Two-step continuous flow process of sodium tanshinone IIA sulfonate using a 3D circular cyclone-type microreactor
Molin Sun ,Choming Ling ,Liming Co ,Yguo Wng ,Jisheng Yng ,Shiyu Hou ,Wei Yu,d,Yueyue M,Ruihu Cheng,∗,Jinxing Ye,,∗
a School of Biomedical and Pharmaceutical Sciences,Guangdong University of Technology,Guangzhou 510006,China
b School of Pharmacy,East China University of Science and Technology,Shanghai 200237,China
c School of Chemical Engineering,East China University of Science and Technology,Shanghai 200237,China
d Shanghai No.1 Biochemical& Pharmaceutical Co.,Ltd.,Shanghai 200080,China
Keywords:Continuous flow Sodium tanshinone IIA sulfonate Cardiovascular Microreactor Green chemistry
ABSTRACT A sustainable and practical process is presented for the direct synthesis of sodium tanshinone IIA sulfonate (STS).Our approach was inspired by the well-established and industrially applied batch synthetic route for STS production.We constructed a telescoped two-step continuous flow platform.This involved a continuous tanshinone IIA sulfonation and in-line salt formation.For the setup,we constructed a 3D circular cyclone-type microreactor using femtosecond laser micromachining.Compared to the 68% yield for 2 h in batch,the two-step continuous flow had an STS yield of 90%,achieved for a total residence time of <3.0 min under optimal conditions.The proposed continuous flow method vastly simplified the operation and improved procedural safety,while significantly reducing the required acid content and wastewater production.
Tanshinone (Tan) IIA is a lipophilic active constituent extracted from the roots and rhizomes of the Chinese medicinal herbSalvia miltiorrhizaBunge (Bge.) [1–3].Tan IIA is sulfonated to produce sodium tanshinone IIA sulfonate (STS;Fig.1) to improve its solubility in water.STS has a wide range of pharmacological properties,such as anti-coagulation [4],anti-inflammation [5,6],anti-oxidant[7],anti-viral [8],anti-cancer [9,10],anti-apoptosis [11],interaction with iron channels [12].In clinical practice,STS has been developed as an injection,widely used in the treatment of coronary heart disease,angina pectoris,myocardial infarction,and other cardiovascular or cerebrovascular diseases [13].
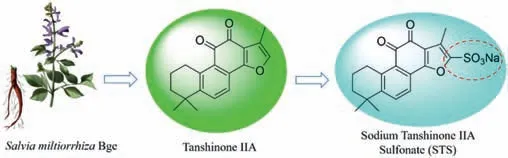
Fig.1. Diagram of Salvia miltiorrhiza Bunge with a magnified image of the roots,and the chemical structures of tanshinone (Tan) IIA and sodium tanshinone IIA sulfonate (STS).
The sulfonation of Tan IIA is a key step in the preparation of STS.Although several sulfonation methods have been reported previously [14–16],large-scale commercial applications have been limited due to low productivity and high cost.Currently,H2SO4is the most commonly used sulfonation reagent for STS production (Scheme 1a).However,precise control of the temperature and reaction time of sulfonation processes in batch reactor can be quite difficult,leading to various side reactions,such as oxidation and the formation of condensation polymers [17].In addition,the sulfonation process in a large-scale batch reactor have to deal with large amount of extremely corrosive sulfuric acid,acetic acid (AcOH),and acetic anhydride (Ac2O).Moreover,after sulfonation,several tedious steps such as hydration,purification,and salt formation are required to prepare STS.The productivity of these methods has long been affected by the challenging step of filtering the crude product during the complex post-treatment process [18].Therefore,there is an increasing demand for a more sustainable way to produce STS.
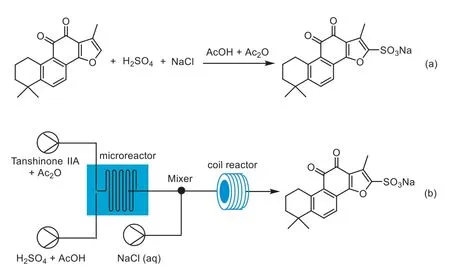
Scheme 1. (a) Conventional STS synthesis process and (b) continuous STS synthesis process presented in this work.
