Different hydraulic strategies under drought stress between Fraxinus mandshurica and Larix gmelinii seedlings
Dandan Luo ·Chuankuan Wang ·Ying Jin ·Zhimin Li·Zhaoguo Wang
Abstract Persistent and severe drought induced by global climate change causes tree mortality mainly due to the hydraulic imbalance of conduit systems,but the magnitude of injury may be species dependent.A water-exclusion experiment was carried out on seedlings of two tree species with distinct characteristics,i.e.,Fraxinus mandshurica and Larix gmelinii to examine hydraulic responses of leaf,stem,and root to drought stress.The two species displayed different hydraulic strategies and related traits in response to drought stress.L.gmelinii reduced its leaf hydraulic conductance by quick stomatal closure and a slow decline in leaf water potential,with a more isohydric stomatal regulation to maintain its water status.In contrast,F.mandshurica was more anisohydric with a negative stomatal safety margin,exhibiting strong resistance to embolism in stem and leafstem segmentation of hydraulic vulnerability to preserve the hydraulic integrity of stem.These differences in hydraulic behaviors and traits between the two species in response to drought stress provide a potential mechanism for their coexistence in temperate forests,including which in the forest modeling would improve our prediction of tree growth and distribution under future climate change.
Keyword Embolism resistance·Hydraulic vulnerability·Stomatal safety margin·Stomatal regulation·Temperate species·Drought stress
Introduction
With ongoing global climate change,more frequent and extreme climate events and changes in precipitation patterns aggravate drought at both local and regional scales,leading to tree hydraulic dysfunction,carbon (C) imbalance,and eventual morality (McDowell et al.2011;Allen et al.2015;Clark et al.2016).Catastrophic damage to hydraulic systems is a main cause of tree mortality during drought (Anderegg et al.2016;Adams et al.2017).However,tree responses to drought may vary with species that have different hydraulic characteristics and regulation strategies,even co-existing in the same ecosystem (Johnson et al.2018).Therefore,it is critical to understand species-specific drought-response strategies and underlying mechanisms for accurately modeling and predicting tree growth and distribution under future climate change scenarios (Choat et al.2018).
Trees adopt several trade-offstrategies to cope with drought stress.Stomatal regulation is the frist line of defense (Martínez-Vilalta and Garcia-Forner 2017).Plants can often be classif ied into isohydric and anisohydric species based on their stringency of stomatal limitation to transpiration in drying soils (Klein and Niu 2014;Martínez-Vilalta et al.2014).Isohydric species have strict stomatal control to maintain a relatively constant minimum leaf water potential (Ψleaf) as soil water potential (Ψs) and/or vapor pressure def icit decline,while anisohydric species have a low stomatal sensitivity to environmental changes and allowΨleafto decline nearly parallel withΨsas drought proceeds (Tardieu and Simonneau 1998;Klein and Niu 2014).However,plants often lie between these two extremes (Martínez-Vilalta et al.2014).Previous syntheses suggest that the primary mechanisms of tree mortality,particularly under prolonged drought conditions,are different between isohydric and anisohydric species,that is,the former are mainly constricted by C O2assimilation due to earlier stomatal closure,whereas the latter have more resistant xylems to negative water potential and can maintain gas exchange under moderate drought stress (McDowell et al.2008;Pou et al.2012).However,Mitchell et al.(2013) reported thatPinus radiataD.Don (more isohydric) survived longer than twoEucalyptusspecies (more anisohydric) under drought because the former used the storage of nonstructural carbohydrates as a carbon source after stomatal closure,while the prolonged stomatal opening of theEucalyptusled to more rapid water loss and thereafter,hydraulic failure.
Xylem embolism resistance is another mechanism of tree survival under drought stress.Embolism resistance has been characterized with several proxies for hydraulic failure,such asΨ50orΨ88(the water potential causing 50% or 88% loss of hydraulic conductivity;Brodribb and Cochard 2009;Choat et al.2012),HSM (hydraulic safety margin,the difference between the minimum water potential (Ψmin) andΨ50orΨ88; Anderegg et al.2016;Adams et al.2017),and SSM (stomatal safety margin,the difference betweenΨgs88(the leaf water potential causing 88% loss of the maximum stomatal conductance;Skelton et al.2015) andΨ50orΨ88; Creek et al.2018).Each proxy has its limitation that varies with species.For example,Ψ50orΨ88often inaccurately represents tree drought tolerance because of interactions between stomatal regulation and xylem embolism (Blackman et al.2009;Hochberg et al.2017;Skelton et al.2018);and conifers tend to haveΨ50as the threshold,while angiosperms haveΨ88(Choat et al.20,122,018;Urli et al.2013).SinceΨminintegrates multiple responses of plant structure (e.g.,rooting depth) and physiology (e.g.,stomatal behavior) to environmental changes (Choat et al.2012,2018),it is difficult to quantify the inf luence of stomatal regulation on water potential,which leads to uncertainty of HSM-based prediction (Skelton et al.2015;Chen et al.2019).Moreover,the HSM for angiosperms is less than that for conifers,because the former has greater capacity to reverse embolism and riskier embolism threshold for survival (Choat et al.2012,2018;Urli et al.2013).Chen et al.(2019) reported that SSM was a better predictor for the mortality of temperate broadleaf species thanΨ50/Ψ88and HSM because it integrates both stomatal regulation and xylem resistance and represents the degree of stomatal regulation over cavitation (Creek et al.2018).Nevertheless,few verifications on SSM have been conducted.
Another strategy for plant mitigation of drought stress has been proposed as the within-plant hydraulic vulnerability segmentation,i.e.,plants “sacrifice” their terminal organs (i.e.,leaves and/or roots) in favor of the C-costly stems when stomatal regulation fails,maintaining a safe water balance (Tyree and Ewers 1991;Creek et al.2018).However,there has been no generality on vulnerability segmentation reached to date (Pivovaroffet al.,2014).For example,Johnson et al.(2016) reported that the leaves and roots of four angiosperms and four conifers were more vulnerable than their trunks that were more vulnerable than their branches.However,Hao et al.(2013) found similar vulnerabilities across the organs of mature trees ofBetula papyriferaMarsh.McCulloh et al.(2014) reported interspecific similarity but within-tree variability of vulnerability for large trees of four co-occurring conifers in western USA.Vulnerability segmentation is possibly species and/or age specific,and deserves more whole-plant studies.
