Eco-friendly iron-catalyzed oxidation of unstrained tertiary aromatic alcohols to ketones
Shanmei Zhu ,Penghui Hu ,Mengying Guo,Linlin Zhao,Linlin Yang,Wei-Jin Gu,Wei Han
Jiangsu Collaborative Innovation Center of Biomedical Functional Materials,Jiangsu Key Laboratory of Biofunctional Materials,Key Laboratory of Applied Photochemistry,School of Chemistry and Materials Science,Nanjing Normal University,Nanjing 210023,China
Keywords:Iron catalysis C−C bond cleavage Oxidation H2O2 Late-stage functionalization
ABSTRACT A general,facile and eco-friendly iron catalysis enables oxidation of unstrained tertiary aromatic alcohols to ketones through C−C bond cleavage even with H2O2 as the oxidant.Notably,this transformation can tolerate oxidation-labile functional groups.The robustness of this method is further demonstrated on the late-stage oxidation of complex bioactive molecules.
The oxidation of alcohols to ketones and aldehydes is among the most widely used class of oxidation reactions in organic chemistry [1–3].However,this process is mainly limited to secondary and primary alcohols.Currently,oxidation of tertiary alcohols to ketones remains a challenge,as the transformation involves inert C−C single bond cleavage [4–17].Consequently,examples of tertiary alcohol oxidations are rather rare,and their oxidative conversion generally requires a sufficient intrinsic reactive allylic alcohols,strained cyclic alcohols,and diols [18] Thus,expanding the scope of simple tertiary alcohol oxidations remains an important challenge.
Tertiary alcohols are readily accessible alkoxy radicals,leading to a subsequentα-C−C bond cleavage throughβ-scission to realize deconstruction/functionalization processes [18–40].Among them,deconstruction/hydrogenation reactions can transform tertiary alcohols to ketones.For instance,Knowles and co-workers first reported the hydrogenative C−C scission of unstrained tertiary aryl alcohols with ap-methoxyphenyl group (PMP) adjacent to the hydroxyl group to give the corresponding 4-methoxyarylketones by using an Ir photocatalyst [41,42].Subsequently,Hu realized the transformation with a broader scope of substituted phenyl tertiary alcohols by applying FeCl3as the photocatalyst [36].However,the scope of the tertiary aromatic alcohols is limited top-substituted phenyl tertiary alcohols.In addition,these examples rely on the use of the combination of a Brønsted base,and a thiol as hydrogen atom donor.Obviously,the development of new protocols with efficient catalytic activities to overcome the strict substrate limitation is highly desirable.To this end,Huang’s group demonstrated Ag-catalyzed oxidation of various tertiary aromatic alcohols to arylketones by using Bi(OTf)3as the promoter and K2S2O8(3 equiv.) as the oxidant under mild conditions (Scheme 1a) [43],although the catalytic system is probed only on simple aromatic alcohols.
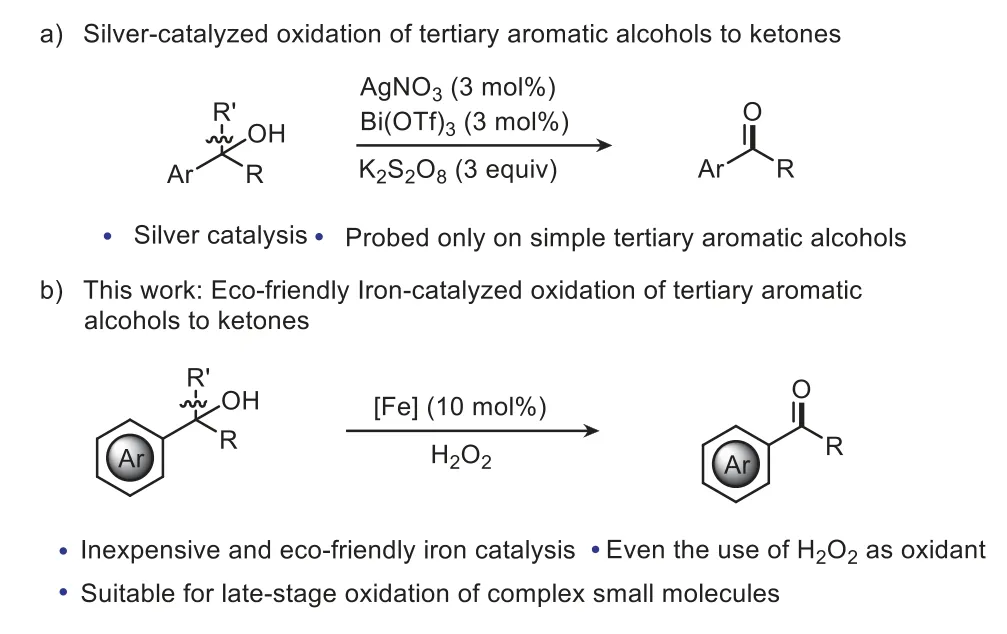
Scheme 1. Strategies for oxidation of tertiary alcohols to ketones.