As an emerging synthetic technology,continuous flow techniques have greatly improved organic synthesis [19–22].These techniques have the added advantages of rapid mixing,efficient heat transfer,narrow residence time distribution,good reproducibility,and rapid system response,along with easy-to-automate controls compared to the traditional batch reactors [23–26].These techniques have evolved into an excellent toolkit for handling the challenge of chemical transformations over the past few decades[27–29].For example,several prior studies have pointed out the difficulty in handling highly exothermic and kinetically fast reactions in conventional batch reactors even at a small scale [29–31].For STS synthesis,so far,only one report has involved the usage of a continuous flow tubing reactor for the sulfonation using H2SO4,with the subsequent salt formation and product purification steps still conducted in the conventional batch reactors;this method had relatively low yields of 48%-73% [32].
The challenge of continuous scale-up demonstration in the STS synthesis is the handling of the solid products,which usually leads to irreversible clogging.The most commonly used strategy is to use solvents which can solubilize products.However,it is diffi-cult to eliminate the solid produced during the formation of the salt due to its poor solubility.The synthesis of inorganic materials has been successfully achieved in continuous flow systems,which involve a transfer of solid materials during the flow process [33–35].Several studies have addressed the clogging issue to varying extents through microreactor designs,flow modulation,or applying external forcing using ultrasonic waves [36–38].Motivated by these advances,we designed a fully continuous flow process,from sulfonation to in-line salt formation,by optimizing the microreactor and the reaction conditions.Our proposed method greatly simplified the operation process and improved STS yield to meet the production requirements (Scheme 1b).
Firstly,the extensive preliminary experiments were performed to obtain the standard reaction conditions for the continuous flow system and assess the feasibility of the proposed protocol.Generally,the input feed of a continuous flow reactor must have the consistency of a liquid.Considering the concentration limit imposed by the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) of 60 ppm of chloroform and 600 ppm of dichloromethane (DCM),for the sulfonation process,DCM was selected as the solvent to make the Tan IIA solutions (Table S1 in Supporting information).Further,we tested and verified the feasibility of sulfonation in a polyfluoroalkoxy (PFA) coil reactor.The Pump A fed a DCM solution of tanshinone IIA and Ac2O,and the Pump B fed a mixed solution of H2SO4and AcOH.The highest STS yield of 77.0% was achieved for the single-step mechanism through a fast screening of the reaction conditions (Table S2 in Supporting information).During post-processing,the viscosity of the reaction solution increased to paste-like consistency,making it quite difficult to filter,with only partial separation even after centrifugation (Fig.2a).The HPLC analysis showed that various organic impurities,primarily present in the raw Tan IIA,led to this difficulty in filtration.Therefore,we increased the purity of Tan IIA from 60% to 84% and repeated the process.This significantly improved the process of separating the reaction solution through filtration to obtain the crude product of STS (Fig.2b).

Fig.2. Post-processed products obtained after the sulfonation of (a) 60% pure Tan IIA and (b) 84% pure Tan IIA.
The use of high-performance microreactors can improve mixing efficiency,heat exchange,and mass transfer during the reaction,directly improving the productivity of the continuous flow system [39].Based on the several designed microreactors with unique internal structures on glass by femtosecond laser micromachining [40–42],a new three-dimensional (3D) circular cyclone-type microreactor was designed and manufactured for the continuous flow sulfonation due to its computational and experimental performances (Fig.3) [43–45].Computational fluid dynamics (CFD) simulations revealed that the 3D circular cyclone-type microchannel would be able to guide the fluid to produce sets of vortices in opposite directions within the mixing unit.Moreover,within a single chamber,the straight channels with alternating top and bottom further guide the twisting of the vortices.This columnar behavior allows the fluid to achieve turbulence within the theoretical laminar flow.