Given the multiple mechanisms of hydraulic responses to drought,trees in natural settings display a suit of acclimatization and life-history strategies with different physiological,morphological,and anatomical traits (Choat et al.2018),such as the “fast-slow” plant economics spectrum proposed by Reich (2014).Typically,angiosperms have higher water transport capacity and resource use efficiency to maintain faster growth rates,while gymnosperms have higher embolism resistance and less vulnerability to drought (Reich 2014;Jin et al.2016).
In this study,a water-exclusion experiment was carried out with seedlings of Manchurian ash (Fraxinus mandshuricaRupr.) and Dahurian larch (Larix gmelinii(Rupr.) Rupr.) during the growing season of 2019 to examine dynamics in the hydraulics and related traits of roots,stems,and leaves.Ash and larch co-exist in Chinese temperate forests,and represent distinct plant functional types with contrasting anatomical,morphological,and physiological traits,e.g.,a broadleaf angiosperm with ring-porous wood and wider and longer vessels versus an evergreen gymnosperm with nonporous wood and narrower and shorter tracheids.Our specific objectives were to:(1) examine temporal dynamics in hydraulics of the two species during the drought treatment;(2) explore leaf-stemroot hydraulic coordination in response to drought stress for the two species;and (3) compare their water regulation strategies under drought.We hypothesized that:(1) the hydraulic conductivity of both species would decline in response to drought stress but the magnitude of reduction would vary with species;(2) the two species would exhibit hydraulic vulnerability segmentation in response to severe drought,but with different temporal patterns;and (3) the two species would develop distinct strategies of hydraulic regulation to cope with drought stress,with ash being more anisohydric and larch more isohydric.
Materials and methods
Study site and experimental design
Our study was carried out at the Maoershan Forest Ecosystem Research Station,Northeast China (45°20' N,127°30' E,400 m a.s.l.).This region is significantly influenced by a temperate continental monsoon climate,with humid,warm summers and dry,cold winters.Annual precipitation varies from 600 to 800 mm,of which~ 62% falls during the growing season (June-September).Mean annual temperature is 3.1 °C,and January and July are the coldest and warmest months,with mean temperatures of -18.5 °C and 22.0 °C,respectively.The frost-free period is 120-140 days (Wang et al.2013).
Two-year-old seedlings of ash and larch were obtained from the local nursery and planted into individual pots (~22 L capacity),f illed with soil from the adjacent forest in April 2019.The soil was passed through a 5-mm sieve and mixed well before using.At the beginning of the experiment,the seedlings were placed in an open area with full sunlight and watered 2-3 times per week to maintain soil moisture at~ 80% of field capacity.In July 2019,four plants per species were randomly sampled to determine the background values of soil moisture and hydraulic variables of root,stem,and leaf.The plants were randomly assigned into two groups,i.e.,treatment (SD) and control (CK).The SD group was left unwatered and blocked from rainfall using a transparent rain shelter to simulate drought,while the CK group was watered regularly as previous.As the experiment proceeded until almost completely dried out,the plants were sampled five times based on the continuously monitoring of soil volumetric water content (VWC) and physiological characteristics of the plants (Fig.1).At each sampling period,eight plants per species were randomly sampled,of which four were used to measure predawn and midday leaf water potentials (ΨPDandΨMD,respectively),and the rest four to in situ measure leaf stomatal conductance (gs) .The seedlings were brought back to the laboratory for measuring hydraulic conductivity of leaf,stem,and root.In general,the physiological responses of each plant were measured within the range of -0.2 MPa to -2.7 MPa of the plant water potential.
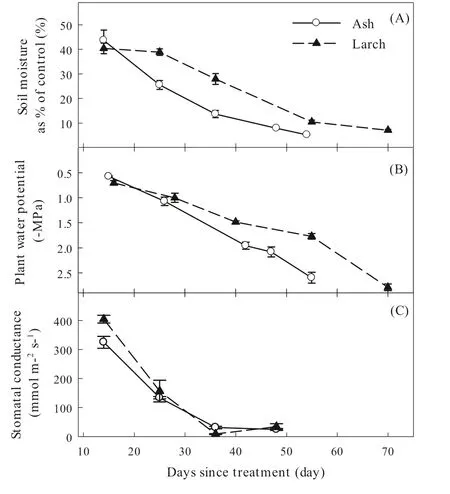
Fig.1 Temporal dynamics in soil moisture (A),plant water potential (B),and stomatal conductance (C) for ash and larch along the drought-stress treatment.The error bars are standard errors (n =4)
Measurements of leaf hydraulic traits
Pre-dawn and midday leaf water potential
ΨPDandΨMDwere measured on the same day,with the sampling times at predawn (03:00-05:00) and midday (12:00-14:00),respectively.The leaves of ash and leafy twigs of larch were excised from the plants andΨPDandΨMDwere measured with a pressure chamber (Model 1505D;PMS Instrument Company,Albany,OR,USA).The relationship betweenΨPDandΨMDfor each sampling time was fitted by Eq.(1) (Martínez-Vilalta et al.2014):

where Λ is the intercept atΨPD=0,σ is the slope.Hydroscape area (HA) was fitted by the trajectory ofΨMDvs.ΨPDduring soil drying (Meinzer et al.2016;Fu et al.2019).
Pressure-volume (PV) curves
The PV curves of leaves were measured before the drought treatment.Four seedlings per species were randomly sampled and the leafy twigs of larch or the compound leaves with petioles of ash were excised from well-irrigated plants before dawn (03:00-05:00),immediately sealed in black plastic bags that contained moist filter paper and transported to the laboratory where they were rehydrated in deionised water for 30-60 min to full rehydration.They were then measured for saturated fresh weight with a digital balance (0.001 g resolution),and then immediately re-measured for the initial leaf water potential (Ψleaf) with a pressure chamber.The leaves were dehydrated,and the mass andΨleafwere repeatedly measured until the relationship between 1/Ψleafand water loss at least five successive points became linear.The samples were then oven-dried at 70 °C for 48 h and weighed for dry mass.PV curves were established by plotting 1/Ψleafagainst relative water content and used to calculateΨleafat turgor loss point (ΨTLP) .Leaf capacitance (Cleaf) was calculated from the slope of the relationship betweenΨleafand water loss (Tyree and Hammel 1972).