In a constant search for cleaner methods,there is a definite need for catalytic oxidations that use an inexpensive biorelevant metal as the catalyst and dioxygen (O2) or H2O2as the stoichiometric oxidant [43–52].Herein,we describe an unprecedented iron-catalyzed oxidation of diverse unstrained tertiary aromatic alcohols to arylketones even with H2O2as the oxidant.This newly developed methodology utilizes inexpensive and eco-friendly reagents and tolerates oxidation-labile functional groups,and enables late-stage oxidation of complex molecules(Scheme 1b).
Encouraged by our recent studies on iron-catalyzed oxidations[53,54],we initially investigated the iron-catalyzed oxidation of 2-phenyl-2-propanol (1a) with K2S2O8as an oxidant in MeCN/H2O and at 60 °C (Table 1).When Fe(acac)2was used,14% of the desired ketone2awas observed (entry 1).Other iron sources such as FePc and Fe(OAc)2resulted in poor results,whereas FeCl3and FeCl2can give much better results (81% and 93%,respectively)(entries 2–5).The use of various oxidants was also examined:(NH4)2S2O8,TBHP,and DTBP were inferior to K2S2O8,and environmentally benign H2O2can give the optimal result (entries 6–9).Without H2O2,the reaction didn’t work (entry 18).None of other solvents employed (MeCN,H2O,EtOH/H2O,DMSO/H2O,DMF/H2O,and CH3COCH3/H2O) could replace the MeCN/H2O (entries 10–15).No reaction was observed without an iron catalyst (entry 16).Further,the utilization of ultrapure FeCl2(99.99% based on trace metals) resulted in a slightly better yield of2a.These results indicated that trace quantities of other metals in the iron sources do not affect the efficiency of the transformation (entry 17).
With the optimized iron-catalyzed oxidation in hand,we explored its versatility with a range of diversely substituted tertiary aromatic alcohols (Scheme 2).Tertiary aromatic alcohols having neutral,electronically activated,and deactivated moieties gave the desired arylketones in good to excellent yields with high selectivities.A variety of electron-donating groups such as isopropyl,alkoxy,benzoyloxy,and chloromethyl on the benzene ring were well tolerated (2b-2e,2k-2o,and2u).As seen with2b-2e,2f-2hand2m,ortho-,meta-,para-substituted,as well as sterically hindered tertiary aromatic alcohols proceeded successfully.The reactions also worked smoothly with aromatic alcohols bearing ester and even reactive carboxy moieties,and provided good yields(2p-2q) in the presence of extra K2S2O8(1 equiv.).Gratifyingly,oxidation-labile boronic acid groups were tolerated by this catalytic system,so that they can be used as a handle for further modifications (2r-2t).
