Review of advanced oxidation processes for treating hospital sewage to achieve decontamination and disinfection
Si-Ying Yu ,Zhi-Hui Xie ,Xiaoyu Wu ,Yun-Zhe Zheng ,Yang Shi ,Zhao-Kun Xiong ,Peng Zhou,Yang Liu,Chuan-Shu He,,∗,Zhi-Cheng Pan,Kai-Jun Wang,Bo Lai
a State Key Laboratory of Hydraulics and Mountain River Engineering,College of Architecture and Environment,Sichuan University,Chengdu 610065,China
b Sino-German Centre for Water and Health Research,Sichuan University,Chengdu 610065,China
c State Key Joint Laboratory of Environment Simulation and Pollution Control,School of Environment,Tsinghua University,Beijing 100084,China
d Water Safety and Water Pollution Control Engineering Technology Research Center in Sichuan Province,Haitian Water Group,Chengdu 610041,China
Keywords:Hospital sewage treatment Advanced oxidation processes Pharmaceutical contaminants Disinfection Decontamination
ABSTRACT Hospital sewage contains various harmful pharmaceutical contaminants (e.g.,antibiotics,antiinflammatory agents,and painkillers) and pathogens (e.g.,bacteria,viruses,and parasites),whose direct discharge into the environment will induce diseases and pose a powerful threat to human health and safety,and environmental ecology.In recent years,advanced oxidation processes (AOPs),particularly photocatalysis,electrocatalysis,and ozone catalysis have been developed as widespread and effective techniques for hospital sewage treatments.However,there is a lack of systematic comparison and review of the prior studies on hospital sewage treatment using AOPs systems.This review elaborates on the mechanisms,removal efficiencies,and advantages/disadvantages of these AOPs systems for hospital wastewater decontamination and disinfection.Meanwhile,some novel and potential technologies such as photo-electrocatalysis,electro-peroxone,Fenton/Fenton-like,and piezoelectric catalysis are also included and summarized.Moreover,we further summarize and compare the capacity of these AOPs to treat the actual hospital wastewater under the impact of the water matrix and pH,and estimate the economic cost of these technologies for practical application.Finally,the future development directions of AOPs for hospital wastewater decontamination and disinfection have been prospected.Overall,this study provides a comparison and overview of these AOP systems in an attempt to raise extensive concerns about hospital wastewater decontamination and disinfection technologies and guide researchers to discover the future directions of technologies optimization,which would be a crucial step forward in the field of hospital sewage treatment.
1.Introduction
Hospital sewage is a kind of complicated wastewater containing pathogenic microorganisms,refractory drugs,metabolites,resistance genes,heavy metals,and contrast agents [1,2].In recent years,a huge quantity of pharmaceutical contaminants (e.g.,antibiotics,anti-inflammatory agents,and painkillers) have been abused and discharged,and harmful pathogens (e.g.,bacteria,antibioticresistant bacteria,and viruses) have been spread due to epidemic outbreaks [3,4].Particularly,these pharmaceutical pollutants in hospital sewage have multiple types,complex structures,diffi-cult biochemical degradability,and high biological toxicity,and pathogens in hospital wastewater are tiny in size,ubiquitous,strongly latent,and infectious [5,6].As a result,traditional biological technologies are difficult to treat hospital sewage effectively.However,incomplete removal can cause significant adverse effects on the aquatic environment and pose a serious threat to aquatic life and public health [7,8].
Antibiotics,anti-inflammatory agents,and painkillers,as the pharmaceutical contaminants commonly existed in hospital sewage,have drawn broad concern due to their semi-volatile,refractory,high toxicity,and the concentration that could achieve the level of mg/L in hospital effluent [9].Moreover,trace amounts of antibiotics in hospital wastewater probably increase microbial resistance,leading to the development of antibiotic-resistant bacteria (ARB) [10,11].Beside ARB,Escherichiacoli(E.coli),Salmonella,and hepatitis virus are also commonly found in hospital wastewater and are highly susceptible to bacterial infections and illnesses in humans [6,12].Thus,it is essential to seek an economical and effective way to remove pharmaceutical contaminants and bacteria from aqueous solutions.In order to realize the rational treatment,resource utilization,and risk management of hospital wastewater,chemical treatments such as advanced oxidation processes (AOPs)[1,13],physical treatments such as sedimentation and filtration[14],and biological treatments such as membrane bioreactors(MBR) [15] have been widely studied.Among them,AOPs have displayed remarkable performance and gained extensive attention.Moreover,researches on AOPs related to hospital wastewater decontamination and disinfection continue to increase due to the spread of Corona Virus Disease 2019 and the monkeypox virus around the world [3,4].
AOPs are characterized by reactive oxygen species (ROSs) generation with strong oxidation capacity,which can oxidize the refractory macromolecule organic matter into low/nontoxic small molecules with light irradiation,electricity,ultrasonic,or catalyst[16,17].Generally,AOPs can be classified as photocatalytic oxidation,electrocatalytic oxidation,catalytic ozonation,etc.,and the research focus is mainly on improving their efficiency in treating hospital wastewater currently [1,14].Whereas,the synchronous elimination ability of contaminants and bacteria treatment,water matrix and pH impact,and the cost and economy of actual water treatment in hospital sewage still lack comprehensive comparison and summaries.Meanwhile,the removal mechanism and application possibility of various AOPs treatments also require to be a further demonstration to guide the future development direction of AOPs in treating hospital wastewater.
The hierarchical structure of this review is listed as follows.After briefly introducing the characteristics of hospital sewage and AOPs,the second part concludes and elaborates on the removal mechanisms,efficiencies,and advantages/disadvantages of various AOPs for pharmaceutical contaminants in hospital wastewater in detail.Moreover,some promising and emerging technologies,such as combination technologies (photo-electrocatalytic,electroperoxone),Fenton/Fenton-like,and piezoelectric catalysis oxidation for hospital sewage disposal,are discussed.Then,the third section summarizes and contrasts the simultaneous decontamination and disinfection capabilities of the above AOPs.The fourth part discusses the impact of common water matrixes (e.g.,,effluent organic matter) and pH on hospital sewage disposalviaAOPs.The fifth section estimates the costs of various systems through the investigation of treating actual hospital sewage.Finally,some constructive perspectives on the feasibility and practical engineering application of AOPs technologies for hospital wastewater are put forward.This review will help AOPs to play a more crucial role and guide the development direction in the prevention and control of hospital wastewater pollution in the future.
2.Degradation mechanisms and efficiencies of pharmaceutical contaminants removal by AOPs
Previous papers demonstrated critical reviews of pharmaceutical contaminants’degradation from wastewaterviavarious treatment approaches [18,19].The conventional methods,such as MBR,cannot availably remove residual pharmaceutical contaminants in hospital sewage [15].Fortunately,AOPs (e.g.,photocatalysis,electrocatalysis,and ozone catalysis) have displayed splendid effects and manifested to be appropriate methods for recalcitrant and non-biodegradable substances degradation rapidly in water,such as pharmaceutical contaminants in hospital sewage.
2.1. Photocatalytic oxidation
2.1.1.Thecatalyticmechanismofphotocatalyticoxidation
In general,photocatalytic oxidation technology includes homogeneous and heterogeneous photocatalytic oxidation [10].Homogeneous photocatalytic degradation usually takes Fe2+/Fe3+and hydrogen peroxide (H2O2) as media and generates hydroxyl radicals (•OH) through a photo-assisted Fenton reaction to remove pollutants (Eq.1) [20].Furthermore,sulfate radicals (SO4•−) are the main reactive species when peroxysulphate (PS) is an oxidant(Eq.2),while when peroxymonosulfate (PMS) is selected as an oxidant,•OH and SO4•−are the dominating reactive species (Eq.3)[21].These ROSs possess extremely high oxidation potential and strong oxidation ability to degrade organic pollutants like antibiotics to CO2,H2O,and inorganic ions (Eq.4) [22].