Stomatal conductance (gs)
Stomatal conductance was measured for the sun-exposed leaves between 08:00-10:00 a.m.using a LI-6400 portable photosynthesis (Li-6400;Li-Cor Inc.,Lincoln,NE,USA),with the environmental settings as:the block temperature at 25 °C,the photosynthetically active radiation at 1200 mol m-2s-1,and the CO2concentration at 400 μmol mol-1.The adjacent leaves orleafy twigs were then taken to measureΨleaf.The relationship betweengsandΨleaf(orΨPD) for each sampling time was fitted by Eq.(2) (Blackman et al.2019):
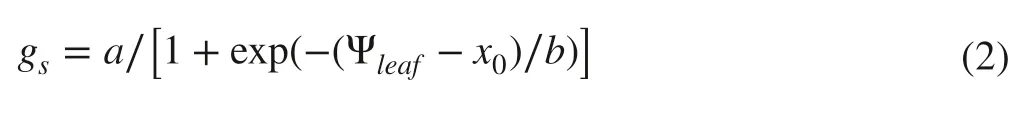
whereais the maximumgs,bthe coefficient determining the slope of the curve,andxothe water potential causing 50% loss of stomatal conductance.A similar three-parameter sigmoidal function was used to fit the vulnerability curve ofgs,from which the leaf water potential and pre-dawn water potential causing 12% and 88% loss of stomatal conductance (Ψ gs12andΨ gs88) were calculated,which represent the beginning of stomatal closure and complete closure,respectively (Skelton et al.2015;Creek et al.2018).
Measurements of whole-plant hydraulics and related traits
Hydraulic conductance (K)
The high-pressure flowmeter (HPFM) method (Hochberg et al.2014) was used to measure the whole-plant hydraulics,because it is rapid and easy to operate in both laboratory and field.It can also be used to measure hydraulic conductance of the whole plant and organs (Tsuda and Tyree 1997),which considers both xylary and extra-xylary pathways for water.Hydraulic conductance was measured with an HPFM-Gen3 (Dynamax Corp.,Houston,TX,USA).The sampled plants were moved to the laboratory before dawn (03:00-05:00) and covered with black plastic bags for 1.5 h to allow equilibration of the whole-plant water potential (Ψplant) .The leaves or leafy twigs were cut to measureΨplantand the wounds caused by water potential measurements were sealed with epoxy.Stems were then removed 5 cm above the soil surface,the cut end immediately submerged into deionized water and~ 5 cm segments cut underwater.A bark strip (~3 cm in length) next to the cutting point was then removed to facilitate the connection to the HPFM tubing system.Shoot hydraulic conductance was measured using a quasi-steady-state measuring mode.The stem was connected with the HPFM and perfused at a pressure of~0.5 MPa until a stable flow rate was reached (about 30-45 min).All the needles (for larch) or leaf lets (for ash) were removed before stem hydraulic conductance was measured.The stable flow rate for stems was reached in 2-3 min.Root hydraulic conductance was measured using the transient measuring mode.The HPFM tubing system was connected to the remaining portion in the pot,and the applied pressure increased to 5 kPa s-1while the pressure and flow rate were recorded every 2 s.Root hydraulic conductance was obtained from the slope of the linear part of the relationship between water flow rate and the applied pressure.Whole plant hydraulic resistance (Rplant,the inverse of plant hydraulics) was the sum of hydraulic resistance of shoot and root of each plant (i.e.,Rplant=Rshoot+Rroot),while leaf hydraulic resistance (Rleaf) was the difference betweenRshootandRstem(Tsuda and Tyree 1997;Wang et al.2016).Leaf area,stem length,and diameters at both ends of the stem were measured.The samples of leaf,stem,and root for each plant were then oven-dried at 70 °C for 72 h and weighed for dry mass.Leaf,stem,and root hydraulic conductance were expressed on a leaf area basis (Kleaf,Kstem,andKroot,respectively) for direct comparisons.The leaf specific hydraulic conductance of the CK group and the background values measured before the treatment were designated as the maximum plant hydraulic conductance (Kmax) .Kleaf,Kstem,andKrootduring the whole drought treatment were correlated to the corresponding water potential.
The percentage loss in hydraulic conductivity (PLC) was calculated as:
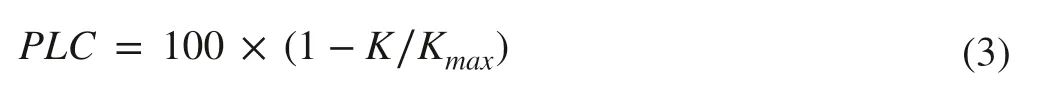
The vulnerability curve for each species was fitted by the least square methods based on an empirical function (Tsuda and Tyree 1997) as:

whereΨis plant water potential,athe maximum PLC,bthe maximum slope of the curve,andcisΨ50.At the same time,the water potential associated with 12% and 88% loss of hydraulic conductivity (Ψ12andΨ88) were calculated,which represent the thresholds of the beginning ofKdecline and plant death,respectively.
Wood density (WD) and root to shoot mass ratio (R/S)
Whole plants were harvested when the experiment was finished and separated into different biomass tissues.Each tissue from each plant was separately bagged,oven-dried at 70 °C for 48 h and weighed for dry mass.Stem volume was measured by the displacement method.WD was calculated as the dry mass of stem without bark divided by stem volume.R/S ratio was calculated as the ratio of the aboveground dry mass to the belowground.
