Safety profile of 0.0015% tafluprost eye drops in China: a post-marketing observational study
Xue-Li Chen, Yan-Jing Fu, Bo Qu, Ye-Wei Wang, Xin Tang, Yu-Hong Wang, Guo-Yi Zhou,Ming-Kai Lin, Jing-Yuan Shen, Jin Yao0, Su-Yan Li, Miao-Qin Wu, Hua-Zong Peng,Ming-Ying Lai, Ren-Yi Wu, Yi-Nong Zhang, Yan Li, Xiao-Jun Wu, Ming-Chang Zhang,Su-Ping Guo0, Xing-Huai Sun
1Department of Ophthalmology, Eye & ENT Hospital of Fudan University, Shanghai 200000, China
2Department of Ophthalmology, Daqing Ophthalmologic Hospital, Daqing 163000, Heilongjiang Province, China
3Department of Ophthalmology, the Fourth Affiliated Hospital of China Medical University, Shenyang 110000, Liaoning Province, China
4Department of Ophthalmology, Da Lian He Eye Specialist Hospital, Dalian 116000, Liaoning Province, China
5Department of Ophthalmology, Beijing Tongren Hospital,Beijing 100000, China
6Department of Ophthalmology, Xiamen Eye Center of Xiamen University, Xiamen 361000, Fujian Province, China
7Department of Ophthalmology, Yueqing People’s Hospital,Wenzhou 325600, Zhejiang Province, China
8Department of Glaucoma, Zhongshan Ophthalmic Center, Sun Yat‐sen University, Guangzhou 510000, Guangdong Province,China
9Department of Ophthalmology, Shengzhou Shen’s Eye Hospital, Shaoxing 312400, Zhejiang Province, China
10Department of Ophthalmology, the Affiliated Eye Hospital of Nanjing Medical University, Nanjing 210000, Jiangsu Province, China
11Department of Ophthalmology, Xuzhou No.1 Peoples Hospital, Xuzhou 221000, Jiangsu Province, China
12Department of Ophthalmology, Zhejiang Provincial People’s Hospital, Hangzhou 310000, Zhejiang Province, China
13Department of Ophthalmology, Wuhan Eyegood Ophthalmic Hospital, Wuhan 430014, Hubei Province, China
14Department of Ophthalmology, Shenzhen Eye Hospital,Shenzhen 518001, Guangdong Province, China
15Department of Glaucoma, Shanghai Heping Eye Hospital,Shanghai 200000, China
16Department of Ophthalmology, Wuxi Second People’s Hospital, Wuxi 214000, Jiangsu Province, China
17Department of Ophthalmology, First Affiliated Hospital of Kunming Medical University, Kunming 650000, Yunnan
Province, China
18Department of Ophthalmology, Union Shenzhen Hospital(Nanshan Hospital), Shenzhen 518000, Guangdong Province, China
19Department of Ophthalmology, Wuhan Union Hospital,Wuhan 430000, Hubei Province, China
20Department of Ophthalmology, Shengyang He Eye Specialist Hospital, Shenyang 110000, Liaoning Province, China
Abstract
● KEYWORDS: tafluprost; glaucoma; ocular hypertension;intraocular pressure; adverse drug reactions
INTRODUCTION
Glaucoma is a leading cause of blindness and decreased vision‐related quality of life[1]. The prevalence of glaucoma in China during 1990‐2015 ranged from 2.58%to 2.59%, which account for almost half of patients with glaucoma worldwide[2]. Elevated intraocular pressure (IOP)is deemed as the most important risk factor for progression of glaucoma and lowering IOP is the only proven treatment[3].
The Chinese Glaucoma Guidelines and European Glaucoma Society (EGS) Glaucoma Guidelines recommended prostaglandin analogs (PGAs) as the first‐line treatment for primary open angle glaucoma (POAG) and ocular hypertension (OH)[4‐5].Among them, tafluprost is a PGA with higher affinity and selectivity[6]. The efficacy of tafluprost in lowering IOP has been verified in many countries, even in patients who were insufficiently controlled with latanoprost, while the common safety issues involved eyelid pigmentation, ocular hyperemia,eyelash changes and eyelid hypertrichosis[7‐12].