Heterogeneous photocatalysis is a promising approach to accelerate the removal of contaminants by increasing the reaction efficiencies of reactants on the catalyst surface or pore channel.As illustrated in Fig.1,when a semiconductor (e.g.,TiO2,ZnO,CdS,WO3) is irradiated by light,it absorbs photons with energy (e.g.,hv) equal or exceeds the band gap,and excites electrons transit from the filled valence band (VB) to the empty conduction band(CB) of the photocatalyst Fig.1,(1) and (2)) [23,24].The catalyst generates photo-induced electronand electron-holewith the irradiation of ultraviolet light (UV) (Fig.1,(3)) (Eq.5).Noticeably,is an assistant for the decontamination of organic pollutants leading to the increasing production of •OH (Eq.6).Furthermore,theon the CBreacts with O2to formviaa reduction process on the surface of the semiconductor (Eq.7).Subsequently,it creates hydroperoxyl radicalviaprotonation,and then these radicals combine with the trapped electrons,yielding H2O2and•OH (Eqs.8 and 9) [22,25].It is well known that•OH is a strong oxidant,degrading pollutants non-selectively and effectively [10].Moreover,may recombine in the interior or surface of the photocatalyst,leading to the appearance of charge recombination (Fig.1,(4) and (5)).Finally,the generation of ROSs will effectively mineralize organic pollutants into relatively small nontoxic molecular substances (Fig.1,(6) and (7)) (Eq.10) [26,27].
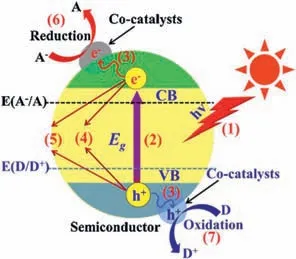
Fig.1. The fundamental mechanism of heterogeneous photocatalysis: (1) light harvesting;(2) charge excitation;(3) charge separation and transfer;(4) bulk charge recombination;(5) surface charge recombination;(6) surface reduction reactions and (7) surface oxidation reactions.Reprinted with permission [27].Copyright 2017,Elsevier.
2.1.2.Theremovalefficiencyand(dis-)advantagesofphotocatalytic oxidation
During homogeneous photocatalysis,UV/H2O2has been successfully used to remove pharmaceutical contaminants from various hospital wastewater.Chenetal.[28] adopted the UV/H2O2method to treat 50 μmol/L nitro-hydroxybenzoic acid with 0.5 mmol/L H2O2at pH 7.0,reaching a 96.0% removal rate in 20 min.Moreover,Zhangetal.[29] and Yangetal.[30] reported the mechanism of effective treatment of sulfonamide antibiotics in human urine and 20 mg/L norfloxacin (NOR) by UV/H2O2at 254 nm,respectively,and reached the conclusion that•OH was dominant.By comparing the removal efficiencies of 2,4-dichlorophenol (2,4-DCP) at different pH values,Shenetal.[20] found that UV/H2O2mainly produces•OH to degrade 2,4-DCP in acidic or neutral solution,while 2,4-DCP directly remove by UV photolysis with low efficiency in alkaline conditions because of H2O2is not easily hydrolyzed.In conclusion,the current studies disclose that homogeneous photocatalysis requires a low pH as well as the consumption of a large amount of H2O2.
Actually,heterogeneous photocatalysis based on TiO2is most widely applied due to the low energy consumption and cost.Supplementary material Table S1 (Supporting information) concludes the literature references of the heterogeneous photocatalytic process for removing pharmaceutical contaminants from wastewater.It is firstly noticed that the photocatalytic performance of each catalyst is different because of the photocatalytic experimental conditions.In addition,the band gap plays a key role in photocatalytic activity.The band gap varies by the properties of the catalyst since it is related to the catalyst structure and also to the elements contained in the catalyst [10].Reyesetal.[25] manifested that 0.5 g/L TiO2could photo-catalytically remove 50%tetracycline (40 mg/L) within 120 min at pH 6.0.Moreover,Elmollaetal.[31] and Malakootianetal.[23] completely degraded 105 mg/L amoxicillin (AMX),ampicillin (AMP),cloxacillin (CLX),and ciprofloxacin (CIP) respectively,using 1.0 g/L TiO2at pH 5.0 within 30 min.Besides,ZnO,CoBi2O4,Bi2WO6,and other novel catalysts have also been applied to achieve better efficiency without the usage of H2O2[24,26,32–34].Delightedly,it was discovered that complete mineralization and conversion of pharmaceutical contaminants after heterogeneous photocatalysis were simpler processes with less harmful products.
Nevertheless,the pharmaceutical contaminants of hospital wastewater with photocatalytic treatment are still in the research stage,and the method has not yet been industrialized.Hence,the development direction of photocatalysis is to exploit new photoreactors and semiconductor photocatalysts.On the catalyst aspects,doping of metals (e.g.,Ag,Pt),semiconductor metal oxides (e.g.,Fe2O3,CdS),and inorganic atoms (including N,C,S),is an effective approach to broaden the absorption spectrum range and slow down the recombination of electron-hole pairs [27,35–38].Moreover,advanced photoreactors are progressing towards high effi-ciency,large scale,good light transmission,simple operation,and economic investment.
2.2. Electrocatalytic oxidation
2.2.1.Thecatalyticmechanismofelectrocatalyticoxidation
Pharmaceutical contaminants’decontamination in the EC process can be attributed to two oxidation processes: (1) direct oxidation: electron transfer between pharmaceutical contaminants and the anode surface [39];(2) indirect oxidation: the direct oxidation of OH−or anions on the anode surface generates plentiful intermediate oxidative species,such as•OH,•O−2[39,40].Text S1 (Supporting information) and Fig.2 suggest the detailed difference between direct oxidation and indirect oxidation.Notably,the critical conditions for the EC process start-up are good adsorption of pharmaceutical contaminants onto the surface of the anode,and the applied voltage must be higher than the H2O oxidation voltage[5,39].
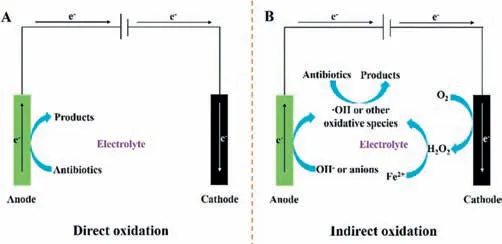
Fig.2. Scheme for (A) direct oxidation and (B) indirect oxidation in electrocatalysis systems.Reprinted with permission [5].Copyright 2021,Elsevier.
2.2.2.Theremovalefficiencyand(dis-)advantagesofelectrocatalytic oxidation
In practical application,electrocatalytic oxidation takes advantage of the anode and cathode in a synergistic manner [41].Namely,the cathode can electrically adsorb and discharge harmful substances,and the anode can stimulate water molecules to produce radicals to decompose and degrade organic pollutants.Furthermore,EC does not occur as secondary pollution generally since it only requires electricity without the addition of agents and the introduction of new impurities.In fact,many factors could obviously impact the EC removal of pharmaceutical contaminants,for example,current density,pH,electrolyte concentration,and electrode materials [5].The electrode catalysts of EC have the most significant impact on the effectiveness of pharmaceutical contaminants degradation.Currently,carbon-based catalysts (e.g.,borondoped diamond (BDD),carbon nanotubes (CNTs),and graphene)and metal oxide-based catalysts (e.g.,PbO2,SnO2,TiO2) have been mainly investigated for removing pharmaceutical contaminants in the EC [42–47].
As described in Table S2 (Supporting information),metal oxidebased electrodes exhibited great decontamination on quinolones(e.g.,levofloxacin,ofloxacin,ciprofloxacin) during EC processes[45,48–50].Chloramphenicols (chloramphenicol and florfenicol),β-lactams (ceftazidime,cefadroxil,and amoxicillin),and imidazole (metronidazole) were indicated to be removed by over two types of metal oxide-based electrodes,such as Ti/PbO2-based,Sb/SnO2-based,Ti/RuO2-based electrodes [44,45,51].Moreover,a high removal rate of sulfonamides (sulfamethazine,sulfamethoxazole,trimethoprim) was displayed in carbon-based electrodes during EC oxidation [45,52].Excitingly,BDD electrodes are considered an emerging material,achieving ∼90% of the mineralization rate in SMX and trimethoprim removal due to their strong oxidant ability [53].According to previous reports [5],it was probably related to the functional group of the pharmaceutical contaminants.For instance,piperazinyl,ethylamine,and cyclopropyl existed in quinolones which are efficiently mineralized by CeO2-based electrodes [48].Summarily,the following functional groups are more prone to be mineralized and degraded during EC processes: (1) quinolones: piperazinyl,ethylamine,and cyclopropyl groups,(2) chloramphenicols: phenyl-nitryl,O–H,and C-Cl bonds,(3) imidazoles: hydroxyl groups,nitro groups,and imidazole ring,(4)β-lactams: peptide bond and benzoquinone group,(5) sulphonamides: C-N,C-S,S-N bond and benzene ring[45,48,51,53,54].