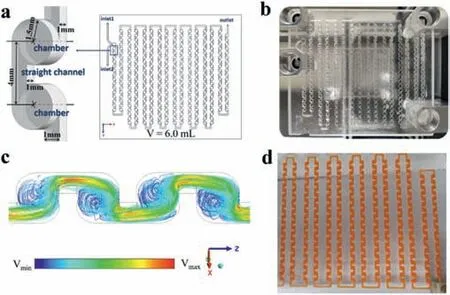
Fig.3. (a) Geometry;(b) Photo of the 3D circular cyclone-type microreactor;(c)3D streamlines of the microreactor;(d) Experimental observation photo at the total flow rate of 24 mL/min (oil-water ratio 1:2,oil phase staining with Sudan III).
Subsequently,the sulfonation of Tan IIA (84% purity) was conducted using the newly constructed microreactor in a single-step continuous flow.Since sulfonation is an exothermic reaction,the batch reactor operation needs to be conducted at the temperature range of–10–0°C.By the microreactor,Table 1 shows that the yield of the reaction could reach more than 80% at 0-20°C under continuous flow conditions (Table 1,entries 1-3) due to the high mass and heat transfer efficiency.Although the reaction temperature was increased,it did not lead to an increase in yield.Instead,it made it more difficult to control the temperature (Table 1,en-tries 4 and 5).Considering the cost of temperature control and the yield of STS,the optimal reaction temperature was determined to be 20°C.Since the microreactor could intensify the reaction rate and vastly reduce the reaction time,the residence time (RT) of sulfonation reaction was reduced to 2.5 min,which produced an 87%STS yield.However,a lower yield was observed for RT=2.0 min(Table 1,entries 6-9).
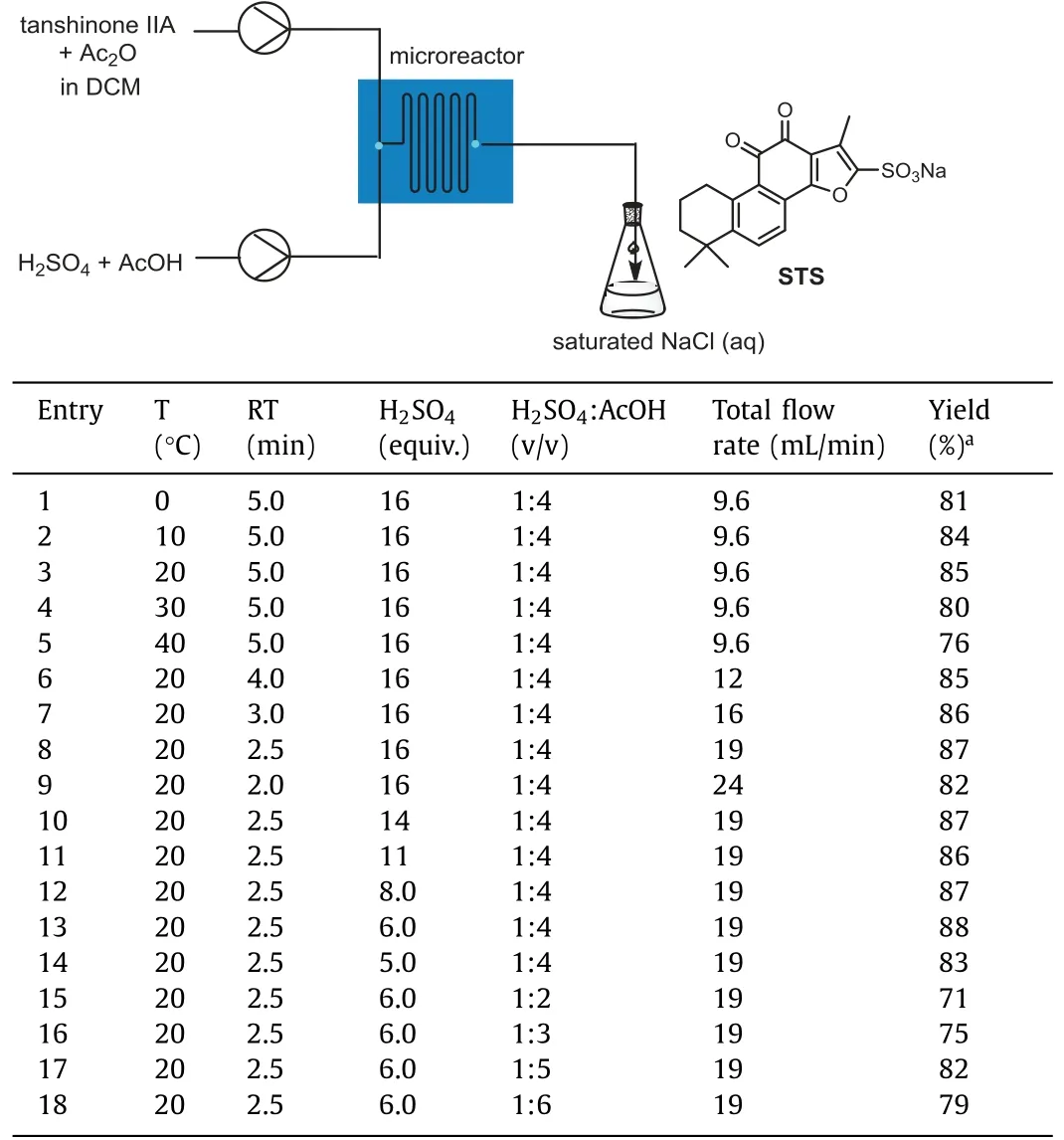
Table 1 Optimization of reaction conditions for the sulfonation reaction of tanshinone IIA in continuous flow with microreactor.