Post‐marketing studies in Japan and Philippines[8‐10]proved that safety profile of tafluprost was manageable in patients with glaucoma and OH in real‐world clinical setting, alone or in combination with other eye drops. Phase III randomized controlled trial conducted at five Chinese clinical centers in 2015[13]stated that efficacy and safety of tafluprost was comparable to latanoprost in patients with POAG and OH with major adverse reactions being conjunctival hyperemia, eye irritation, eye pain and foreign body sensation. Nevertheless,the characteristics of Chinese patients receiving tafluprost has not been well described yet. Furthermore, there is still a paucity of large‐sample real‐world evidence regarding treatment pattern and the safety of tafluprost in actual clinical practice.Thus, this multicenter study aimed to investigate the safety and treatment pattern of tafluprost for glaucoma and OH in clinical practice, providing real‐world evidence of safety profile of tafluprost in Chinese patients.
SUBJECTS AND METHODS
Ethical ApprovalThe study was approved by the ethics committees of each participating center. The approval number at the leading center was No.2017022‐1. The study was conducted in accordance to the Declaration of Helsinki.Informed consent was obtained from all patients or their legal guardians.
Study Design and ParticipantsThis study was a post‐marketing observational study. A cross‐sectional survey was conducted in patients with POAG and OH who received tafluprost at departments of ophthalmology in 20 hospitals in China between September 2017 and March 2020.The inclusion criteria were patients who were being treated with tafluprost or had been treated with tafluprost within 30d,regardless of age. The exclusion criteria were patients who participated in other trials within 30d.
Calculation of Sample SizeAccording to 95% statistical test efficacy analysis, data of 2996 patients ought to be collected in order to observe the occurrence of adverse drug reactions(ADRs) with incidence of 0.1%. It was planned to recruit 3000 participants to meet the requirements of regulatory agencies.
Data CollectionDemographics, diagnosis, medical history,complications, previous medication, combined therapy for IOP‐lowering, and other tafluprost treatment details were collected. Adverse events (AEs) occurred during tafluprost treatment and within 30d after the last administration of tafluprost were collected using a safety questionnaire filled out by patients. Patients with AEs were followed for 30d and the treatment and outcomes of AEs assessed by the investigators were recorded.
AEs was defined as any medically harmful and unfavorable signs, symptoms or diseases after the use of tafluprost,including aggravation of the original disease and abnormality of laboratory test values, whether related to tafluprost or not.AEs were classified as mild (completely tolerable without influence on daily life), moderate (leading to discomfort that affected normal activities) and severe (leading to inablility in working or daily activities). If correlation with tafluprost cannot be ruled out, it is considered as an ADR. Iris pigmentation, eyelash changes, eyelid pigmentation, and eyelid hypertrichosis were ADRs of special interest.
在数学教学中,老师应尽可能地了解数学原理产生的背景,与学生一起探讨新的数学思想萌芽的过程,在这过程中,使学生认识到数学原理的发展过程是经过曲折而又漫长的过程,这对学生的数学学习有很大的作用。
Serious adverse events (SAEs) was defined as AEs which resulted in death; was life‐threatening; required inpatient hospitalization or prolongation of existing hospitalization;resulted in persistent or significant disability/incapacity; or was a congenital anomaly/birth defect.
Statistical AnalysisStatistical analysis was conducted using SAS (version 9.4, SAS Institute Inc., Cary, NC, USA). AEs were classified according to the International Conference on Harmonisation International Dictionary of Medical Terms. The incidence of ADRs was defined as patients with at least one ADR during treatment divided by the total number of patients in safety analysis, expressed as numbers and percentages.Subgroup analysis was carried out by patient age, by combination therapy, and by actual clinical application.
RESULTS
Baseline Characteristics of PatientsOwing to the Coronavirus Disease 2019 outbreak, a total of 2544 participants were recruited, and 2544 patients were eventually included in the study. There were 1367 (53.7%) males and 1177 (46.3%)females, and the average age was 50.8±19.1y. The majority(98.6%, 2509/2544) were Chinese Han ethnicity. Tafluprost was prescribed as topical treatment of both eyes in 63.1%(1604/2544) of all cases. The reason for using tafluprost was POAG and OH in 58.5% (1488/2544) and 21.9% (556/2544)of the participants respectively, while 19.7% (500/2544) of participants had other reasons. Only 1.6% (40/2544) of the patients used contact lenses. There were 38.9% (989/2544)of the patients who had other concomitant ocular diseases,and 24.3% (618/2544) had other systematic diseases. Over half of the participants (60.2%, 1532/2544) were receiving other medications or treatments. Most patients (82.5%,2099/2544) did not stop taking other drugs within 30d before using tafluprost. Among all participants, 65.6% (1670/2544)were prescribed with tafluprost in combination with carbonic anhydrase inhibitors (CAIs), sympathomimetics, β‐blockers or other PGAs. Detailed baseline characteristics are showed in Table 1.
