hydroxyl groups in aromatic systems is not only affected by hydrogen bond donors and acceptors but also affected by temperature.We detected the fluorescence emission spectra changes ofNapH1in eight solvents from melting point temperature (m.p.) to room temperature (r.t.)(Fig.4,Figs.S5–S9 in Supporting information).In solvents such as methanol and ethanol to form intermolecular hydrogen bonds withNapH1at room temperature,as the temperature decreases,the long-wavelength fluorescence gradually weakens and finally disappears,while the short-wavelength fluorescence intensity gradually increases.The fluorescence color changes from white light at room temperature to blue at melting point temperature.This indicates that at a lower temperature,the ESPT process no longer occurs.However,the opposite trend appeared in apolar solvents.With toluene as a typical sample,although there was no intermolecular hydrogen bond formed withNapH1,as the temperature decreased,the short-wavelength fluorescence weakened,and the long-wavelength fluorescence appeared and increased.This may be due to the stable existence of the deprotonated productNap1at a lower temperature.
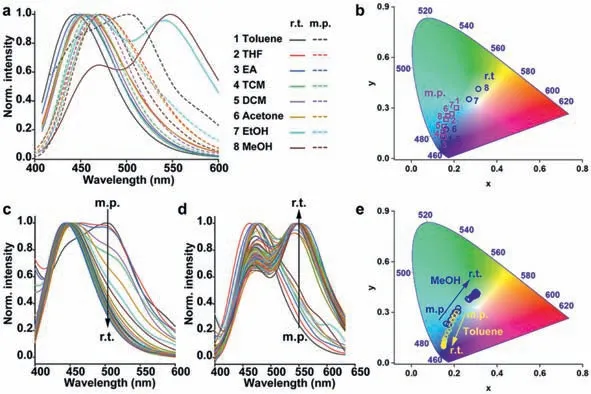
Fig.4. (a) Fluorescence emission spectra;and (b) CIE 1931 diagrams of NapH1 at room temperature and melting point temperature.The fluorescence emission spectra from melting point temperature to room temperature of NapH1 in toluene (c);and methanol (d).(e) The corresponding CIE 1931 diagrams of (c) and (d).
Finally,we attachedNapH1andNapH2to the papers.Due to the existence of oxygen-containing functional groups on the paper surface,NapH1andNapH2can still undergo ESPT on the paper surface after being excited by light,and emitting two-color mixed fluorescence.The difference in the mixing ratio of the two shows different luminescence on the two kinds of paper,which implies the different environment of the paper (Fig.5a).The acidity and alkalinity of paper are related to the writing quality and aging life of the paper,and the acidity and alkalinity of different papers are different.As shown in Fig.5b,NapH1sprayed onto the filter paper and writing paper emits different fluorescence colors.And the filter paper is easily changed by the alkali vapor,which changes the luminous color ofNapH1;while the writing paper is easily affected by the acid vapor so that the sprayedNapH1has no color on the paper and emits almost white light.These results indicate thatNapH1can be used to identify the acidity and alkalinity of paper,and it can also be used as a test strip for detecting acidity and alkalinity vapor.
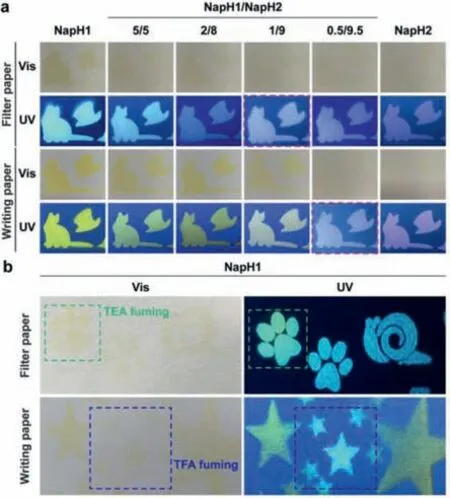
Fig.5. The luminescence on the paper.(a) The color and luminescence of different mixing ratios of NapH1 and NapH2 on filter paper and writing paper respectively;(b) the color and luminescence of NapH1 on two kinds of paper and the change of color and luminescence after acid or base stimulation.
It should be noted here that since the absorption of the deprotonated productsNap1andNap2is significantly different from that ofNapH1andNapH2,most of the long-wavelength fluorescence obtained when using 380 nm as the excitation light comes from the deprotonation ofNapH1andNapH2in the excited state,instead ofNap1andNap2produced in the ground state (Fig.S10 in Supporting information).Therefore,the acidity and alkalinity of the environment can be judged by the absorption color of the solution,that is,whether there is a stable existence ofNap1andNap2in the ground state,and ESPT is used to judge the intermolecular hydrogen bond.Meanwhile,NapH1andNapH2exhibit good photostability (Fig.S11 in Supporting information),which makes this environmentally sensitive multicolor luminescence system potential for further applications.
In this paper,we found that the 4-and 3–hydroxyl substituted naphthalimidesNapH1andNapH2have similar absorption and emission spectra,but the fluorescence wavelengths of the deprotonated productsNap1andNap2have significant differences,thus obtaining the blue and yellow emission ofNapH1,and three-color emission of blue,yellow and red in whichNapH1andNapH2are mixed.Ideal white light can be obtained through proper deployment.The dual-channel emission ofNapH1andNapH2is generated by proton transfer between excited state molecules,which makes the white light emission be affected by environmental factors and can recognize polarity,temperature,and pH.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work is supported by the National Natural Science Foundation of China (Nos.22225806,22078314,22278394) and Dalian Institute of Chemical Physics (Nos.DICPI202227,DICPI202142).
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2023.108348.
 Chinese Chemical Letters2023年12期
Chinese Chemical Letters2023年12期
- Chinese Chemical Letters的其它文章
- Spin switching in corrole radical complex
- Benzothiadiazole-based materials for organic solar cells
- Mono-functionalized pillar[n]arenes: Syntheses,host–guest properties and applications✰
- Recent advances in two-step energy transfer light-harvesting systems driven by non-covalent self-assembly✩
- From oxygenated monomers to well-defined low-carbon polymers
- Doping-induced charge transfer in conductive polymers