The sulfonation reagent,H2SO4,is well known for its extreme corrosiveness and high handling risk,and hence,minimizing its use is necessary for environmentally sustainable chemistry.Therefore,the effect of the amount of H2SO4on the sulfonation of Tan IIA in continuous flow was investigated while keeping a constant volume ratio of H2SO4: AcOH.Upon the analysis of the required acid content,we observed that 6.0 equiv.of H2SO4produced a satisfactory yield of 88% (Table 1,entries 10-14).Various H2SO4:AcOH volume ratios were also analyzed to optimize the concentration of the latter.The STS yield peaked in the volume ratio range 1:5 ≤H2SO4: AcOH ≤1:3 (Table 1,entries 15-18).Therefore,the selected optimal reaction conditions for the continuous flow setup — temperature of 20°C,RT of 2.5 min,6.0 equiv.of H2SO4,H2SO4:AcOH=1:4 (v/v),and total flow rate of 19.0 mL/min — produced the highest isolated yield of 88%.
The synthesis of STS includes the processes of sulfonation and salt formation reactions.In the sulfonation reaction in continuous flow,the resulting solution produced through the sulfonation of Tan IIA was then added dropwise to saturated sodium chloride (NaCl) to proceed with salt formation.However,after longterm operation,the accumulation of the product led to the wrapping of fat-soluble impurities,making the solid-liquid separation increasingly difficult.Furthermore,a significantly large quantity of NaCl was required,adding to the cost of acquiring reagents and waste treatment.Therefore,we first introduced an in-line purification strategy to reduce the organic impurities (Fig.4).Water was added to the outlet of the microreactor 1 followed by in-line extraction in the subsequent microreactor 2.The organic phase was separated by a liquid-liquid separator,while the aqueous phase was then dripped into aqueous NaCl to obtain the STS.Despite the higher purity of the product,the yield was only 72%.