Adverse Drug Reactions of TafulprostIn this study, a total of 359 ADRs occurred in 258 patients, with an incidence rate of 10.1% (258/2544). Table 2 presents the ocular ADRs with incidence of over 1% and non‐ocular ADRs over 0.1%.Of all participants with ADRs, 21.7% (56/258) recovered,32.9% (85/258) were improving, and 48.1% (124/258) did not show improvement. Of the 58 patients who had 74 ADRs of special interest, 5.2% (3/58) recovered, 24.1% (14/58) were improving, and 72.4% (42/58) did not show improvement.Among the 42 patients who did not show improvement, the 55 ADRs included 21 eyelid pigmentation, 5 iris pigmentation, 25 eyelash changes and 4 eyelid hypertrichosis.
In the 258 patients who had ADRs, 85.3% (220/258) continued to use tafluprost, while 2.3% (6/258) suspended tafluprost treatment due to ADRs, and 12.0% (31/258) terminated tafluprost. Among all 124 patients whose ADRs did not improve, 111 patients still continued using tafluprost.
The most common ADR was conjunctival hyperemia (128events in 124 patients, 4.9%, 124/2544). Conjunctival hyperemia was mainly mild (91.41%, 117/128) and moderate (7.03%, 9/128). There was no severe conjunctival hyperemia reported. Furthermore, 35 (27.34%) among all 128 conjunctival hyperemia recovered, 46 (35.94%) were improving, and 43 (33.59%) did not show improvement.Additionally, 87.5% (112/128) continued to use tafluprost,while 29.69% (38/128) of which conjunctival hyperemia did not improve also continued to use tafl uprost.
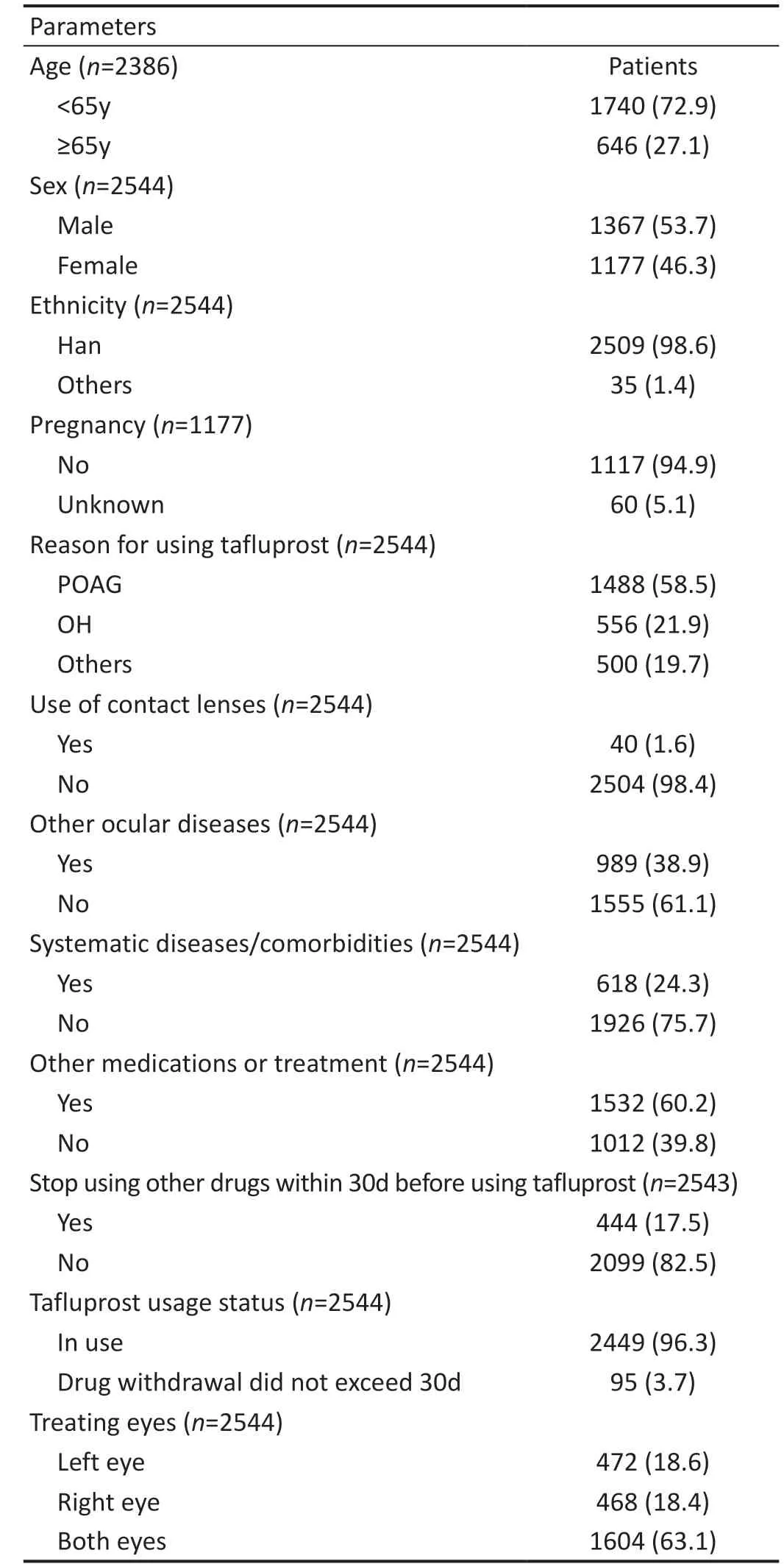
Table 1 Baseline characteristics of patients n (%)
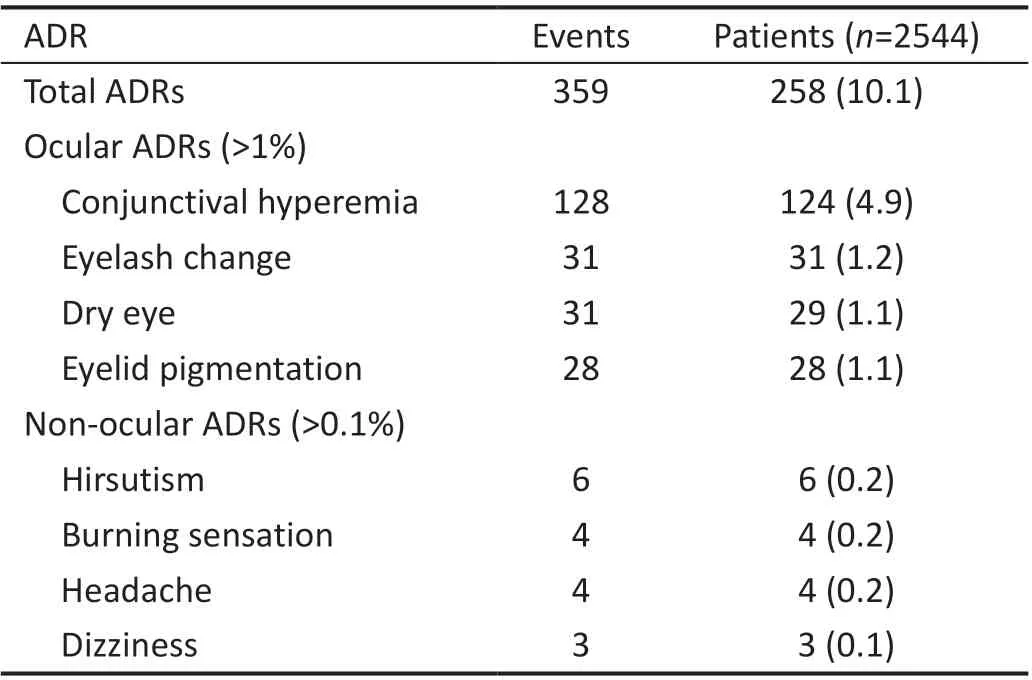
Table 2 Adverse drug reactions of tafluprost n (%)
The safety of tafl uprost in elderly patients was also evaluated.A total of 646 participants were 65 years old or older, with 322 males and 324 females. Among them, 7.0% (45/646) reported 65 ADRs, and all of the ADRs were non‐serious. The 15.4%(10/65) of the ADRs recovered, 22.2% (15/65) improved, 1.5%(1/65) unknown, while 60.0% (39/65) did not improve.