Although BDD,PbO2,and graphene-based electrodes have been widely applied for pharmaceutical contaminants’degradation,the ability to deal with multiple types of pollutants and the possible side effects are still unresolved problems [42,49].Fortunately,with the advent of cheap and renewable electricity,electrocatalysis has immense potential as a hospital wastewater treatment technology[39].Moreover,it is relatively flexible and can be used in combination with a variety of processes,achieving further functions of disinfection and flocculation [55,56].Meanwhile,the modification and optimization of traditional electrodes should be further explored and studied in the future for better application in hospital wastewater treatment.
2.3. Ozone catalytic oxidation
2.3.1.Thecatalyticmechanismofozonecatalyticoxidation
Ozone catalytic oxidation can be divided into homogeneous and heterogeneous catalytic ozonation according to the phase state of the catalyst.During the homogeneous catalytic ozonation process,the soluble transition metals (e.g.,Fe(II),Mn(II),Ni(II)) are typically considered excellent catalysts to enhance the degradation of pollutants with O3[57,58].Whereas,the removal mechanisms of organic pollutants are not fully clear in the homogeneous catalytic ozonation process.It is generally reckoned that there are two reaction mechanisms,one is the catalytic decomposition of O3to form•OH,which oxidizes organic contaminants.The detailed catalytic mechanisms are disclosed as follows Eqs.11–14.Notably,according to Eq.14,the generated•OH would be scavenged by the excess metal ions,so the catalytic dosage optimization was also crucial for the catalytic ozonation process.And the other mechanism is that the catalyst is oxidized by O3after forming complexes with organic matter [57].
Heterogeneous catalytic ozonation is more effective,environmentally friendly,and cleaner than homogeneous catalytic ozonation [59].The heterogeneous catalytic ozonation utilizes a solid catalyst like metal oxides (e.g.,MgO,FeOOH,Al2O3,and AlOOH),and carbon materials (activated carbon,CNTs,graphene) to accelerate the liquid/gas phase oxidation with ordinary pressure [60].Three mechanisms for heterogeneous have been proposed for heterogeneous catalytic ozonation as below [61]: (1) the chemisorption of O3on the catalyst surface generates ROSs that can react with non-chemisorbed organic matters;(2) the chemisorption of organics on the catalyst surface and their further reaction with gas-or liquid-phase ozone;(3) both organics and O3are adsorbed on the catalyst surface,followed by the mutual reaction between chemisorption sites.The following Eqs.15–19 can be used to summarize the mechanism of metal oxide catalyzed ozonation proposed by previous researchers and illustrated in Fig.3 [62].
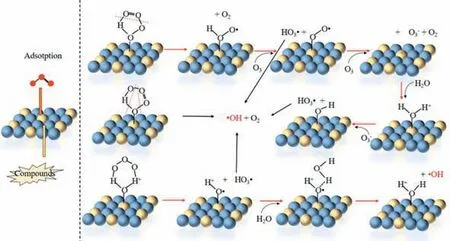
Fig.3. Possible catalytic mechanisms of metal oxides in heterogeneous catalytic ozonation processes.Reprinted with permission [62].Copyright 2020,Elsevier.
2.3.2.Theremovalefficiencyand(dis-)advantagesofozonecatalytic oxidation
In the past decades,it was usually believed that the introduction of catalysts can promote the decomposition of O3to produce more ROSs.It would cause the pharmaceutical contaminants in water/wastewater to be quickly removed and effectively mineralized.Gotvajnetal.[63] researched the degradation of AMX,levofloxacin,and their mixture with vancomycin at 21 °C and 40 °C during the O3process and Fe(II)/O3process.The results disclosed that Fe(II)/O3system had more superior mineralization capacity (93.4%) regardless of the temperature compared to O3alone(75.5%),and significantly reduced the TOC and COD of the pharmaceutical contaminants.Although transition metal/metal ions are low-cost and easily prepared,adding metal ions into wastewater will cause secondary pollution in homogeneous catalytic ozonation[58,64,65].Hence,it is one of the main reasons why it has not been widely applied yet.
Fortunately,heterogeneous ozonation catalysis has received more attention lately due to the convenient separation and recovery of solid catalyst form solution,efficient catalytic capacity,no secondary,and high stability [59,60,66].So far,various catalysts have been tested for catalytic ozonation,for instance,FeOOH,Fe-MCM-48,and Ce0.1Fe0.9OOH (Table S3 in Supporting information)[66–68].Herein,Sunetal.[69] first investigated nano-Mg(OH)2as a catalyst for degrading pharmaceutical contaminants such as sulfathiazole (ST),ofloxacin (OFL),and tetracycline (TC) during ozonation catalysis.The OFL removal rate constant (kOFL=0.512 min−1) was about 2.1 times greater in ozone-catalysis treatment than in single ozone treatment (kOFL=0.249 min−1).Also,the removal efficiencies of ST and TC increased by 23.5% and 32.8%,from 0.298 min−1to 0.368 min−1and from 0.384 min−1to 0.510 min−1,respectively [69].Moreover,Chenetal.[70] have prepared Fe3O4/Co3O4composites to catalyze 15 mg/min O3,resulting in complete degradation of SMX,and successfully achieving 60% of TOC removal.
However,some ozone catalysts require extremely difficult preparation and a complex synthesis procedure,which makes them unsuitable for mass production and industrial applications[71,72].Additionally,despite some materials performing well in the ozonation catalytic process,few studies sufficiently investigate the stability and reusability of the catalysts.As a result,low-cost,reusable,and stable catalysts require to be invented.More significantly,a systematic way to evaluate the catalyst’s ability should be established,including activity,selectivity,lifetime (half-life),stability,etc.Notably,the low O3usage efficiency is also a key issue for catalytic ozonation applications in wastewater treatment,demanding greater attention in future research topics [73].Furthermore,by-products probably be produced during the ozonation catalysis process,which is regarded as a severe hazard to human health and the ecology,as such appropriate mitigation techniques should be adopted in the ozonation application and ozone-based sewage treatment [61].
2.4. Application of other emerging AOPs on pharmaceutical contaminants removal
Except for several common catalytic oxidation approaches mentioned above,various emerging AOPs have been proved to be effective in degrading pharmaceutical contaminants with excellent ability from hospital sewage presently.For instance,combined systems like photo-electrocatalytic oxidation (PEC),electroperoxone processes (E-peroxone),and high-effective systems including Fenton/Fenton-like process,and piezoelectric catalysis oxidation [17,74,75].
2.4.1.PEC
PEC technology is a combination derived from photocatalysis and electrocatalysis processes.It is based on light irradiation to stimulate the semiconductor and is supplemented with external electric fields [10].The degradation of pharmaceutical contaminants in the PEC process is illustrated in Fig.4,firstly caused by the photoactivation of the semiconductor anode,then by separating the light-generated electron-hole pair (e−-h+) with the support of electric fields.Thus,pharmaceutical contaminants on/near the electrode surface are degraded by residual holes on the anode or ROSs (•OH and•O2−) produced on the cathode [10,75].Generally,the PEC processes are influenced by multifarious factors,for instance,light and electric field intensity,electrode materials,solution pH,and temperature [18].Undoubtedly,the photoelectrodes materials are the most vital factor in PEC processes in determining the removal efficiency.
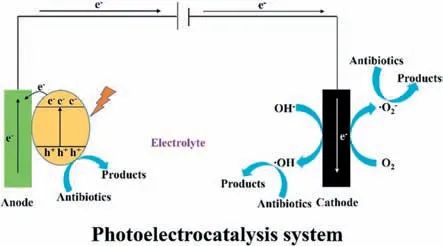
Fig.4. Scheme for photoelectrocatalysis system.Reprinted with permission [5].Copyright 2021,Elsevier.