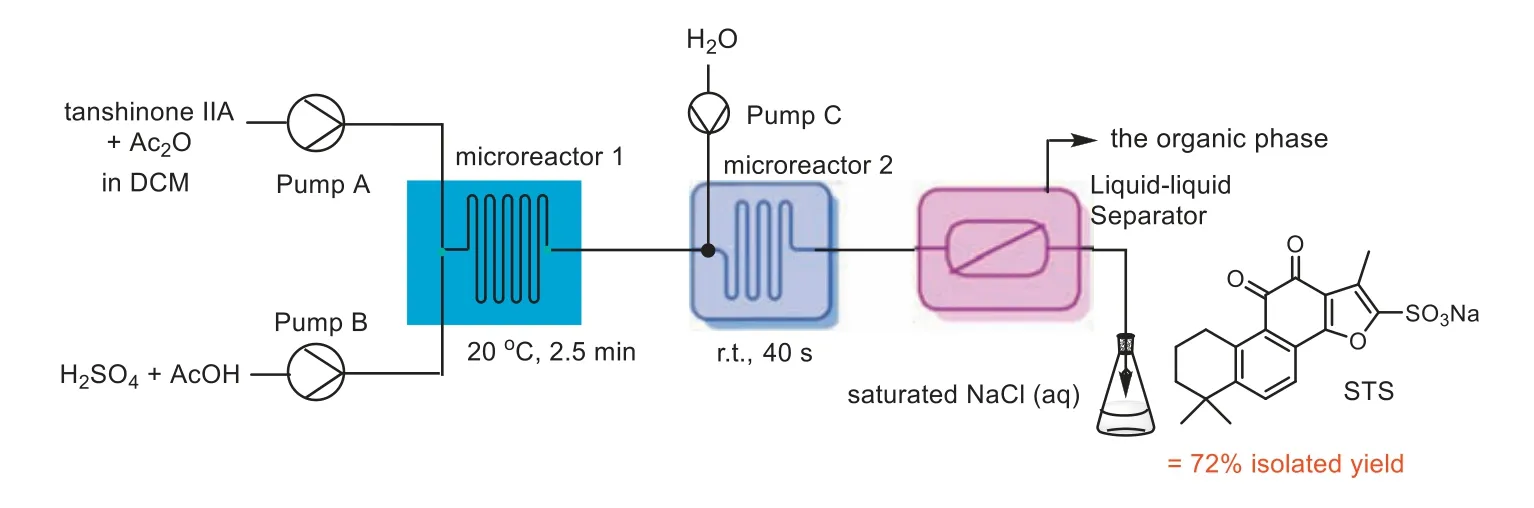
Fig.4. Schematic diagram of single-step Tan IIA sulfonation and the in-line purification process of STS.
The typical scanning electron microscopy (SEM) images show that the STS are basically uniformly distributed,with a long rodlike structure,and the size of the particles is less than 5 μm (Fig.S1 in Supporting information).To improve the STS yield,a twostep continuous synthesis process involving an in-line salt formation reaction was subsequently considered (Fig.5).However,this modified setup also experienced stagnation of the solid product in the conventional mixer region.The flow rate was increased to prevent solid accumulation,but we noticed that the clogging still occurred after long run times.Subsequently,multiple variations of the equipment were investigated in terms of the mixer,reactor flow path sizes,and internal structures (Table S3 in Supporting information).Finally,it was found that the two-step continuous flow process can operated stably for longer run times when the "Y"mixer with inlet and outlet outer diameters of 1/16" and 1/8",respectively,and coil reactor with outer diameter of 1/8" were used for the continuous salt formation reaction.