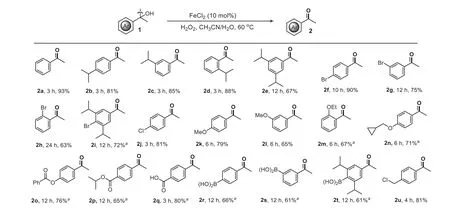
Scheme 2. Scope of tertiary aromatic alcohols.Reaction conditions (unless otherwise noted): substrate (0.25 mmol),FeCl2 (0.025 mmol),H2O2 (0.75 mmol),MeCN (1.0 mL),H2O (1.0 mL),60 °C,air.a substrate (0.25 mmol),FeCl2 (0.025 mmol),H2O2 (0.75 mmol),K2S2O8 (0.25 mmol),MeCN 1.0 mL,H2O 1.0 mL,80 °C,air.
Next,we investigated the scope of the different gem–disubstituted tertiary arylcarbinols (Scheme 3).They worked equally well to deliver aryl ketones in good to excellent yields.3-Arylpentan-3-ols bearing neutral,electronically activated,and deactivated moieties show high reactivity (1v-1y).Generally,the high reactivity and selectivity of the C−C bond cleavage relied on the stability of the release of alkyl radicals.For instance,1A-1C(containing ethyl,benzyl and allyl groups,respectively) transformed smoothly to the corresponding acetophenone2ain 67%,84%,and 91% yields.Also1Dbearing a longer alkyl chain proceeded well.In addition,2-phenylbut-3-en-2-ol (1E) and 2-phenylbut-3-yn-2-ol(1F) underwent selective C-vinyl and C-alkynyl bond cleavages respectively,to provide