Combined Therapy of Tafl uprost and Other IOP-lowering Eye DropsA total of 65.6% (1670/2544) of participants has been found to use tafluprost as a part of combined therapy.The five most common eye drops combing tafluprost are CAIs (37.1%, 620/1670), sympathomimetics (33.5%,559/1670), β‐blockers (33.2%, 555/1670), other PGAs(15.6%, 260/1670) and other medications for ophthalmic use (46.8%, 782/1670; mainly including artificial tears,fluoroquinolones, corticosteroids and antiinfectives in combination, glucocorticoids, vitamin B, mannitol injection,and mydriatics,etc). The highest incidence of conjunctival hyperemia was noted in patients who received tafluprost in combination with other PGAs (23 ADRs in 23 patients, 8.8%,23/260) and the lowest was in combination with CAIs (16 ADRs in 16 patients, 2.6%, 16/620). Among the 5 combination regimens, eyelash changes were reported most often in patients prescribed with combination of tafl uprost and other PGAs (9 ADRs in 9 patients, 3.5%, 9/260). Detailed ADRs in relation to the different combination therapy is showed in Table 3.
Other Clinical ApplicationIn clinical practice, patients were prescribed with tafluprost in the situation other than POAG or OH. Among those 500 patients, 208 (41.6%, 208/500)were primary angle‐closure glaucoma (PACG), 89 (17.8%,89/500) received tafl uprost treatment after glaucoma surgery,and 79 (15.8%, 79/500) were intraocular hypertension after non‐glaucoma surgery. There was no SAE or new ADR different from when used for POAG and OH under various circumstances of clinical application. Of the 89 patients receiving tafl uprost after glaucoma surgery, 20 patients (22.5%,20/89) reported 29 ADRs, while 19 ADRs occurred in 13 patients (6.3%, 13/208) who were treated with tafl uprost due to PACG. No ADR was observed when tafluprost was given to patients after non‐glaucoma surgery. The most common ADR related to tafluprost was also conjunctival hyperemia.Detailed ADRs of tafl uprost under various situations of clinical application were displayed in Table 4.

Table 3 Adverse drug reactions in relation to the combination therapy of tafluprost and other IOP-lowering eye drops events/patients (%)
DISCUSSION
This study was a multi‐center post‐marketing observational study of tafluprost focusing on its safety in real‐world clinical setting in Chinese population. Tafluprost proved satisfying safety profile with merely 359 ADRs occurred in 258 patients, with an incidence rate of as low as 10.1%.This study also proved acceptable safety of tafluprost in combination with various IOP‐lowering eye drops, including CAIs, sympathomimetics, β‐blockers, other PGAs and other medications for ophthalmic use. In clinical practice, tafl uprost was also well tolerated to lower IOP in PACG and after glaucoma and non‐glaucoma surgeries apart from treating POAG and OH.
In Chinese Phase III study published by Geet al[13], the incidence of ADRs reported in patients with POAG after prescription of tafluprost was 31.7%. In this study, the incidence rate was not higher (10.1%) with 359 ADRs reported in 258 patients out of 2544. This may be explained by the 4‐week washout period and 1‐month follow‐up in the randomized phase III study. Furthermore, the relatively older baseline age of participants in this study might also relate to the low incidence rate. Nevertheless, the main ADRs in the two studies were similar, involving conjunctival hyperemia.
Despite the number of observed patients, including 27.1%(646/2386) being 65 years or older and a total of 120 being 14 years or younger, no serious AEs were reported in this study, which was similar to other observational post‐marketing studies[14], indicating that study drug is well‐tolerated in observed populations. The ADRs with relatively higher incidence (over 1%), such as eyelid pigmentation (3.94%,168/4265) and eyelash changes (2.34%, 100/4265), described in observational study by Kuwayamaet al[9]were reported in 1.1% and 1.2% of the patients in this study, respectively.The slightly higher incidence might be owing to the longer observation period (2y) in the Japanese study.The frequency of ADRs in this study is 10.1% (359 ADRs in 258 patients) which is also slightly lower than reported by Tumbocon and Macasaet[8](Philippines, 15% of patients) orKuwayamaet al[9](Japan, 18.64%). The patient age in these two studies [Philippines, 64.8 (14‐94)y; Japan, 66.8±12.7y]were much older than that in this study (50.8±19.1y), which might be associated with the higher incidence of ADRs. As for the common ADRs of tafluprost, this study reported similar safety profile with the most common one of conjunctival hyperemia (128 ADRs in 124 patients, 4.9%), which was consistent with previous study[9]. Conjunctival hyperemia is a common ADR regarding PGAs which is attributed to the ocular surface toxicity of the anti‐glaucoma eye drops components,including the active ingredients, the preservatives as well as the excipients[15]and the effect of PGAs on the retinal blood flow[16]. Studies of bimatoprost[17]and travoprost[18]both reported higher incidence of conjunctival hyperemia compared with that observed in this study of tafluprost. In addition, with the fact that the majority of conjunctival hyperemia was mild and moderate, 87.5% (112/128) continued to use tafluprost,while 29.69% (38/128) continued to use tafluprost despite that conjunctival hyperemia did not improve. This indicated that the most common ADR of tafluprost, conjunctival hyperemia,might not greatly interrupt the treatment.