Up to now,many photoelectrodes have already been successfully prepared for pharmaceutical contaminants removal,for example,WO3,Fe2O3,TiO2,ZnO,and CuO [54,75–77].Among them,TiO2-based catalysts have become the most broadly material during PEC treatment owing to their ubiquity,non/low toxicity,and great optical properties.Luetal.[76] first explored the TiO2nanotube array electrode to degrade tetracycline.Moreover,Liuetal.[77] synthesized highly ordered TiO2nanotubes,achieving better removal efficiency of OFL (90.1%)viathe PEC process than photocatalysis (75.6%) and electrocatalysis (65.6%) alone.It was mainly due to the fact that PEC is more helpful than photocatalysis and electrocatalysis to promote the separation and to impede the recombination of e−and h+with a lower voltage.As a result,the PEC efficiency is increased and the re-reduction of intermediates on the cathode is hindered.More importantly,the efficient design of novel photoelectrochemical reactors will become a challenge for future scientists and engineers [78,79].
2.4.2.E-peroxone
E-peroxone,another promising technology for pharmaceutical contaminants removal,was invented by Professor Wang of Tsinghua University [80].Compared to traditional catalytic ozonation,it makes full use of the mixture of O3and O2exposed to the ozone generator and overcomes the unsafe problems in the transportation,storage,and operation of H2O2[80].The process is a coupling of electrocatalysis and ozonation,where the O2from the ozone generator reacts at the cathode to produce H2O2,and the exposed O3produces•OH catalyzed by the H2O2generatedin-situ[81].In addition,O3acquires electrons to generate free radicals and a series of reactions as listed in the following Eqs.20–23 [82].
The E-peroxone system has been extensively applied in hospital sewage treatment by virtue of its individual and integral process mineralization capacities [83].For instance,Yu and coworkers [4] selected porous graphite as cathodes within supplying the O2/O3mixture directly.Therefore,thein-situH2O2yield and TOC removal efficiencies were higher than those of electrocatalysis and ozone oxidation alone in the actual treatment of hospital wastewater containing 126 pharmaceutical compounds.In addition,the advanced E-peroxone process successfully decontaminates diclofenac (DCF),ibuprofen (IBP),and gemfibrozil (GMZ) from the secondary effluents by enhancing the removal kinetics of individual O3process by ∼40%−170% [82].
In conclusion,the E-peroxone treatment displays a distinct synergistic impact between electrocatalysis and ozonation of wastewater treatment,which can be potentially used in hospital sewage treatment.More critically,it is important to focus on the reaction equipment structure of E-peroxone and optimization of the operational parameters,providing a more detailed pilot evaluation for putting into actual hospital sewage treatment [74].Obviously,PEC and E-peroxone are regarded as the typical combination system for pharmaceutical contaminants’degradation,and further studies are required to investigate how their properties enhance or limit individual/integral efficiency,as well as the cost of the entire treatment system in practice.
2.4.3.FentonandFenton-likeAOPs
In fact,Fenton systems have been put into practical applications for the treatment of pharmaceutical pollutants in hospital sewage owing to the significant production of•OH [84].Especially,Liuetal.[85] demonstrated that Fenton processes are effective for antibiotic elimination.This study systematically reviews the key parameters (such as pH,catalyst,electrode,light source,and device)of various Fenton techniques (e.g.,electro-Fenton,photo-Fenton,photoelectro-Fenton,and solar photoelectro-Fenton) for contaminants removal.Moreover,the reaction mechanism,degradation pathways,toxicity assessment of pollutants,and practical cost are also comprehensively discussed in this article [85].The mechanism details of the Fenton reaction are described in Supplementary material Text S2 (Supporting information).Fortunately,Fentonlike AOPs including Fe0/H2O2process,PS-based system,and PAAbased AOPs remain the effective performance of Fenton and try to broaden the reaction pH and optimize the separation and recovery of ions [86,87].
In particular,PAA-based AOPs,as a novel Fenton-like system,have received increasing attention due to their high oxidizing capacity (1.96 Vvs.standard hydrogen electrode (SHE)),easy activation performance,and excellent decontamination efficiency [17].Generally,PAA-based AOPs mainly depend on activation by energy(e.g.,UV,US),metal ions/oxides (e.g.,Fe2+,Co2+,Mn2+),carbon materials (e.g.graphene oxide (GO),CNTs) to generate multiple ROSs (e.g.,•OH,organic radicals,and high-valent iron species) to rapidly oxidizing contaminants in water/wastewater [2,17,88].Current studies reported that PAA-based AOPs manifest outstanding performance in pharmaceutical contaminants removal from hospital wastewater.Xieetal.[2] adopted CoFe-LDH to activate PAA to generate abundant ROSs for various pharmaceuticals degradation with high efficiency from hospital sewage,including SMX,sulfisoxazole (SIZ),naproxen (NAP),and carbamazepine (CBZ).Surprisingly,PAA was also efficiently activated by low-cost,green,and environmentally friendly FeS (25 mg/L) to rapidly oxidize and degrade three pharmaceuticals (NAP,SMX,CBZ) in a wide pH range of 3.0–9.0 with 80%−100% removal efficiencies within 5 min [88].
Nevertheless,water matrices such asand organic matters contained in the actual hospital sewage have an inhibitory effect on the PAA-based system,so further work needs to be done to reduce their impact on the degradation of contaminants.Furthermore,most previous studies have concentrated on the efficiencies of decontamination,while mainly ignoring the identification of potentially harmful by-products.As such,PAAbased AOPs require to be evaluated for toxicity and appropriate control measures should be established to assess the potential environmental impact [17].
2.4.4.Piezoelectriccatalysisoxidation
Recent studies disclose a significant advancement in the properties of piezoelectric materials,due to their high surface area and nanoscale built-in electric field,thus inducing an effective charge carriers generation and a fast reaction mediated by ROSs [89].Piezoelectric catalysis utilizes the combination of the piezoelectric effect and catalytic reaction to achieve an efficient catalytic reaction.Specifically,piezoelectric materials undergo deformation upon application of an external force,resulting in charge separation phenomena that generate electrons and holes and react with water molecules to produce ROSs [89].Lanetal.[90] synthesized BaTiO3piezo-catalyst and applied it for the piezo-catalytic removal and dichlorination of 4-chlorophenol.Surprisingly,the results indicated that the ultrasonic irradiation caused the deformation of BaTiO3,which generated ROSs including1O2,and•OH,leading to the degradation of 4-chlorophenol and other organic pollutants such as phenol,hydroquinone,and cyclohexanone.Actually,the formation of large amounts of ROSsor•OH) as oxidants in external pressure conditions is the key to achieving pharmaceutical contaminants decontamination in piezoelectric catalysis oxidation.Moreover,the synthesis of piezoelectric materials is critical in the future for the piezo-catalysis strategy in pollutant degradation or metal ion/radionuclide reduction/solidification [91].
3.Synchronous decontamination and disinfection mechanism and efficiency of AOPs
3.1. Photocatalytic oxidation
3.1.1.Thebacteriainactivationmechanismofphotocatalyticoxidation
Among multitudinous AOPs technologies,photocatalysis is a safe and effective green-friendly photochemical means,long applied to fight against pathogenic bacteria and inactivate viruses as well [36,92].It was first confirmed in 1985 that semiconductors like TiO2in the existence of light could inactivate bacterial activity by generating ROSs [93].Moreover,previous papers have confirmed that the pivotal target of photocatalysis technology is the bacterial envelope,which can modify membrane permeability and cause cell death [19].
For nearly two decades,there have been many attractive photocatalysis applications in drinking water and hospital sewage disinfection [94].It can be demonstrated that photocatalyst has a strong ability to inactivate bacteria and viruses,and a detailed disinfection mechanism can be disclosed in Fig.5.Similar to pharmaceutical contaminants removal,ROSs (like[31] act as outstanding powerful oxidizing agents that damage microorganisms by interacting with bacterial cells and attacking the cell wall and contents (e.g.,protein,lipids,carbohydrate and DNA) without further chemical oxidants addition [95].Eventually,it causes protein denaturation,inactivation/death and the generation of various end-products,and Eq.24 displays a basic photocatalytic water disinfection process.