Statistical analyses
Linear regression was used to fit the relationship betweenΨPDandΨMDwithin species,of which the intercept (Λ) and slope (σ) were used to determine the tendency in the isohydric to anisohydric continuum for each species (i.e.,larger σ,more anisohydric;Martínez-Vilalta et al.2014).Thet-test was used to compare the initial traits of the two species.For each species,hydraulic conductivity was regressed against water potential with a three-parameter sigmoidal function,from whichΨ12,Ψ50andΨ88and their 95% conf idence intervals (CIs) were obtained.Vulnerability segmentation for each species was assessed by theΨ50differences between leaf,root and stem (LeafΨ50-StemΨ50and RootΨ50-StemΨ50),i.e.,vulnerability segmentation if theΨ50differences were greater than zero and their CIs did not overlap (Creek et al.2018).The relationship betweengsandΨduring drought was fitted with a three-parameter sigmoidal function,from whichΨgs12andΨgs88(orψgs12andψgs88) were obtained.Stomatal safety margins (SSM50or SSM88) were calculated as the difference betweenΨgs88andΨ50orΨ88of leaf,stem,and root by species,respectively (Chen et al.2019).Larger SSM50or SSM88values represented stronger stomatal regulation and xylem resistance coordination (Skelton et al.2015).The loss of whole-plant hydraulic conductivity (%) and relative stomatal conductance (%) were fitted against the treatment duration (day) with a three-parameter sigmoidal function.The duration for plants to reachΨgs88andΨ88were def ined as the times for stomatal closure and plant death threshold,respectively.All statistical analyses were performed in IBM SPSS Statistics 21.
Results
Temporal dynamics in hydraulics of ash and larch during drought-stress
As soil water content decreased along the drought-stress treatment (Fig.1),gsand hydraulic conductance of the two species declined but both exhibited distinct inter-specific temporal patterns (Fig.2).Thegs12for ash occurred earlier than that for larch (Day 14 vs.Day 23),whereasgs88for the former was later (Day 30 vs.Day 25).The duration betweenΨgs12andΨgs88was 16 days and two days for ash and larch,respectively.Conversely,Ψ50andΨ88for ash occurred on Day 41 and Day 57,respectively,while those for larch occurred on Day 33 and Day 77,respectively.The duration betweenΨ50andΨ88was 16 and 43 days for ash and larch,respectively.
Water potential of leaf,stem,and root and whole-plant water transport efficiency for both species also decreased as drought-stress increased,but the thresholds of stomatal closure and vulnerability varied with species and organs (Figs.3 and 4).Specifically,theΨgs88for ash (-1.52 ± 0.06 MPa) was more negative than for larch (-1.40 ± 0.04 MPa),while theΨgs12for the former (-0.92 ± 0.06 MPa) was less negative than that of the later (-1.22 ± 0.04 MPa) (Fig.3 A and C).Theψgs88for ash (-1.59 ± 0.06 MPa) was more negative than that for larch (-0.89 ± 0.04 MPa) (Fig.3 B and D).The whole-plantΨ50for larch (-1.64 ± 0.07 MPa) was higher than that for ash (-1.85 ± 0.11 MPa) (Fig.4 A and B).The stemΨ50andΨ88for ash (-2.13 ± 0.11 and -2.63 ± 0.11 MPa) were more negative than those for larch (-1.70 ± 0.07 and -1.85 ± 0.07 MPa) (Fig.4 E and F).The rootΨ88for ash was lower than that for larch,but an opposite trend occurred for leafΨ50(Fig.4 C and D).The difference betweenΨ50andΨ88was the least for stems,followed by leaves,and the greatest for roots;the wholeplant difference for ash was greater than that for larch.Ash,rather than larch,showed a vulnerability segmentation with leafΨ50> rootΨ50> stemΨ50and non-overlapped CIs ofΨ50for ash,but overlapped CIs ofΨ50for larch despite leafΨ50> rootΨ50> stemΨ50(Fig.4).
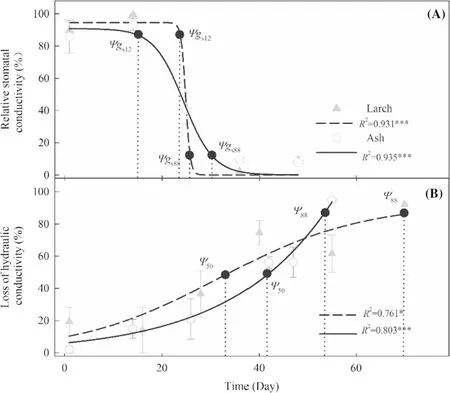
Fig.2 Temporal dynamics in relative stomatal conductance (A) and loss of plant hydraulic conductivity (B) for ash and larch along the drought-stress treatment.The dotted lines represent the time when the water potential caused 12% and 88% loss of stomatal conductance (Ψgs12 and Ψgs88),and 50% and 88% loss of plant hydraulic conductance (Ψ50 and Ψ88) occurred,respectively.* P < 0.05,*** P < 0.0001

Fig.3 Responses of stomatal conductance to decreasing leaf water potential (Ψleaf) and predawn water potential (ΨPD) for ash (A,B) and larch (C,D).The vertical solid and dashed lines indicate the leaf water potential (Ψgs12 and Ψgs88) and pre-dawn water potential (ψgs12 and ψgs88) at 12% and 88% loss of stomatal conductance,respectively.The values in parentheses are 95% conf idence intervals
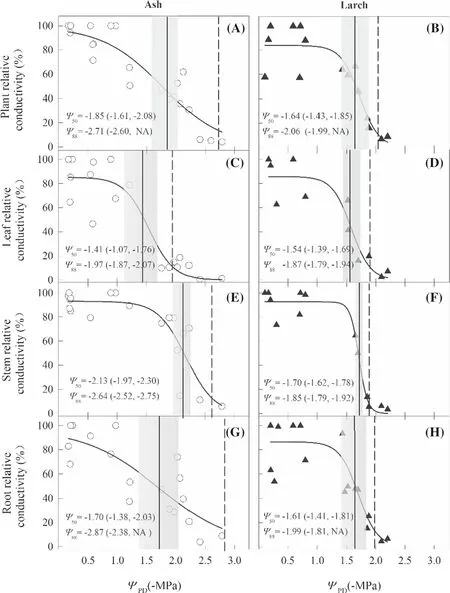
Fig.4 Hydraulic vulnerability curves showing responses of the relative conductivity of whole plant (A,B),leaf (C,D),stem (E,F),and root (G,H) to respective pre-dawn water potential (ΨPD) for ash and larch.The vertical solid lines indicate the water potential causing 50% loss of conductance (Ψ50) and the shaded areas represent 95% conf idence interval.The vertical dashed lines represent the water potential causing 88% loss of conductance (Ψ88)
Comparisons of hydraulics and related traits between ash and larch
Hydraulics and related traits differed significantly between the two species (Figs.5 and 6).Specifically,the means ofΨPD,ΨMD,andΨTLPwere significantly (P< 0.05) higher (less negative) for ash than for larch,while that ofgswas significantly lower for the former than for the latter.TheKleaf,Kstem,andKrootfor ash were 6.7,2.6,and 2.7 times greater than those for larch,respectively.TheCleaffor larch was 2.3 times greater than that for ash,but theWDand R/S ratio for ash (0.59 g cm-3and 1.1) were significantly greater (P< 0.05) than those for larch (0.41 g cm-3and 0.5).