the corresponding arylketones with satisfactory yields.During the reactions of1Eand1F,we observed the formation of CO2by GC,suggesting that vinyl and ethynyl moieties may undergo oxidation cleavage to form a carboxyl group and then decarboxylation to give the desired ketone2a,consistent with a previous study [55].
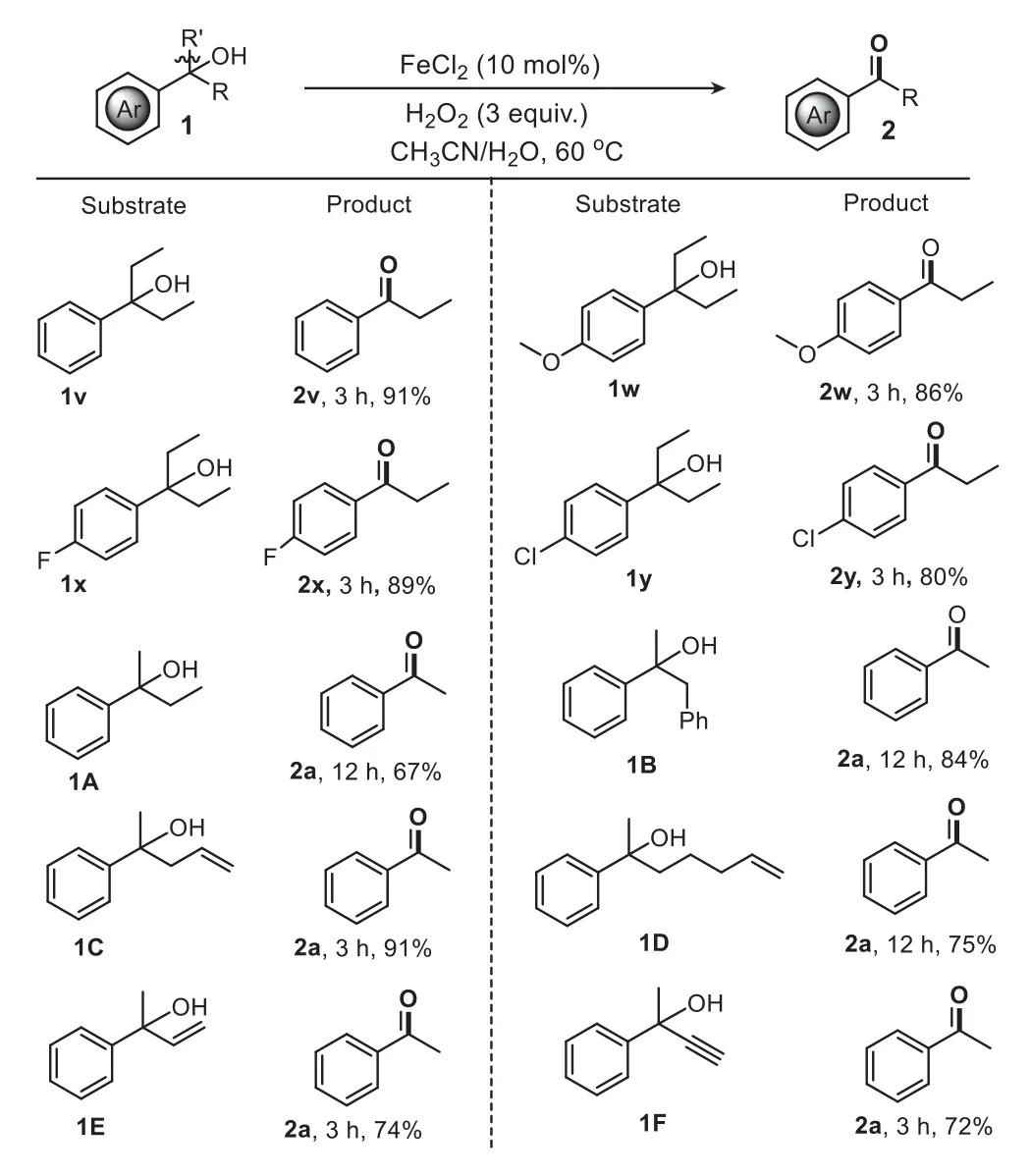
Scheme 3. Scope of tertiary aromatic alcohols.Reaction conditions (unless otherwise noted): substrate (0.25 mmol),FeCl2 (0.025 mmol),H2O2 (0.75 mmol),MeCN(1.0 mL),H2O (1.0 mL),60 °C,air.
To further demonstrate the utility of this method,late-stage oxidation of complex molecules was evaluated (Scheme 4).1Gderived from bioactive epiandrosterone was a competent substrate and provided the desired ketone2Gin a synthetic useful yield.Similarly,a glycyrrhetnic acid derivative bearing an oxidationsensitive hydroxy group also proceeded well.Remarkably,cholic acid-derived tertiary aromatic alcohol containing even three hydroxy groups was identified as a viable substrate,making this method more attractive in organic synthesis.
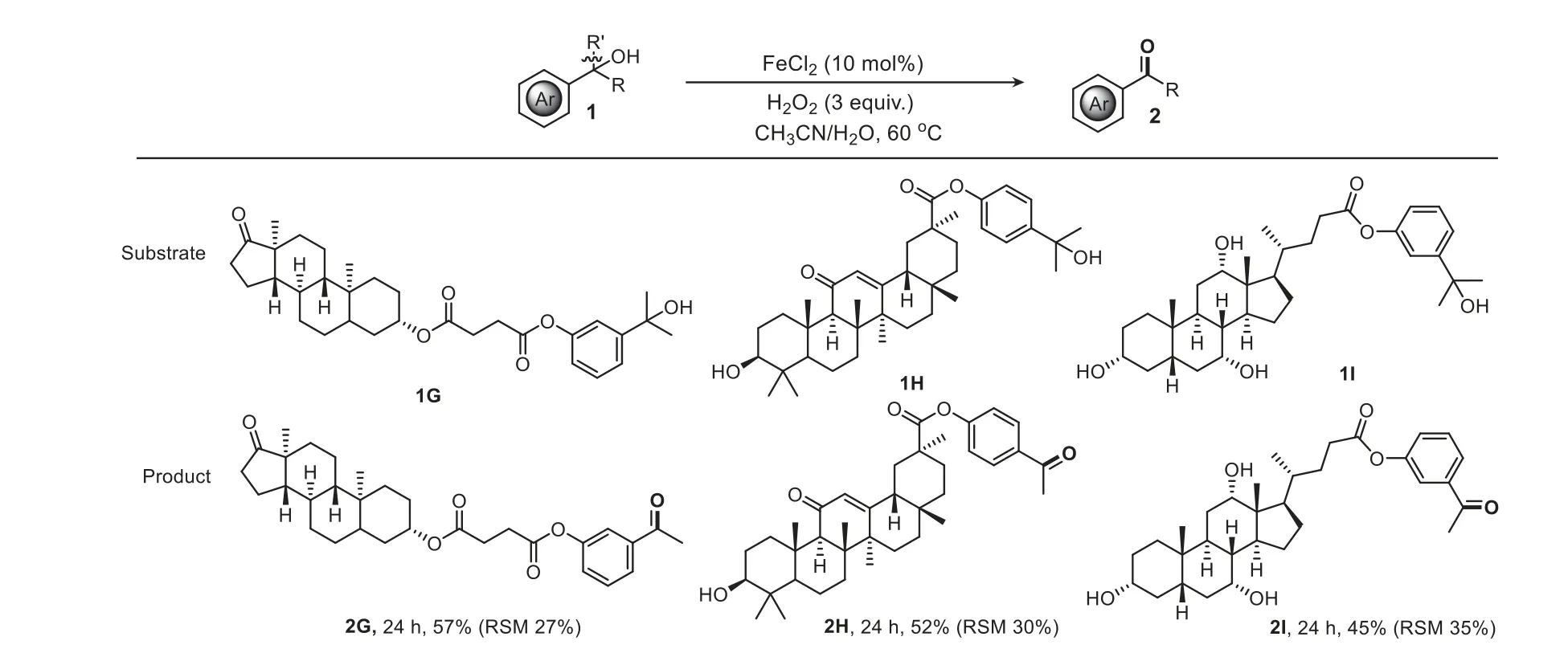