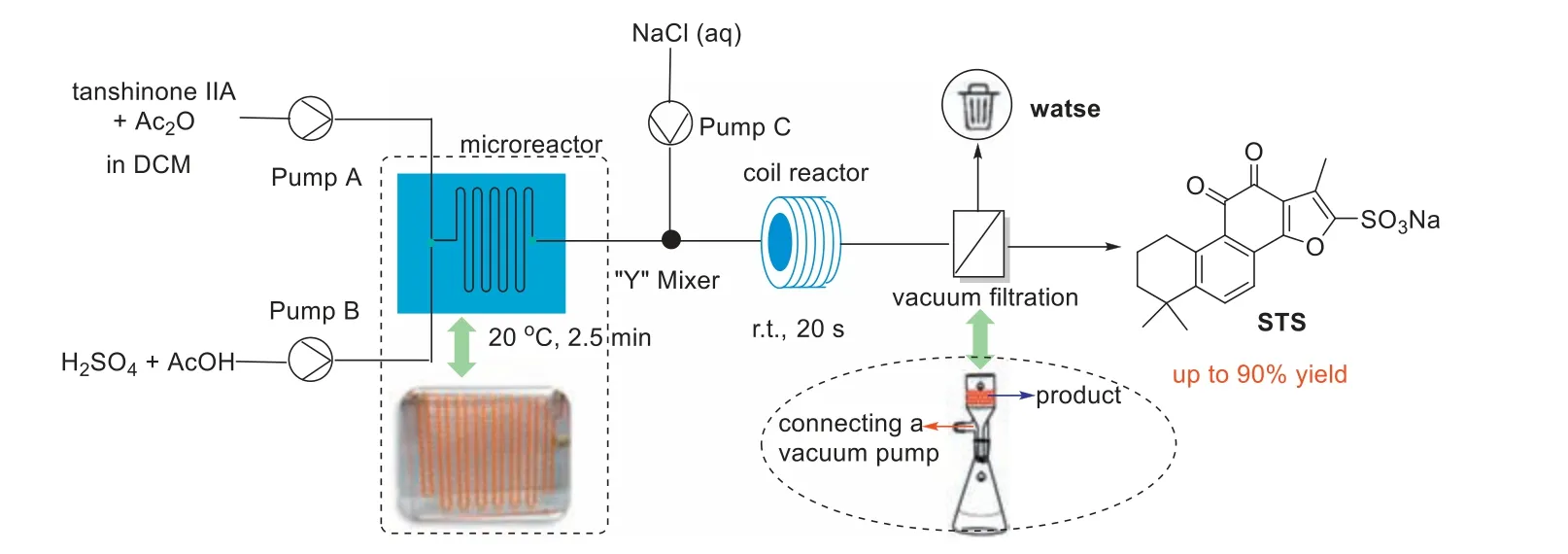
Fig.5. Schematic diagram of two-step continuous flow process of STS.
To further improve the yield results,the effect of the concentration and the quantity of NaCl solution on the salt formation reaction in continuous flow was investigated under the optimal sulfonation conditions.The yield increased with decreasing NaCl concentration,reaching up to 90% for the concentration of 4.0 mol/L(Table 2,entries 1-3).To reduce wastewater production,a similar performance was achieved with 8.0 equiv.of NaCl (Table 2,entries 4-6).It is worth mentioning that the reduction in NaCl quantity significantly reduced the wrapping of the crude product and simplified the purification process.The increase in RT had little effect on the STS yield,although it slightly reduced for RT=10 s (Table 2).Therefore,the optimal RT for this step was chosen to be 20 s.
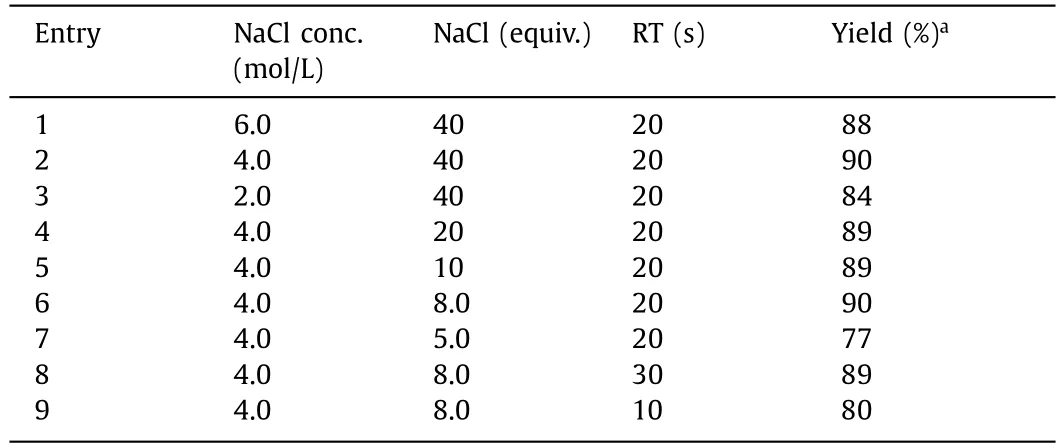
Table 2 Optimization of reaction conditions for salt formation in continuous flow.