The relationship ofΨMDtoΨPDwas different between ash and larch (Fig.7).The slope of the relationship for ash (0.91) was insignificant from 1 (0.91 [0.77,1.06]),while that for larch was significantly less than 1 (0.69 [0.59,0.81]).The intercept for ash (-0.64) was larger (less negative) than that for larch (-0.94).HA was 1.17 for larch and 1.68 for ash.Moreover,leaf SSM50was less than 0 for ash but greater than 0 for larch.However,leaf SSM88was comparable between the two species (0.45 vs.0.48) (Fig.8).Both SSM50and SSM88of the stem and whole plant for ash were greater than those for larch.Nevertheless,ash had less root SSM50than larch (0.19 vs.0.22) but greater root SSM88(1.35 vs.0.60).The SSM88of leaf,stem,root,and whole plant for ash were much higher than those for larch.
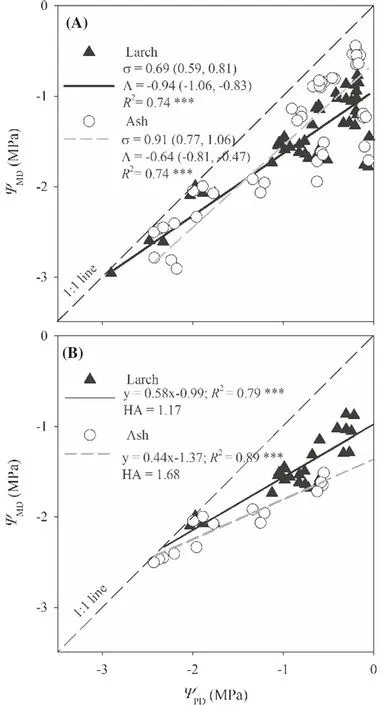
Fig.7 Relationships between predawn (ΨPD) and midday leaf water potential (ΨMD) .(A) Including all the data;(B) stomatal regulation of ΨMD prior to complete stomatal closure.HA is hydroscape area.The regression equations (including R2 ) are given.*** P < 0.0001
Discussion
Temporal dynamics in hydraulics of ash and larch during drought stress
As drought stress increased,stomatal closure occurred early but slowly for ash while it occurred late but rapidly for larch.Consequently,the duration of stomatal closure for ash lasted eight times longer than that for larch (Fig.2).These patterns suggest that larch has a more isohydric regulation,while ash is more anisohydric.Both leaf and stemΨ50for larch occurred afterΨgs88(Figs.3 and 4),suggesting that leaf hydraulic function is maintained to some extent even after stomatal closure,which consequently slows down the decline in plant dehydration and water potential.For ash,however,theΨ50of the stem rather than theΨ50of leaf occurred afterΨgs88(Figs.3 and 4),suggesting that the decline inKleafcauses partial/complete stomatal closure and thereby maintains the hydraulic function of the stem.After stomatal closure,water potential continued to decrease slowly and hydraulic conductivity loss increased (Fig.2),possibly resulting from water loss via cuticular conductance,stomatal leaking and through other tissues such as bark (Choat et al.2018).
The distinct temporal patterns of hydraulics under drought-stress between ash and larch may be attributed to several factors.First,larch has a smallerKleafand total leaf area with less water loss through the epidermis compared with ash (Fig.5;Reich 2014).Second,the higher stemΨ50and less stem safety margin of larch (Figs.4 and 8 B) suggest its greater stem water storage and hydraulic capacitance (Meinzer et al.2009).The release of stored water can supply the transpiration stream and buffer the decline of water potential (Choat et al.2018) induced by drought stress,and consequently reduce the decreases inKplantand water potential (Borchert and Pockman 2005).Third,ash consumed water faster than larch as drought stress increased (Figs.1 and 2),evidenced by its greaterΨPDand R/S ratio (Figs.5 and 6) and the longer period of its stomatal opening (Figs.2 and 3).The greaterΨPDof ash possibly resulted from its larger rooting-zone that translated more soil water intoΨPD,while the longer period of stomatal opening likely depleted more soil water,resulting in faster reduction inΨPDover time (Sperry et al.2002;Martínez-Vilalta and Garcia-Forner 2017;Nolan et al.2017b).Nevertheless,it should be noted that our potted experiment may limit the development of seedling rooting systems (Yoseph et al.2011;Nardini et al.2016),which therefore requires more field studies.