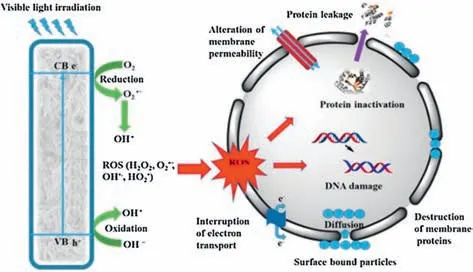
Fig.5. A schematic diagram for the proposed disinfection mechanism during photocatalytic oxidation.Reprinted with permission [95].Copyright 2018,Elsevier.
3.1.2.Synchronousdecontaminationanddisinfectionvia photocatalyticoxidation
The above research results (Part2.1andPart3.1.1) state that photocatalysis can oxidize various pharmaceutical contaminants as well as inactivate different bacteria and microorganisms.Fig.6 illustrates the photodegradation mechanism of pharmaceutical contaminants and the inactivation pathway of bacteria.Excitingly,this green and safe process eliminates chemical pollutants and biological hazards in the hospital sewage at once by using solar energy,without containing by-products harmful to the environment [96].Therefore,literature references for the photocatalytic simultaneous degradation of pharmaceutical contaminants and inactivated antimicrobials in water are collated in Table S4 (Supporting information).
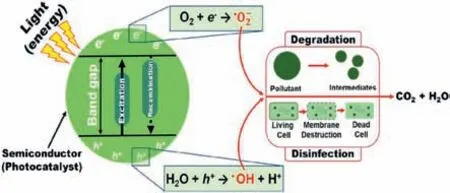
Fig.6. Mechanisms of the organic matter and microbial pollution photocatalytic removals.Reprinted with permission [19].Copyright 2020,Elsevier.
Nevertheless,the result was inadequate to testify to the benefits and advantages of photocatalysis and the specific reasons are as follows [10]: (1) there are few research articles dealing with this topic,and photocatalysis has not been sufficiently studied in hospital wastewater treatment.Additionally,E.coliis usually regarded as the bacteria model discussed in most research,leaving the influence of other bacteria undefined;(2) to simplify the analysis approaches,many researchers commonly treat decontamination and sterilization separately,ignoring the most key aspect of the nonnegligible interaction between antibiotics and ARB.Actually,this interaction can lead to important changes in the removal efficiency of a harmful substance by the formation of a hybrid system with photocatalysis,resulting in enhanced activity.It is well acknowledged that antibiotics have the antibacterial property for treating bacterial infections by preventing the multiplication or destroying the cellular structure [10].Oppositely,ARBs yield corresponding degradation enzymes to resist antibiotics by modification or hydrolysis molecular structure [10].In other words,the interaction of antibiotics and bacteria plays a key role in the microbial removal of those antibiotics as well.To settle this antagonistic process,the photocatalyst/semiconductor is the assistant in terminating this war [18].For instance,it has been disclosed in the former research that the introduction of TiO2can simultaneously remove antibiotics and sterilize bacteria [23,96].This effect creates a new mechanism,namely a hybrid system for each pollutant (photocatalyticbacterial,photocatalytic-antibiotic,bacterial-antibiotic),as demonstrated in Fig.7.Hence,one of the challenges facing the world nowadays is how to find photocatalysis with lower cost and less environmental damage to meet future water purification and disinfection needs.
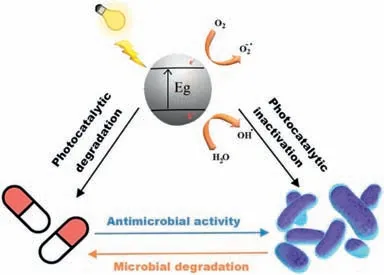
Fig.7. Summary of hybrid mechanisms on the photocatalytic removals of antibiotics and microbial pollution.Reprinted with permission [10].Copyright 2021,Elsevier.
3.2. Electrocatalytic oxidation
3.2.1.Thebacteriainactivationmechanismofelectrocatalytic oxidation
At present,the three dominant bactericidal mechanisms of electrocatalysis mainly include (1) direct electron transfer oxidation on the anode,(2) indirect oxidationviaROS (e.g.,•OH,H2O2,),and(3) electroporation due to high or local high voltage [97].Therein,direct oxidation has been deemed to be the dominant inactivation approach for bacteria and viruses [40].The specific mechanisms are explained below.Firstly,direct oxidation depends on electrons directly transferring from the protein or functional groups of the cell membrane to the anode.This mechanism provides a radical site for lipid peroxidationviaa sequence of radical chain reactions,resulting in cell membrane non-integrity and cell death eventually [56].Unfortunately,the detailed mechanism and exact process have never been elaborated up to now.Secondly,indirect oxidation inactivation is owing to the strongly oxidizing properties of the generated ROSs (e.g.,•OH,H2O2,and O3),which can oxidize sugars,proteins,and nucleic acids,thus destroying the microbial structure and inactivate cells [40,43].And the intermediate reaction equation is listed as Eqs.25–29.Moreover,electroporation’s mechanism is that a strong electric field (>105V/cm) generates the magnetic field,and their interaction induces phospholipids in the surface of the cellular membrane to rearrange.Finally,this situation creates a membrane rupture and cell death [98].
3.2.2.Synchronousdecontaminationanddisinfectionvia electrocatalyticoxidation
Many researchers have manifested that the EC system is strongly impacted by the adopted electrode materials and the applied current density [16,99].Thus,operating conditions and electrode materials determine the type of oxidant produced,which directly affects the rate of oxidant generation required to inactivate bacteria [97].Therein,electrode materials including BDD,mixed metal oxides (MMO),and carbon fiber felt (CFF) is frequently utilized in water disinfection operations and have demonstrated effective disinfectant properties [52].Nietal.[100] have reported the disinfection ability of a CFF through EC against grampositive/negative pathogens (Fig.8).Results disclosed that gramnegative bacteria (E.coliandfecalcoliform) and gram-positive bacteria (BacillussubtilisandE.faecalis) were entirely removed at short hydraulic retention time (1–10 s) and low applied voltages (1–5 V).In addition,since indirect oxidation was ignored at low voltages,the authors used direct oxidation as the primary disinfection mechanism [100].Differently,BDD electrodes generate ROS at low voltages of 4–10 V,which can completely disinfectEnterobacteriaceaeand obviously reduce spores in wastewater [101],and the results of the pilot test also showed a great disinfection effect.
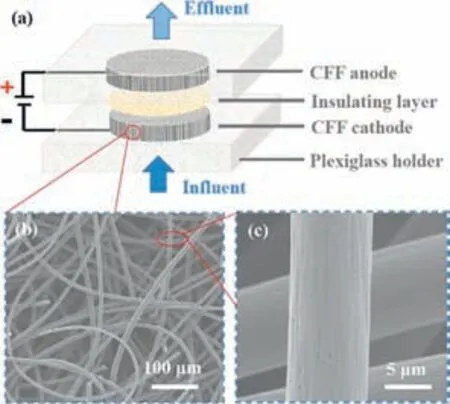
Fig.8. Construction and characterization of the flow-through electrode system(FES).(a) The schematic diagram of the FES includes Plexiglass coaxial electrode holders,filter paper as the insulating layer,and two CFF electrodes connected to the DC power.(b) Scanning electron microscopy (SEM) image of the micro-structure of CFF electrodes.(c) SEM image of the carbon fibers under high magnification.Reprinted with permission [100].Copyright 2020,Elsevier.
Although there are limited articles on electrocatalytic oxidation for simultaneous sterilization and decontamination,Gongetal.[102] and Duetal.[42] manifested that the same electrode material used for disinfection can also be utilized to degrade pharmaceutical contaminants under similar conditions.Meanwhile,an excellent,environmentally friendly,and energy-saving EC has been formed with the continuous development of advanced electrode materials and electrochemical reactors.Additionally,great progress has been made in the joint application research of varied technologies.Many scholars have discussed the application of EC in water/wastewater treatment,but most of them are still in the laboratory stage.Therefore,various problems in the practical application process need to be solved,including electrode plate fouling,low current efficiency,continuous disinfection,and disinfectant concentration control.This review supposed that the future research trend should focus on optimizing electrocatalyst reactors and operation parameters,creating a compact electrocatalysis process for water disinfection and decontamination.