Based on the optimization of continuous flow conditions,a system for the continuous synthesis of STS was built.The equipment system included modules for the input feed,reaction,product collection,and control.The control module enabled the precise regulation of equipment operation,flow rate,temperature,and system pressure (Fig.S2 in Supporting information).In contrast to the batch processes,the continuous manufacturing is characterized by its constant production.Therefore,long-term stable operation is the basis for achieving large-scale production.To demonstrate the feasibility and stability,the continuous flow reaction system had been operated for 8 h under optimal process conditions at a total flow rate of 20.2 mL/min (Fig.6).The samples were taken at 1 h intervals with the fluctuating yield at most by 1% during the process and the throughput can reach 26 g/h.Furthermore,the maximum STS yield of the two-step continuous flow process was 90% compared to the 68% yield for the batch process at the laboratory scale (Table 3).The final product purity also increased to 93%–95% with a reduced total RT of<3.0 min.Additionally,this process demonstrated a 57.5% decrease in the required amount of H2SO4,AcOH,and Ac2O,as well as a 53.0% decrease in the amount of wastewater byproduct.Compared with the batch process,this new continuous flow process represented 63% reduction in E-factor.The above results revealed dramatic improvements in cost reduction and environmental sustainability of the continuous flow process.
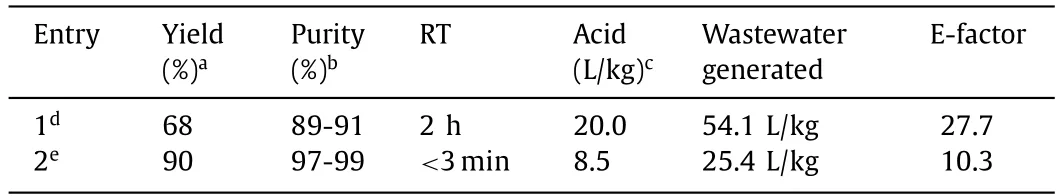
Table 3 Comparison of between the batch and continuous flow process for the synthesis of STS.

Fig.6. The yield from continuous flow synthesis of STS in the long run.Fractions were collected at 1 h intervals.
In summary,we developed a two-step continuous flow process for the synthesis of STS with a total RT<3.0 min and the isolated yield up to 90%.The proposed strategy significantly reduces the reaction time,required acid content,and wastewater byproduct.It improves the safety of the STS production process and increases the final product purity,while being environmentally friendly and cost-efficient.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was partially supported by the National Natural Science Foundation of China (No.22278087).We acknowledge Prof.Ya Cheng and Dr.Miao Wu for their contributions to the manufacture of the glass micromixer chips using femtosecond laser micromachining,and Prof.Xuhong Qian and Prof.Weiping Zhu for their insightful guidance and discussion during the entire research.
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2023.108738.
 Chinese Chemical Letters2023年12期
Chinese Chemical Letters2023年12期
- Chinese Chemical Letters的其它文章
- Spin switching in corrole radical complex
- Benzothiadiazole-based materials for organic solar cells
- Mono-functionalized pillar[n]arenes: Syntheses,host–guest properties and applications✰
- Recent advances in two-step energy transfer light-harvesting systems driven by non-covalent self-assembly✩
- From oxygenated monomers to well-defined low-carbon polymers
- Doping-induced charge transfer in conductive polymers
