Dimesitylboryl-ended oligothiophene with tetrazine as core: Synthesis,structure and Diels–Alder reactivity
Shimin Zhou ,Yang Liu ,Yuyin Hao ,Zhiqiang Liu,∗ ,Xiaoqiang Yu
a State Key Laboratory of Crystal Materials,Shandong University,Ji’nan 250100,China
b Shenzhen Research Institute of Shandong University,Shenzhen 518057,China
Keywords:Tetrazine ligation Bioorthogonal reaction Crystal structure Fluorescence Quadrupolar hybrid-oligothiophene
ABSTRACT A dimesitylboryl-ended oligothiophene with tetrazine as core (BTz) was synthesized and its reactivity and spectral changes toward trans-cyclooctene ((4E)-TCO-OH),cis-cyclooctene and bicyclo[6.1.0]non-4-yn-9-ylmethanol were comprehensively studied.The fluorescence intensity of BTz was enhanced up to more than 100 times upon bioorthogonal reaction with (4E)-TCO-OH.In addition,the first crystal structure of isolated product of tetrazine derivative with cyclooctene was determined,which clearly confirmed a dehydrogenation occurred after Diels–Alder reaction under ambient conditions.
Bioorthogonal chemistries are not affected by and minimally perturbing to biological systems,which can be used to label diverse kinds of biomolecules in cells and other complex environments [1].Since the early successes of Bertozzi group carrying a modified Staudinger reaction on cell surface twenty years ago,a variety of bioorthogonal chemistries have been developed and applied in not only numerous chemical biology studies [2–4] but also synthetic chemistry and materials science [5,6].Among the extensively studied bioorthogonal chemistry,the tetrazine bioorthogonal reactions,including inverse-electron-demand Diels–Alder (iEDDA)reaction with dienophiles and [4+1] cycloaddition with isonitriles,have attracted more and more attention due to their unique fluorescence properties [7].The iEDDA reaction between tetrazines and strained dienophiles are widely used for protein,lipid and glycan labeling because of fast reaction kinetics and good fluorescence quenching efficiency of tetrazine moiety [8].
On the other side,tetrazine was also adapted as electron acceptor in some optoelectronic materials due to its unique electrondeficient property.Several groups have systematically investigated a series of thiophene coupled acceptors,and found that tetrazineembedded oligothiophene demonstrated the lowest unoccupied molecular orbital (LUMO) level among twenty usual electrondeficient units [9–11].Especially,these tetrazine derivatives are electroactive both in oxidation and reduction,they were incorporated into conjugated backbone of some polymer for solar cell [12].Furthermore,it can also be used as precursor of other electroactive unit [13].
In the past 20 years,our group [14,15] and Marderetal.[16–18] have found that many dimesitylboryl ended quadrupolar oligothiophene compounds exhibit large two-photon absorption crosssections (σ2) and emit bright two-photon excited fluorescence(TPEF).Especially,Marder’s group reported theσ2value of 4590 GM on a tetracationic quadrupolar chromophore with two triarylboranes linked by 5,5'-di(thien-2-yl)−3,6-diketopyrrolopyrrole and successfully applied realized TPEF imaging of lysosomes in living cells [19].The systemic comparison of these TPEF emissive dimesitylboryl-ended quadrupolar oligothiophenes clearly indicated the structure ofπ-bridges play significant roles [15–19].
In contrast to the previously adapted electron-donating core,we herein embedded electron-accepting tetrazine into a quadrupolar hybrid-oligothiophenes (BTzin Scheme 1).The synthesis,structure,and photophysical properties and its Diels–Alder (D-A) reactivity toward strained dienophiles,such as TCO-OH,cis-cyclooctene,5-norbornen-2-ol and bicyclo[6.1.0]non-4-yn-9-ylmethanol,were also examined.Especially,the first crystal structure of the cycloaddition product of tetrazine-cyclooctene was obtained,which confirm the dehydrogenation of direct D-A produced dihydropyridazine derivative to an aromatized pyridazine compound under ambient conditions.
As outlined in Scheme 1,the title compoundBTzis a dimesitylboryl-ended quadrupolar oligothiophene derivative with tetrazine as core,which could be readily prepared through common procedure for similar compounds as reported [15,17].Firstly,the dibrominated precursor (BrTz) was prepared starting from 5-cycano-2-bromine-thiophene using hydrazine hydrate as a reductive agent.ThenBrTzwas lithiated and then quenched with dimesitylfluoroborane to finally affordBTz.Although the direct iEDDA reaction ofBTzwith (4E)-TCO-OH was not carried out at preparative scale,we did isolate two compounds from the reaction product ofcis-cyclooctene withBrTzandBTz,respectively,which were characterized asBrOCandBOC.Especially,single crystals ofBrTz,BTz,BrOCandBOCsuitable for X-ray diffraction were successfully grown by slow evaporation of their solution of DCM.To the best of our knowledge,this is the first crystallographic proof of iEDDA product between cyclooctene with tetrazine derivatives.