Scheme 4. Late-stage oxidation of complex molecules.Reaction conditions: substrate (0.25 mmol),FeCl2 (10 mol%),H2O2 (3.0 equiv.),K2S2O8 (1.0 equiv.),MeCN/H2O(1 mL/1 mL),80 °C,and air.Yields of the isolated products are given;RSM: recovered starting material.
To probe the possible catalytic pathway of the transformation,controlled experiments were carried out (Scheme 5).DMSO,a hydroxyl radical scavenger,was applied with the reaction of1a,leading to completely inhibition of the reaction [56,57].This finding suggests that a Fenton process may be present (Scheme 5a).When the reaction was run in the presence of a radical quencher,2,2,6,6-tetramethylpiperidineN-oxide (TEMPO) [58,59],no reaction occurred (Scheme 5b).Furthermore,introduction of a radicalcapture reagent,1,4-benzoquinone to the reaction of1atrapped a methyl radical to give methyl-p-benzoquinone detected by GC–MS(Scheme 5c).In addition,α-methylstyrene was not observed during the reaction of1a,suggesting that a dehydration-oxidation process might be ruled out.
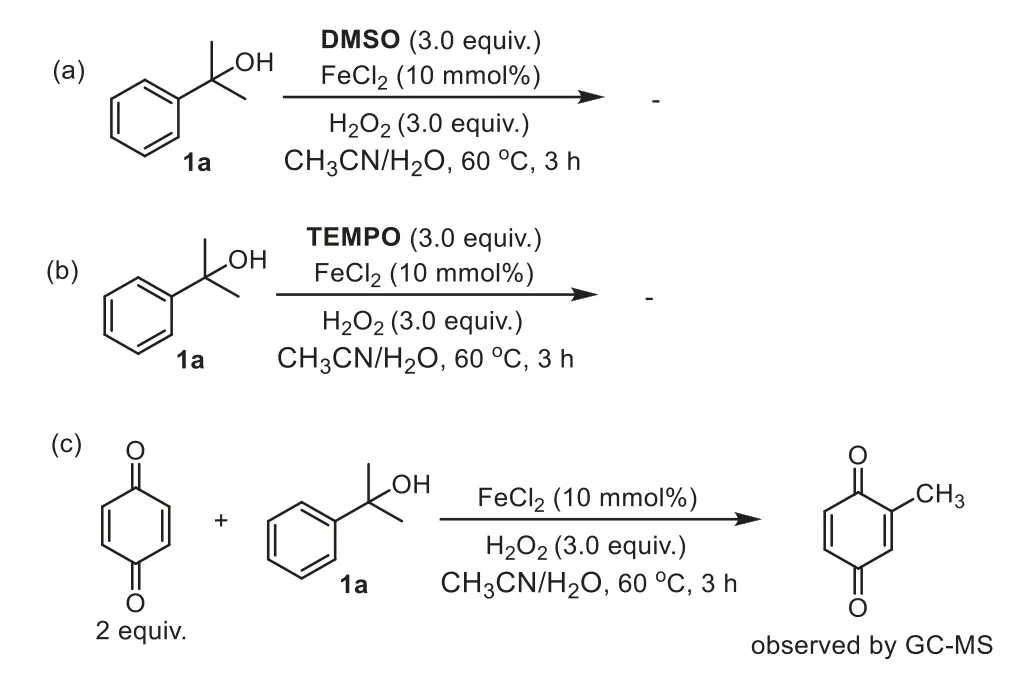
Scheme 5. Mechanistic studies.