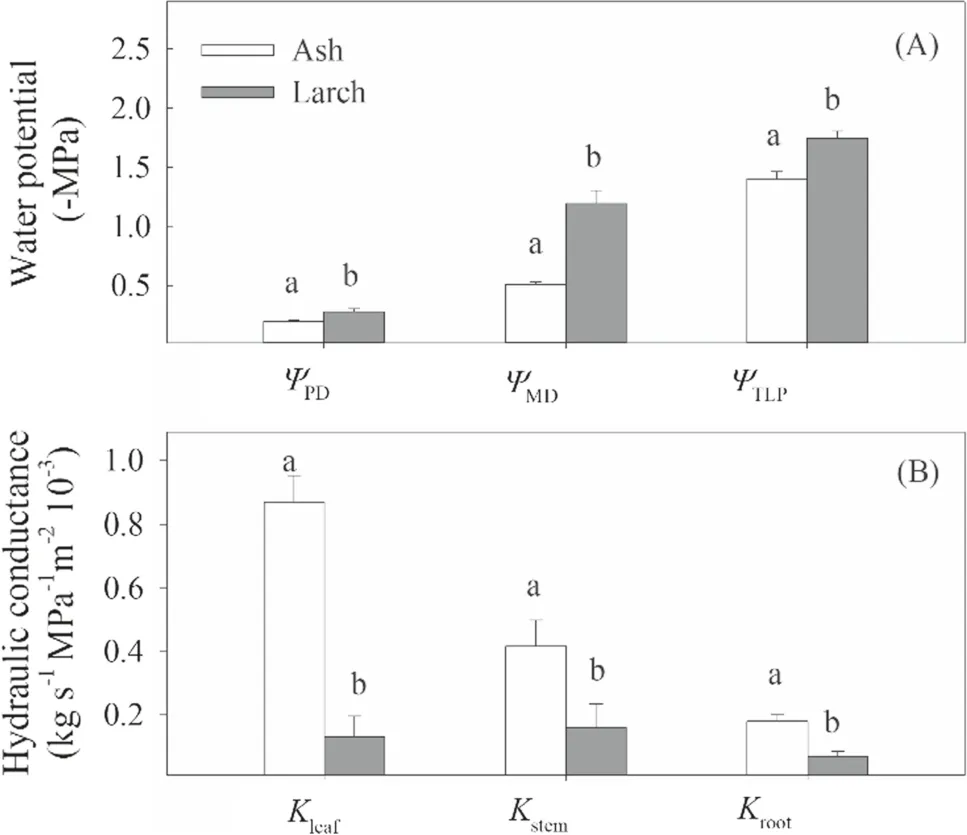
Fig.5 Comparisons of hydraulics traits between ash and larch without drought stress.ΨPD,ΨMD,and ΨTLP represent predawn,midday,and turgor loss point water potential,respectively; Kleaf,Kstem,and Kroot represent leaf-area-based hydraulic conductance of stem,leaf,and root,respectively.Different letters above the bars (mean ± SE,n =4) indicate significant differences between the two species (α =0.05)
Leaf-stem-root hydraulic coordination of ash and larch in response to drought stress
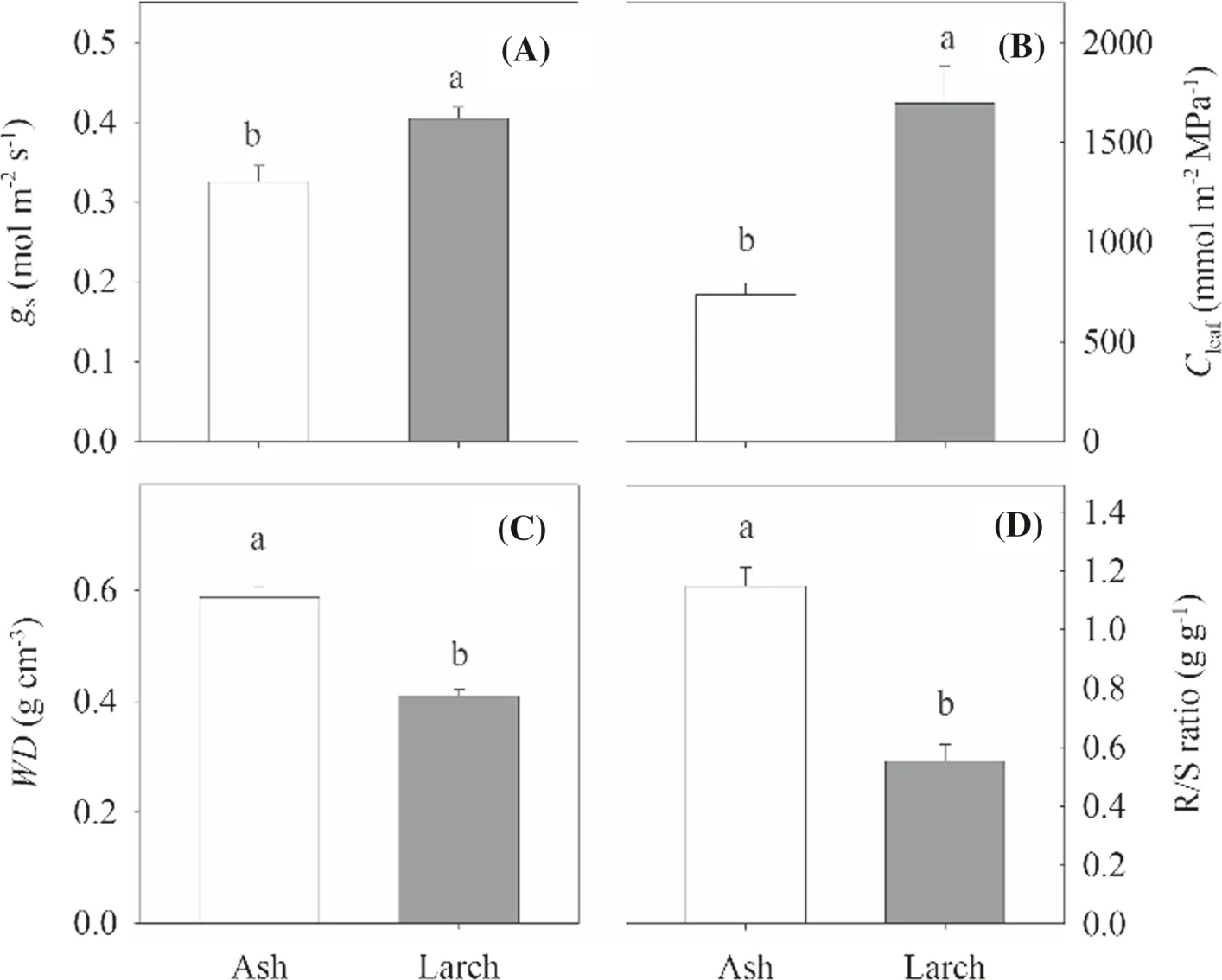
Fig.6 Comparisons of hydraulic-related traits between ash and larch without drought stress; gs,stomatal conductance; Cleaf,leaf capacitance; WD,wood density;R/S ratio,root to shoot mass ratio.Different letters above the bars (mean ± SE,n =4) indicate significant differences between the two species (α =0.05)
Hydraulic vulnerability segmentation often promotes plant physiological recovery once soil rehydrates (Ishida et al.2008) and reflects the combination of plant hydraulics and C economies (Sperry 2000).In this study,a leaf-stem vulnerability segmentation occurred for ash but not for larch (Fig.3),which contradicts a previous study in the same area (Jin et al.2019).The contradiction may be associated with tree status and experimental protocols.Unlike our drought-stress treatment of seedlings,Jin et al (2019) investigated mature trees under natural settings where water availability may not have been a limiting factor.They also used the timed-rehydration method to measureKleafand construct vulnerability curves,while we were measured along a drought-stress process.They measured the hydraulic conductivity and vulnerability of stems without bark,while we measured the vulnerability of stems with bark that included both xylary and extra-xylary pathways.Nevertheless,it should be noted that the high-pressures applied in our HPFM measurements might remove some of the xylary embolism,although the HPFM and conventional evaporative flux methods were demonstrated to yield consistent values of plant hydraulic resistances (Tsuda and Tyree 1997).In addition,the leaves of larch from different nodal positions,basal versus apical leaves,had varied vulnerability,in line with previous studies (e.g.,Hochberg et al.2017).When the water potential drops low enough,trees can massively shed basal leaves while retaining apical leaves,and leaf embolism resistance to drought can be enhanced by osmotic regulation.This may result in the smaller embolization differences between the stem and leaves for larch (Fig.4).