As presented in Fig.1 and Table 1,bothBrTz(Fig.S1 in Supporting information) andBTzadapt central symmetric configurations,with S atoms of thiophene moieties pointing to opposite directions.The lengths of the central N=N bond ofBrTzandBTzare 1.320 ˚A,which is slightly shorter than typical N–N single bond but longer than that of isolated N=N bond,indicating the aromaticity of tetrazine.However,the planarity of thiophenetetrazine-thiophene backbone inBrTzandBTzare much better than other reported tetrazine derivatives [20].The dihedral angle between tetrazine and thiophene are only 2.26° forBrTzand 3.43°forBTz,respectively.
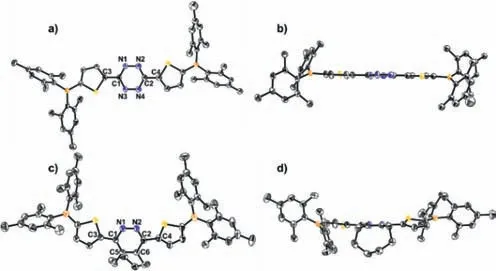
Fig.1. Top and side viewed molecular structures of BTz (a,b) and BOC (c,d) from single crystal X-ray diffraction.Thermal ellipsoids are drawn at the 50% probability level.All the H atoms are omitted for clarity.
Importantly,the structural information ofBrOCandBOCclearly indicated that no hydrogen could be reasonably added on the central C4N2 rings and they are all pyridazine derivatives rather than theoretically predicted dihydropyridazine as direct product of D-A reactions.All the bond lengths in the central C4N2 ring clearly indicated the aromatic character.Among them,the length of bridged C–C bond between pyridazine and cyclooctene moiety inBOCis 1.399 ˚A,which indicates that these three C···C bonds are between single and double bonds.The cyclooctene moiety adapts a halfchair conformation.Although the thiophene-pyridazine-thiophene backbone still keep nearly planar conformation,the dihedral angle between pyridazine-thiophene obviously increased to 19.72°.Interestingly,the two thiophene unit take acis-conformation,that is,with S atoms of thiophene moieties pointing to same directions.
The ultraviolet–visible (UV–vis) absorption and emission properties ofBTzandBOCwere summarized in Table S4 (Supporting information).Generally,similar with the reported quadrupolar dimesitylboryl-endded oligothiophenes [15],the absorption and fluorescence emission spectra ofBTzandBOCin the common solvents show negligible solvent effects.However,the spectra patterns ofBTzandBOCare distinctly different (Fig.S3 in Supporting information).BTzdisplays a strong absorption band around 410 nm and maintains the well-resolved vibronic structures,while the main absorption band ofBOCis degenerated to a broad structureless peak and blue-shifted to around 370 nm.
To track the tetrazine bioorthogonal reaction process and avoid possible distractions of oxygen in air,BTzand (4E)-TCO-OH were mixed and degassed in a quartz pool and the absorption and emission behavior were recorded in real-time.It seems that tetrazine very effectively quenched the fluorescence ofBTz,but the ligation with (4E)-TCO-OH will rapidly turn on the fluorescence(Fig.2).Liuetal.has proposed two kinds of quenching mechanism of tetrazine-functionalized labels as “energy transfer to a dark state (ETDS)” and “internal conversion to a dark state (ICDS)”[21].In ourBTz,the tetrazine moiety is directly ‘‘embedded’’to the oligothiophene fluorophore,resulting in an integratedπconjugation.The time dependent density functional theory (TDDFT) quantum chemical calculations show that the n–π∗transition of the tetrazine fragment will produced low-lying dark states (S1,f=0.003;S2,f=0.004) during the vertical excitation (Table S5 in Supporting information).So,we believe the fluorescence quenching mechanism ofBTzcould be attribute to the “internal conversion to a dark state”.The subsequent iEDDA reaction destroyed the tetrazine part,eliminated the corresponding dark state and turned on the bright fluorescence [21].
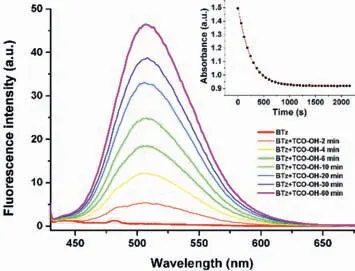
Fig.2. The emission spectra of BTz ligation with (4E)-TCO-OH.
Especially,the emission peak intensity enhanced by more than 100 times and peak position remarkably shifted to 510 nm.Whencis-cyclooctene was used as dienophile,the emission spectra of the reactant mixture changed very slowly,even with the aid of irradiation of UV-lamp [22].However,the emission spectra ofBTz+ciscyclooctene is very similar with that ofBTz+(4E)-TCO-OH,which indicated the formation of similar intermediate dihydropyridazine derivative.In addition,the existence of dihydropyridazine derivative were also verified byin-situhigh-resolution mass spectrometry (MS) spectra (Figs.S9–S11 in Supporting information).