3.3. Ozone catalytic oxidation
3.3.1.Thebacteriainactivationmechanismofozonecatalytic oxidation
The disinfection mechanism of catalytic ozonation includes direct O3disinfection and indirect oxidation which produces radicals like•OH,[43,56] as Eqs.11–19 described in the previous chapter (Part2.3).The specific ROSs inactivate bacteria by a mechanism similar to photocatalysis (Part3.1.1),destroying microorganisms’structure by oxidants for the purpose of disinfection[103,104].
3.3.2.Synchronousdecontaminationanddisinfectionviaozone catalyticoxidation
In general,ozone catalytic oxidation is very effective in the disinfection of pathogens owing to its great oxidizing capacity,which is higher than that of traditional chemical disinfectants and has no harmful by-products [105].Catalytic ozonation is used to disinfect the secondary effluent treatment of hospital sewage,a 99.9%removal rate can be achieved in the case ofE.coliin the water exceeding 7-log units at the disinfection contact reaction time of 10 min [12].Furthermore,Malvestitietal.[64] first assessed the effectiveness of homogeneous catalytic ozonation by various metal ions (Co2+,Al3+,Fe2+) in urban sewage disinfection.Subsequently,the degradation of contaminants in secondary effluent was also evaluated.The results manifested that the inactivation ofE.coliandP.aeruginosawas increased by nearly 20% with 1 mg/L Co2+,Al3+,and Fe2+,and by above 40% with 10 mg/L Fe2+compared with single ozonation.Simultaneously,it can save 30%−50%of ozone dose compared to single ozone oxidation and achieve complete degradation of pollutants.Moreover,the simultaneous removal of chloro–tetracycline (CTC) and ARB by catalytic ozonation technology (O3/H2O2,O3/UV,O3/UV/H2O2) was investigated by Leeetal.[106].The results demonstrated that the O3/UV and O3/UV/H2O2systems were superior to O3/H2O2system with almost complete degradation of CTC within 20 min,and the combination of O3and UV reduced the number of CTC-resistant bacteria by a 6-log within 40 min [106].
Delightfully,Kolosov and co-workers [105] studied and compared five novel materials applied to ozone catalytic oxidation for enhancing the degradation of pharmaceutical contaminants and disinfection.Therein,wollastonite and zeolite did not improve disinfection efficiency and pharmaceutical contaminants removal,including IBP,NAP,and GMZ.Differently,AL-1010S and TiO2-Al2O3displayed enhancement for both facts,and Polonitedid not promote pharmaceutical contaminants degradation but resulted in the higher inactivation ofE.coli.In summary,TiO2-Al2O3,AL-1010S,and Polonitecan be regarded as great catalysts and provide mechanisms to reduce the O3dose requirements for disinfection with great reusability (Fig.9).
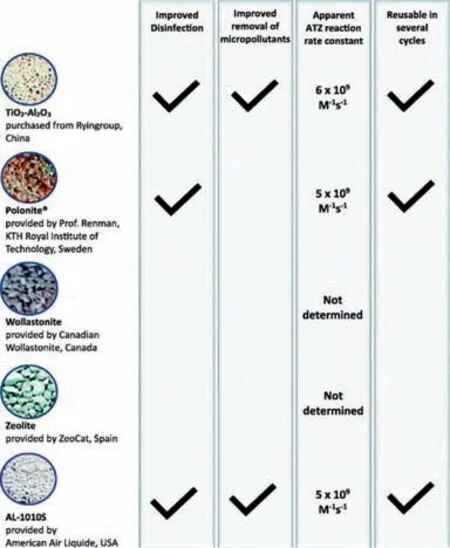
Fig.9. Five novel materials for wastewater ozone catalytic oxidation treatment for disinfection and removal of micropollutants.Reprinted with permission [105].Copyright 2018,Elsevier.
The above studies suggested that both homogeneous/heterogeneous catalytic ozonation have the potential to promote the treatment of hospital sewage,especially for pharmaceutical contaminants removal and disinfection to some extent,helping to meet more strict emission regulations.However,considering the difficulties in production,operation,and maintenance,there are still relatively few successful cases of the practical application of catalytic ozonation in municipal wastewater and hospital sewage.Meanwhile,disinfectants are expensive and require high concentrations,limiting the application of large-scale disinfection[6,105].In addition to the invention of materials for efficient O3utilization,further research is needed to verify the performance in real waters,to investigate the properties and toxicity of transformation products that might be generated during the catalytic ozonation,and to assess the economic feasibility of catalytic ozonation for sewage treatment.
3.4. Application of other emerging AOPs on synchronous decontamination and disinfection
At present,increasingly emerging AOPs are researched and applied in hospital wastewater [8,41],mainly including the combination of several popular processes,and strongly Fenton-like systems,and they are proven for disinfection and decontamination purposes [107,108].Several novel advanced oxidation processes,covering PEC,E-peroxone,Fenton/Fenton-like AOPs,and piezoelectric catalysis oxidation,are described in detail below.
3.4.1.PEC
Photo-assisted electrocatalytic advanced oxidation process (PEC)acts as a promising and valuable option for wastewater disinfection [18].During the PEC process,the cooperation between electrocatalytic-produced radicals and photo-generated reactive species results in enhancing their capabilities when compared with the respectively separate process or with other chemical or physical methods [13,75].The inactivation of germs occursviamechanisms at different distances from the anode,which is illustrated in Fig.10,and the following are detailed mechanisms [18]: (1) direct oxidation: directly on the surface of the electrode,(2)quasi-direct oxidation: at the anode’s surrounding rely on electrocatalytic produced•OH species,(3) and indirect oxidation: active species generated by photoelectrons in bulk solution away from the electrode surface.From this scheme (Fig.10),it is clear that multiple ROSs are generated in diverse reactor areas,where they interact with the bacterial membrane and the most exposed structures,leading to deactivation.As a result,the disinfectant’s oxidation potential is crucial to the inactivation process.Namely,the standard potential values of ROSs generated in the PEC listed in Table S5 (Supporting information) match the mechanisms as displayed in Fig.10.Particularly,a favorable reactor arrangement is especially necessary for direct disinfection oxidation to carry out the disinfection reaction effectively,and some obvious examples list in the Text S3 (Supporting information).

Fig.10. Mechanisms and zones of reaction for direct,quasi-direct,and indirect oxidization in PEC.Me+=metal,X=Cl or Br,Rads=microorganism,Pads=inactivated microorganism.Reprinted with permission [18].Copyright 2021,Elsevier.
Obviously,the majority of disinfection research on the PEC process has focused onE.coliinactivation more than other pathogens,due to its ubiquity and the simplicity of detection in the laboratory[109].Nevertheless,it is essential to broaden the pathogen inactivation spectrum of these technologies by testing various pathogens(e.g.,viruses,ARBs,and helminth eggs),especially virus rampage currently [3].On the one hand,TiO2is by far the most researched in PEC oxidation because of its non-toxicity,high chemical oxidation,and effective photoactivity.On the other hand,UV irradiation is the most commonly used photon source for PEC studies [110].However,it is significant for the development of cost-optimized decontamination and disinfection treatment,and novel materials,and strategies for harnessing solar energy will be investigated in the future.
3.4.2.E-peroxone
The E-peroxone technology is an electricity-based oxidation process that producesin-situH2O2electrochemically through the reduction of cathodic O2during ozonation,enhancing the conversion of O3to•OH [4,111].In recent years,Wangetal.[112] have comparatively researched the decontamination and disinfection of synthetic antibiotics wastewater and real secondary effluents including hospital sewage by E-peroxone and ozonation.Meanwhile,a study on the synchronous degradation of 126 types of pharmaceutical contaminants and the inactivation of pathogenic microorganisms from real hospital sewageviaE-peroxone treatment was realized by Yuetal.[4].Moreover,Zheng and co-workers [111] further manifested the elimination of ARB and plasmid-encoded antibiotic resistance genes after E-peroxone treatment.
According to the above literature,they used carbonpolytetrafluorethylene (carbon-PTFE),graphite felt as cathode materials,and Pt,IrO2/RuO2mesh as anode orderly.Surprisingly,all of them obtained high efficiency in contaminants elimination and bacteria disinfection synchronouslyviain-situgeneration of H2O2-promoted O3depletion and accelerated•OH generation during E-peroxone treatment [4,111,112].By comparison,it is a more efficient,economical,and reasonable approach than single O3and EC in actual wastewater (e.g.,hospital sewage,secondary wastewater effluent).Nevertheless,the removal and elimination rates did not represent the actual effluent handling capacity [14,113],and further exploration of changes in pH,biological oxygen demand(BOD5),chemical oxygen demand (COD),total coliform (TC),and pharmaceutical contaminants concentration is urgently needed[14,113].