Although the stemΨ50of ash was more negative than that of larch,its leafΨ50was slightly higher,which may be caused by higher leafΨTLP.WhenΨleafdrops close toΨTLP,the extra-xylary conductance decreases,leading to leaf hydraulic dysfunction (Blackman et al.2010).The decoupling of stem and leaf embolism resistance suggests that these traits may have evolved independently,and the multiple combinations may reflect the diversity of drought strategies (Pivovaroffet al.2016;Laughlin et al.2020).
Nevertheless,no root-stem vulnerability segmentation under drought stress was detected for ash or larch (Fig.4),illustrating that water movement between root and stem is coupled more strongly than that between leaf and stem.Collectively,hydraulic vulnerability varies with species,organ,and the environmental conditions,highlighting the importance of considering multiple factors in assessing tree resistance to and recovery from drought.
Water regulation strategies of ash and larch under drought stress
Occupying different ecohydrological niches is a primary mechanism of resource partitioning for co-existing species,among which differing responses of stomatal conductance to variations in soil water content (i.e.,isohydric/anisohydric continuum) is an important strategy (Nolan et al.2017a).Within the tolerance of hydraulic systems,stomatal regulation takes full advantage of the range of xylem pressure (Choat et al.2012).In this study,larch is classif ied as more isohydric while ash is more anisohydric based on three metrics.First,the σ for ash was close to 1,while that for larch was less than 1 (Fig.7;strict isohydric (σ=0),partial isohydric (0 < σ < 1),strict anisohydric (σ=1),extreme anisohydric (σ > 1);Martínez-Vilalta et al.2014).This result forFraxinus mandchuricaagrees with previous studies forF.excelsiorandF.americana(Gu et al.2015;Leuschner et al.2019),but forLarix gmelinii,it is inconsistent with other larch species.For example,L.kaempferisustainedΨleafand showed an isohydric stomatal regulation (Bhusal et al.2020;Sasani et al.2021),whileL.deciduawas considered anisohydric (Streit et al.2014;Sasani et al.2021) or isohydric (Peters et al.2019).The second metric used was the hydroscape area (HA) that integrates multiple mechanisms of regulatingΨplantand stomatal behavior during soil drying (Meinzer et al.2016;Fu and Meinzer 2019;Li et al.2019).Ash had a larger HA than larch (1.68 cf.1.17),showing an anisohydric tendency.Based on SSM50(Skelton et al.2015),ash was also def ined as anisohydric (negative leaf SSM50) while larch was isohydric (positive leaf SSM50; Fig.8).
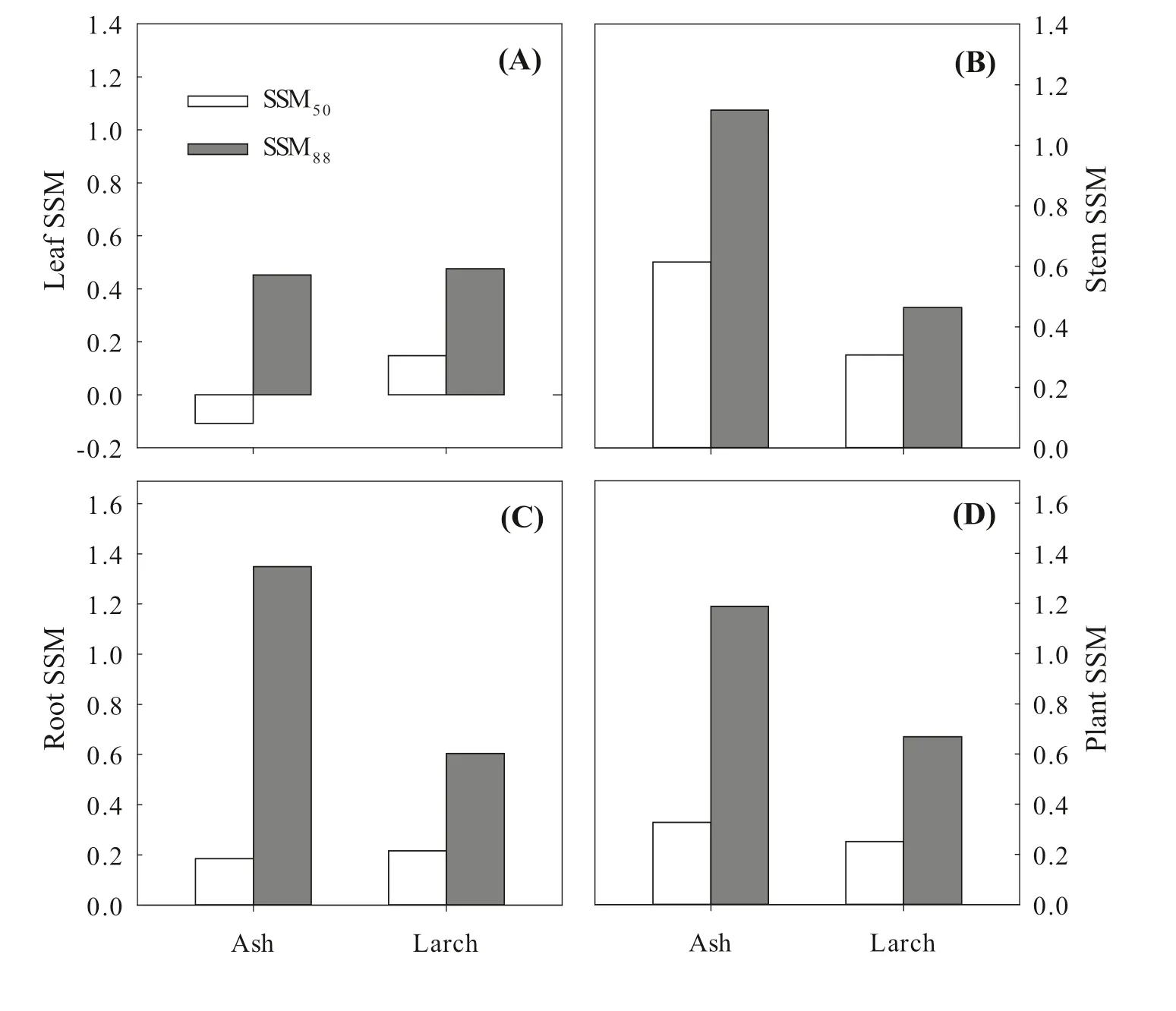
Fig.8 Comparisons of stomatal safety margins (SSM) at leaf (A),stem (B),root (C),and whole-plant (D) levels between ash and larch.SSM50 or SSM88 is the difference in the water potential between causing 88% loss of stomatal conductance (Ψgs88) and 50% or 88% loss of hydraulic conductance (Ψ50 and Ψ88)
In addition to stomatal regulation,embolism resistance to drought is another key strategy for co-existing species to avoid direct competitive interactions.The stomatal safety margin (SSM) has been suggested as a good proxy for characterizing tree hydraulic dysfunction (Chen et al.2019) because it is jointly controlled by stomatal sensitivity and xylem embolism resistance to drought.In this study,larch had a higher leaf SSM50and longer survival period under drought stress than ash,consistent with the findings of Chen et al.(2019),but larch had lower stem and whole-plant SSM (Fig.8).Theoretically,angiosperms show hydraulic failure with more negative stem water potential (i.e.,Ψ88) than conifers (i.e.,Ψ50; Urli et al.2013).In our case,theΨ88and SSM88of stem and whole plant for ash were much higher than theΨ50and SSM50for larch.This suggests that ash has much higher embolism resistance and a wider safety margin than larch.These results also show that ash had a significant hydraulic vulnerability segmentation,which may be another strategy to prevent excessive embolization.