Beside the reaction of tetrazine with alkene,we also studied the D-A reaction of tetrazine with alkyne.Thein-situreaction ofBTzand bicyclo[6.1.0]non-4-yn-9-ylmethanol (BCN) was also performed in a degassed quartz pool.As shown in Fig.3,bothBOCandBTz+BCNpresent strong emission band at 380–520 nm with similar vibration structures,which should result from the formation of aromatized pyridazine core.The quantum yield ofBOCwas determined as 3.05% in toluene,which is much higher than that ofBTz(Φ=0.20%).
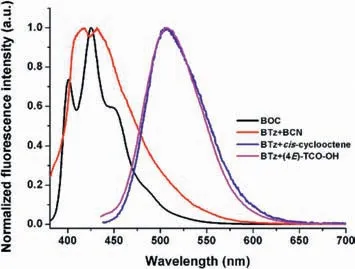
Fig.3. The normalized emission spectra of BTz with different dienophiles.
The reaction kinetics ofBTzwith dienophiles was also examined.The second-order rate constant forBTz+(4E)-TCO-OH at 25 °C isk20.3 L mol-1s-1in toluene,which is obviously slower than that of reported phenyl or pyridinyl substituteds-tetrazine [22].According to the crystal structures ofBTzandBOC,it seems one thienyl ofBTzneed to be rotated before it matches the D-A reaction In addition,Houketal.had found that the reactivity of substituted tetrazines correlate with the electron-withdrawing abilities of the substituents [23].InBTzmolecule,thiophene group is a weak electron donor inBTz,which will increase the LUMO+1 energy and decreases the activity of the D-A reaction.So,relatively slow reaction kinetic ofBTzcould be attribute to the rotation barrier and electronic donating properties of the adjacent thienyl groups.Furthermore,the TPEF spectra ofinsituBTz+(4E)-TCO-OH indicatedBTzcould be precursor of TPEF emitter (Fig.4).
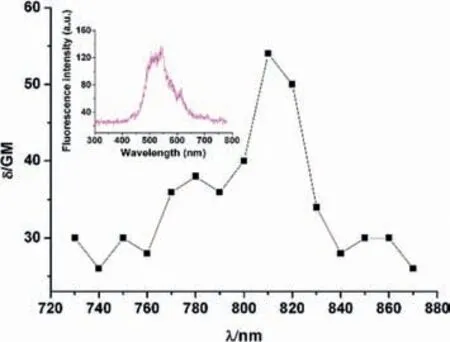
Fig.4. Two-photon excitation and emission spectra of BTz+(4E)-TCO-OH.
In order to understand the spectroscopic results in depth,TDDFT calculation at B3LYP/6–31G(d) basis set with Gaussian 09[24] were carried out onBTz,BOCand possible intermediate dihydropyridazine derivativeBOC-H.As shown in Fig.5,both highest occupied molecular orbital (HOMO) and LUMO energy levels ofBOCare higher than those ofBTz.But since the enhancement of LUMO is bigger than HOMO,so the HOMO-LUMO gap ofBOC(3.77 eV) is obvious larger than that ofBTz(3.54 eV).Furthermore,both the HOMO and LUMO ofBOCis lower than that ofBOC-H,which is also consistent with the experimental results.
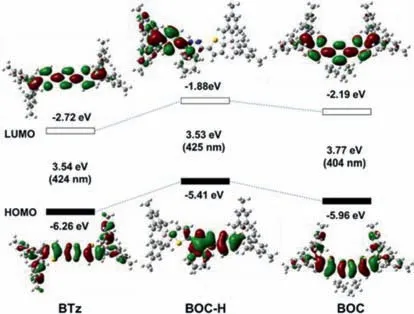
Fig.5. Calculated [B3LYP/6–31G(d,p)] frontier orbitals and their energy levels of BTz, BOC-H and BOC.
In summary,a dimesitylboryl-ended oligothiophene with tetrazine as core (BTz) was synthesized and its bioorthogonal chemistry toward cyclooctene was comprehensively studied by spectral technique and crystal structures of isolated product compounds.The first crystal structure of isolated bioorthogonal product clearly confirmed that a dehydrogenation occurred under ambient conditions after bioorthogonal iEDDA reaction between tetrazine and cyclooctene.We believe this finding will prove useful in designing new ICDS type of tetrazine-based bioorthogonal probes.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by National Natural Science Foundation of China (Nos.52150222,21672130 and 52073163),the Shenzhen Science and Technology Research and Development Funds(No.JCYJ20190806155409104),and the State Key Laboratory of Crystal Materials.We acknowledge Prof.Dr.Cuihua Zhao of Shandong University for her valuable suggestions.
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2023.108325.
 Chinese Chemical Letters2023年12期
Chinese Chemical Letters2023年12期
- Chinese Chemical Letters的其它文章
- Spin switching in corrole radical complex
- Benzothiadiazole-based materials for organic solar cells
- Mono-functionalized pillar[n]arenes: Syntheses,host–guest properties and applications✰
- Recent advances in two-step energy transfer light-harvesting systems driven by non-covalent self-assembly✩
- From oxygenated monomers to well-defined low-carbon polymers
- Doping-induced charge transfer in conductive polymers