3.4.3.FentonandFenton-likeAOPs
In general,the fundamental principle of Fenton reaction disinfection is to attack various pathogens by the role of•OH oxidation capacity,or the direct oxidation of H2O2.Successfully,some researchers have proved that Fenton oxidation is effective for the treatment of hospital wastewater with 25 mg/L Fe3+,1 g/L H2O2and 70–90 °C [114].Under these operating conditions,considerable COD reduction (70%),significant TOC mineralization (50%),and complete elimination of harmful phenolic compounds were achieved,resulting in a nontoxic effluent at the end of treatment[114].In addition,Fenton-based processes have been demonstrated to efficiently inactivate ARBs,as evidenced by a 6-log reduction of colistin-resistantE.coliS115with the dosage of 0.18 mmol/L of Fe(II) and 0.6 mmol/L of H2O2[115],and 5-log decrease of grampositive clarithromycin-and sulfamethoxazole-resistantEnterococcusfaecalisin photo-Fenton (PF) process [116].
Moreover,Fenton-like AOPs,such as PAA-based AOPs,are attracting increasing research and application interests for removing contaminants,which are also extensively investigated in hospital sewage disinfection since it has strong disinfection potential and low generation of hazardous DBPs [17,117].Furthermore,the application of PAA-based disinfection allows for simple and relatively low-cost optimization of current chlorination equipment.Currently,a limited number of studies on pathogen inactivation by PAA-based AOPs have already been reported,mainly with UV/PAA process [17].
According to some studies,the UV/PAA system has a stronger bacterial disinfection effect than the total of the single roles,demonstrating the synergy impact between the two methods [118].There are various mechanisms in the UV/PAA process to disinfect pathogens.Specifically,UV radiation could directly cause damage to nucleic acids,whereas PAA disinfectants might target microbial,membranes,cell walls,enzymes,or transport mechanisms [119].Additionally,radicals generated in UV/PAA systems also probably act as disinfectants to limit the ability of microorganisms to restore cellular structures,as illustrated in Fig.11 [120].Some examples are enumerated in Text S4 (Supporting information).
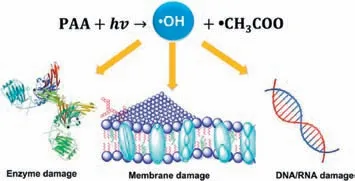
Fig.11. Schematic diagram of general inactivation mechanisms of microorganisms by •OH.Reprinted with permission [17].Copyright 2021,Elsevier.
Therefore,Fenton/Fenton-like AOPs are feasible for simultaneously pharmaceutical contaminants removal and bacterial inactivation,especially for emerging microorganisms and pharmaceuticals in hospital sewage.Further studies should systematically determine the effects of degradation/inactivation kinetics,operational parameters,and water matrix and mechanisms to fully evaluate these technologies.
3.4.4.Piezoelectriccatalysisoxidation
The emergence of piezo materials as a disinfector of pathogens was adopted extensively in recent years owing to the advanced green oxidation approach [89].For instance,sodium niobate(NaNbO3) nanorods with a diameter of 100 nm and a length of 1–2 μm have a strong piezoelectric effect and are used forE.coliinactivation tests.Sharmaetal.used solid agar to measure the inhabitation zone ofE.coliand the tested inactivation zone was 23 mm in size [121].After 24 h of incubation with the addition of 1 g/L NaNbO3at 120 W and 40 kHz vibration,no bacterial growth was found on the sample plates treated for 120 min.This effective bacterial inactivation was due to anionic•OH which disrupts the bacterial cell wall,allowing H2O2to penetrate inside the cell and kill the bacteria immediately.Furthermore,the large impressed output voltage of 16 V enhances the effective charge generation during piezo-catalysis,which subsequently produces higher ROSs [121].Surprisingly,this work also achieves 100% degradation of organic pollutants within 80 min,displaying the feasibility of piezoelectric catalysis oxidation in synchronous decontamination and disinfection of hospital sewage treatment.More significantly,apart from internal parameters (e.g.,size,doping material),external conditions obviously affect the inactivation rate,including vibration,thermodynamics,pressure,and luminescence [91].Improvements in these areas can improve the overall material catalytic performance.
4.The impact of water matrix and pH on decontamination and disinfection of advanced oxidation processes
4.1. Water matrix
In actual hospital wastewater,pharmaceutical contaminants coexist with various inorganic ions (e.g.,Cl−,and effluent organic matter (EfOM),which may have an impact on how efficiently the pharmaceutical contaminants degrade [5].For example,Cl−is the most common water matrix in waters,especially with a high concentration in hospital sewage and pharmaceuticalcontaminated wastewater [122].In general,Cl−scavenges highly reactive•OH and forms less reactive chloride radicals (e.g.,Cl•,ClOH•−,Cl2•−),thus reducing degradation kinetics in various catalytic processes [122,123].Additionally,the more specific influence and references of inorganic ions and organic matter are disclosed in Text S5 (Supporting information).In conclusion,the degradation efficiencies of AOPs for individual pharmaceutical contaminants are the result of the combined action of the aqueous matrix components.Depending on the reaction mechanism and process of these aqueous components,the effect of inhibition,neutralization,or enhancement may be observed.Organic matter can act as inhibitorsviaphoto attenuation,scavenging,or adsorption on the catalyst.Moreover,it also performs as a promoter by generating ROSs to improve indirect photolysis,or through regenerating the catalyst.More significantly,inorganic matter can also be inhibitors by scavenging,iron complexation,forming radicals less active than•OH,reduction of their effective surface area,or adsorption of catalysts.Also,it can consider iron ions as an additional source of catalysts or through the formation of nitrate ions by ROS to facilitate pharmaceutical contaminants removal.The different effects of the above water matrix on AOPs are summarized in Fig.12.
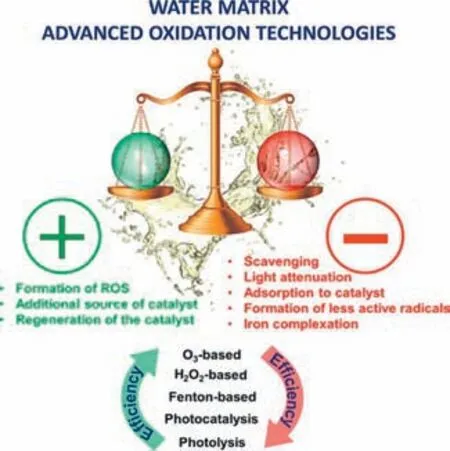
Fig.12. The influence of water matrix on advanced oxidation technologies.Reprinted with permission [124].Copyright 2019,Elsevier.
Nevertheless,there are only a few reviews and data that are now available,and it is still not fully understood how individual components of water work and how it functions.Further research is called to evaluate the wide range of reactions occurring in complex wastewater and to increase their application in hospital wastewater treatment [124].
4.2. pH
The pH of the solution plays a crucial role in the removal rate of pharmaceutical contaminants and the degree of inactivation of pathogens [22,39].In the PC/PEC process,pH affects the removal and adsorption capacity of target contaminants and pathogens by influencing the surface charge properties of the photocatalyst and determining the ionization state of the catalyst surface.Taking TiO2as an example,many reports have used the point of zero charge(PZC) of TiO2to investigate the effect of pH on PC performance[125].PZC is the condition where the surface charge of TiO2is zero/neutral when the pH is in the range of 4.5–7.0.At the PZC value of TiO2,there is almost no interaction between the photocatalyst particles and the contaminant/pathogen due to the absence of electrostatic force.When the operating pH is lower than the PZC(TiO2),the surface charge of the catalyst changes to be positive and gradually exerts an electrostatic gravitational force on the negatively charged compounds.Whereas when the pH is greater than PZC(TiO2),the catalyst surface becomes negatively charged and repels anionic compounds in water.In conclusion,varied pH values have different effects on the charge density of the TiO2catalyst surface [125].