Additionally,the difference in SSM between ash and larch may be attributed to their ability of embolism repair and other traits (e.g.,wood density,hydraulic capacitance).TheΨ88of leaf,stem,and roots were comparable for larch but significantly different for ash,suggesting that ash may have greater regeneration capacity after experiencing a severe drought.This repair ability may be associated with the content of nonstructural carbohydrates that can induce an osmotic gradient to drive ref illing of embolized vessels (Adams et al.2017).Ash may have weaker repair ability due to lower sugar concentrations in the stem than larch (unpublished data).Additionally,larch had less WD and embolism resistance of root,stem,and whole plant to drought (i.e.,less negativeΨ50andΨ88) than ash (Figs.4 and 6),in agreement with previous studies (Klein and Niu 2014;Martínez-Vilalta et al.2014;Fu and Meinzer 2019).The larger WD and stem SSM of ash resulted in a smaller stem hydraulic capacitance (Fu et al.2019).Along the iso-anisohydric continuum,there is a tendency for decreasing reliance on capacity to buffer changes in xylem tension to avoid embolism and increasing reliance on structural reinforcement of xylem to resist embolism (Yi et al.2017;Fu and Meinzer 2019).It is also suggested that high leaf capacitance is correlated with slow stomatal closure (Martins et al.2016),and leaf and stem capacitance may be inversely related (Fu et al.2019).But in this study,theCleafof larch was higher than that of ash,which confers a protection mechanism to avoidΨleafdecline.The lowerCleafallows ash to have a higher capacity of foliar water uptake (Berry et al.2019) and to mitigate the decline inKplantandΨleaf(Fuenzalida et al.2019;Binks et al.2020),which makes theΨ50of ash reached later than larch (Fig.2 B).
Larch and ash diverge their hydraulic strategies under drought stress.Larch displays a conservative strategy but a risky C economy with isohydric regulation,low embolism resistance and no leaf-stem vulnerability segmentation.Conversely,ash shows a risky hydraulic strategy with anisohydric behavior,high embolism resistance,and leaf-stem vulnerability segmentation.Such hydraulic differentiations facilitate species coexistence (Nolan et al.2017a) and productivity maintenance (Roman et al.2015) under drought conditions.
Conclusions
Our water-exclusion experiment on Manchurian ash and Dahurian larch seedlings displayed contrasting responses of hydraulic and water regulation strategies to drought stress,which provide potential mechanisms for their co-existence.Larch mainly uses stomata as a “safety valve” to maintain hydraulic functions of the whole plant and delays dehydration time under drought;ash uses leaves as “hydraulic fuses” (i.e.,leaf-stem vulnerability segmentation) to preserve the hydraulic integrity of the stem.Larch adopts drought avoidance and an isohydric strategy with strong stomatal sensitivity,while ash is more anisohydric with a strong drought resistance.Hydraulic coordination among different organs is central to our understanding of how trees and forest communities respond to drought,and the occupation of ecohydrological niches by co-existing species is a primary strategy for their resource partitioning in the ecosystem (Peñuelas et al.2011;Yoseph et al.2011).Our findings highlight the significance of considering different hydraulic responses of coexisting species in modelling and prediction of tree growth,survival,and distribution under global climate changes.
 Journal of Forestry Research2023年1期
Journal of Forestry Research2023年1期
- Journal of Forestry Research的其它文章
- Hormetic effects of abiotic environmental stressors in woody plants in the context of climate change
- Epigenetic modification associated with climate regulates betulin biosynthesis in birch
- Growth trends and environmental drivers of major tree species of the northern hardwood forest of eastern North America
- Different xylogenesis responses to atmospheric water demand contribute to species coexistence in a mixed pine-oak forest
- Interannual dynamics of stemwood nonstructural carbohydrates in temperate forest trees surrounding drought
- Allocation patterns of nonstructural carbohydrates in response to CO 2 elevation and nitrogen deposition in Cunninghamia lanceolata saplings