Based on these observations,we propose the mechanism depicted in Scheme 6.The reaction is initiated by a hydroxyl radical generated from a Fenton process [60–62],followed by proton abstraction of tertiary aromatic alcohol to yield the corresponding alkoxyl radicalM[63].The alkoxyl radicalMcan subsequently undergoβ-scission to form arylketone and methyl radical that can produce methanol observed by GC [64,65].
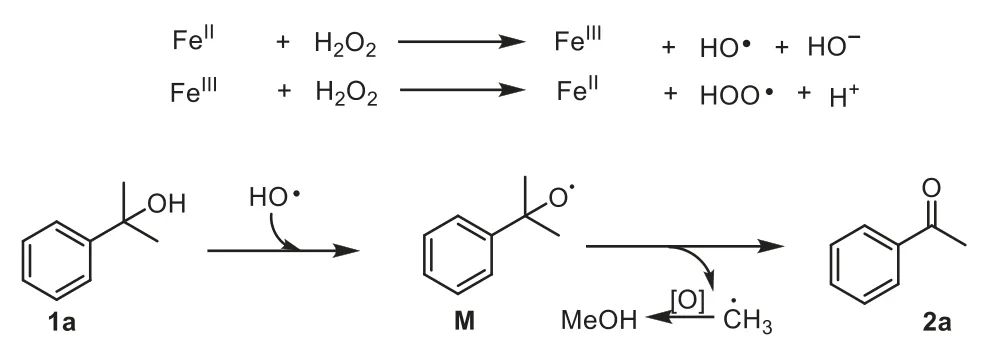
Scheme 6. Proposed pathway of iron-catalyzed oxidation of tertiary aromatic alcohols to ketones.
In summary,we developed a highly efficient and eco-friendly iron-catalyzed oxidation of unstrained C−C single bond cleavage of tertiary aromatic alcohols to prepare arylketones even with H2O2as the oxidant.The established approach is generally applicable to a wide range of tertiary aromatic alcohols and has good functional group tolerance.Notably,late-stage oxidation of structurally complex molecules containing hydroxy groups also works well,revealling great potential in practical organic synthesis.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The work was sponsored by the Natural Science Foundation of China (No.21776139),the “Qing Lan Project” Young and Middleaged Academic Leaders of Jiangsu Provincial Colleges and Universities,and the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2023.108835.
 Chinese Chemical Letters2023年12期
Chinese Chemical Letters2023年12期
- Chinese Chemical Letters的其它文章
- Spin switching in corrole radical complex
- Benzothiadiazole-based materials for organic solar cells
- Mono-functionalized pillar[n]arenes: Syntheses,host–guest properties and applications✰
- Recent advances in two-step energy transfer light-harvesting systems driven by non-covalent self-assembly✩
- From oxygenated monomers to well-defined low-carbon polymers
- Doping-induced charge transfer in conductive polymers