In EC systems,abundant H+enriched on the electrode surface can react with O2to produce H2O2and•OH (E0=2.85 V) eventually under acidic conditions [126].Additionally,strongly acidic conditions also probably improve the conductivity of the solution by generating additional oxidants (e.g.,H2SO4-sulfate ions) to enhance the decontamination rate [53].In contrast,the increased OH−and the reaction between OH−and•OH decreases the dosage of•OH under alkaline conditions and results in a low oxidation capacity of•OH (E0=2.02 V) under alkaline conditions [127].In conclusion,high pH probably reduces the elimination efficiencies of pollutants[126].More importantly,pH values also impact the charge of contaminant molecules.For instance,negatively charged deprotonated CIP molecules might be more readily adsorbed in the anode,causing an easy degrading of the CIP molecules by•OH [128].
During ozone catalytic oxidation processes,some research demonstrates that Lewis’s acids are the active sites for the production of free O3and ROSs,while others suggest that Lewis’s basic sites play a crucial role in the catalytic ozonation process[62].These discrepancies are obviously evident when using carbon materials as catalysts [129].Developing a universal comprehension and accepted active catalyst site is significant and needs to be elucidated.To achieve this goal,full and complete studies are needed in the future.Moreover,apart from controlling and monitoring pH values in laboratory experiments,the value of pH changes in actual sewage samples must be considered and monitored in ongoing research.Hence,future research must focus on understanding how pH impacts the AOPs process and finding optimal pH values for various catalysts and contaminants.
5.Economic aspects and scale-up of the advanced oxidation process
For catalytic ozonation treatment,the ozone catalytic oxidation method with iron-based monolithic catalyst packing by Maetal.is an example [130].Due to the O3generator and catalyst,the investment cost used is relatively high but has the strength of comparatively small occupation,low chemical input,low running cost,and no secondary waste.Furthermore,by depreciation calculation of catalyst and equipment,the common life of iron-based monolithic catalyst packing is 5 years and the O3generator is 8 years,obtaining the investment cost is about 0.00102 CNY/L.And depreciation plus actual operating costs (including electricity only)were calculated at 0.00122 CNY/L (O3/ΔCOD=1.2).Fortunately,the iron-based monolithic catalyst packing catalytic ozone oxidation process with a high COD removal rate is an economical choice for the deep treatment of industrial wastewater.
The economic cost of PC and EC for wastewater decontamination and disinfection can be divided into the initial investment,primarily associated with lamps,electrodes,and the operating costs,which are involved in energy demands and electrode and parts replacement [18].Conventional anode materials,such as dimensionally stable anodes (DSA) and BDD are recognized to be costly but extremely stable,resulting in low maintenance and replacement costs.On the other hand,semiconductors,metallic electrodes (e.g.,aluminum or iron),and carbonaceous materials are affordable choices for PC/EC but have the associated weakness of higher replacement frequency.At this point,PEC is a better means of decontamination and disinfection,in terms of energy requirements for PEC to promote the inactivation of pathogens,the specific electrical energy (EEO) can be calculated using Eq.30 in kWh m−3order−1[131].
whereEis the cell voltage (V),Wlampis the lamp power (kW),Iis the current intensity (kA),tis the operating time (h),Vis the volume (m3),andC0andCfare,respectively,the removal of 1storder pathogens after initial and final concentrations (CFU/mL).
Herraiz-Carbonéetal.[131] disclosed that the adoption of MMO and BDD anode at the low current density resulted in reduced electric energy demands compared to the UV disinfection process.Thus,PEC potentially successfully competes with single AOPs in terms of cost-saving and sustainability.
Overall,the above highlights the future trend in the practical application of AOPs that requires a combination of multiple therapeutic processes to mitigate the drawbacks of the respective techniques.However,economic assessment of hybrid processes on hospital effluent is scarce and more insights are needed to make a broad comparison of the costs and efficiencies of different processes and their combinations to achieve full application early.
6.Conclusions and future perspectives
For the first time,this manuscript mainly provides a comprehensive review of various promising advanced oxidation processes for hospital sewage treatment,including photocatalytic oxidation,electrocatalytic oxidation,ozone catalytic oxidation,and other emerging technologies (PEC,E-peroxone,Fenton/Fentonlike,and piezoelectric catalysis oxidation).Delightfully,the abovementioned AOPs are regarded as effective and promising technologies for pharmaceutical contaminants removal and bacterial inactivation.Therein,the PC process completely mineralizes pollutants easily with less toxicity and energy consumption;EC does not occur secondary pollution generally because it only requires electricity without agents adding and new impurities introducing;ozone catalytic oxidation possesses high efficiency and excellent stability;PEC circumvents the drawbacks of EC and PC to achieve better degradation and inactivation;E-peroxone enhances the utilization of O3;Fenton/Fenton-like AOPs have the potential to simultaneously decontaminate and disinfect,and piezoelectric materials are diverse and effective.Nevertheless,the reported literature has manifested some challenges in the practical application of these AOPs.
Based on the progress achieved so far and the identification of existing difficulties for disinfection and decontamination by AOPs in hospital wastewater,we propose the following future research directions:
(1) Development of materials and renewable energy.The optimum electrode catalysts for EC and PEC systems must satisfy the following criteria: (1) good catalytic activity for the desired reaction;(2) high enough conductivity for electron transfer;(3) good chemical and mechanical stability to maintain catalytic activity during long-term operation.Moreover,cheap and renewable energy sources should be vigorously developed to reduce the cost of the PC/EC.It is also crucial to accelerate the simplification of the synthesis process of ozone catalysts and to optimize the preparation conditions,helping their mass production and industrial application.
(2) Synergistic degradation of complex pharmaceutical contaminants and inactivation of pathogens.These reported process catalysts for AOPs have been evaluated by single pharmaceutical contaminant/pathogen removal (mainly focusing on sulfonamides andE.coli).In fact,real hospital wastewater contains multiple pharmaceutical contaminants/pathogens and intricate interactions.It is essential to evaluate the synergetic removal performance of the catalysts under complex pharmaceutical contaminants/pathogen systems in future studies.
(3) Evaluating the potential environmental effect of AOPs.Currently,existing research has focused on the removal effectiveness of contaminants and pathogens,while the identification of potential biotoxicity/disinfection byproducts has received less attention.As a result,the toxicity of AOPs required to be evaluated,and if any harmful by-products are discovered,appropriate control measures should be created.
(4) Optimization of advanced oxidation processes and equipment.The application of AOPs places strict requirements on the equipment of wastewater treatment plants.For instance,highly-efficient new photoelectrochemical reactors need to be designed and applied.Additionally,the structure of the reaction equipment in AOPs systems requires to be studied to further optimize the operating parameters (e.g.,catalyst dosage,solution pH,and field intensity) and provide more detailed pilot evaluations for putting into actual hospital wastewater treatment.
(5) Comprehensive evaluation and recognition of the treatment process.For a comprehensive assessment of these AOPs technologies’ feasibility in hospital sewage treatment,further accurate determination of degradation/deactivation kinetics and mechanisms,effects of operational parameters,and anti-interference ability in the practical application should be carried out.
(6) Monitoring and control of antibiotic-resistant genes (ARGs).Apart from ARBs,the production and dissemination of ARGs in hospital sewage cannot be ignored.Especially,analyses of antibiotics removal by AOPs and subsequent characterization of the byproducts revealed that the degraded intermediate products of some antibiotics can induce resistance genes.Therefore,it is necessary to evaluate the abundance of ARGs in AOPs treatment and further identify the AOPs technologies to effectively remove both the antibiotics and the ARGs.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors wish to thank the National Natural Science Foundation of China (Nos.52170088 and 52070133),China Postdoctoral Science Foundation (No.2021M690844),and Sichuan Science and Technology Program (No.2021JDRC0027) for financially supporting this study.
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2023.108714.
 Chinese Chemical Letters2023年12期
Chinese Chemical Letters2023年12期
- Chinese Chemical Letters的其它文章
- Spin switching in corrole radical complex
- Benzothiadiazole-based materials for organic solar cells
- Mono-functionalized pillar[n]arenes: Syntheses,host–guest properties and applications✰
- Recent advances in two-step energy transfer light-harvesting systems driven by non-covalent self-assembly✩
- From oxygenated monomers to well-defined low-carbon polymers
- Doping-induced charge transfer in conductive polymers