Table 4 Adverse drug reactions related to different circumstances of clinical application of tafluprost events/patients (%)
It is important to note that 65.6% (1670/2544) of participants used other drugs, which reflect the actual clinical practices in China. Among the patients receiving combined therapy of tafluprost and β‐blockers (33.2%, 555/1670), the incidence of conjunctival hyperemia did not increase tremendously compare with that of tafluprost monotherapy (3.7%vs2.9%),supported by previous studies which also demonstrated the relatively lower incidence of hyperemia and eye irritation under such combination[12,19]. A Meta‐analysis revealed that the fixed combination of PGAs and timolol did not increase the incidence of hyperemia comparing with PGAs monotherapy[20].In this study, the highest incidence of conjunctival hyperemia was observed in patients who used tafluprost in combination with other PGAs (23 ADRs in 23 patients, 8.8%) and the lowest in combination with CAIs (16 ADRs in 16 patients, 2.6%).
The safety of tafluprost in 646 patients with 65 years old or older was evaluated in this study. All 65 ADRs reported among 7.0% (45/546) of these patients were not serious,which indicated satisfying safety profile of tafluprost in the application of elderly patients. This study included a total of 120 patients who were 14 years old or younger and 14 of them (11.67%) reported 18 non‐serious ADRs. Furthermore,this study observed no AE of tafluprost for lowering IOP in a one‐year old infant with congenital glaucoma. Tafluprost was administered for over 5mo and the infant was still receiving tafluprost by this report of study, with no ADR occurred.Tafluprost may be another PGA option for juvenile patients.
This study proved manageable safety profile regarding the combination therapy of tafluprost and other IOP‐lowering eye drops, including CAIs, sympathomimetics, β‐blockers, other PGAs and other medications for ophthalmic use. Previous studies reported similar results when tafluprost was combined with timolol[6,21], which ADRs occurred in 5.01% patients with conjunctival hyperemia, blepharitis, and punctate keratitis[6].This study provided multiple options for tafluprost‐based combined therapy other than timolol, widening the choices of IOP‐lowering regimens for doctors in real‐world clinical practice.
In this study, tafluprost could also be applied in patients with PACG and lowering the IOP after both glaucoma and non‐glaucoma surgeries, indicating that tafluprost might be tolerable under various circumstances in clinical practice.Other PGAs also showed acceptable safety and tolerability in effectively lowering IOP for patients with angle‐closure glaucoma[6,21].
This study had some limitations. First, ADRs were possibly underestimated owing to loss of follow‐up in some patients who stopped taking tafluprost by themselves due to ADRs. In addition, the sample size included was less than the calculated sample size. Second, patients in this study were all Asian,98.6% of Chinese Han, and the observed frequency of AEs may be different in patients with POAG and OH in other ethnicities. Third, 65.6% (1670/2544) of patients used other drugs in our study. ADRs due to other drugs might be present,which could be a potential confounding factor. Finally, it was not sufficient to observe the long‐term safety of tafluprost since AEs in this study were collected only within 30d after the treatment started.
Tafluprost is safe to be applied in patients with POAG and OH. The combination of tafluprost with CAIs, sympathetic agents, β‐blockers, other PGAs or other eye drops all proved manageable safety profile. Furthermore, tafluprost can also be applied in patients with PACG and after both glaucoma and non‐glaucoma surgery.
ACKNOWLEDGEMENTS
Authors’ contributions:Chen XL had full access to all of the data in the study and take responsibility for the integrity and accuracy of the data. Chen XL: Acquisition, analysis, or interpretation of data; Drafting of the manuscript; Critical revision of the manuscript for important intellectual content;Statistical analysis. All authors: Acquisition.
Foundation:Supported by Santen Pharmaceutical (China)Co., Ltd.
Conflicts of Interest: Chen XL,None;Fu YJ,None;Qu B,None;Wang YW,None;Tang X,None;Wang YH,None;Zhou GY,None;Lin MK,None;Shen JY,None;Yao J,None;Li SY,None;Wu MQ,None;Peng HZ,None;Lai MY,None;Wu RY,None;Zhang YN,None;Li Y,None;Wu XJ,None;Zhang MC,None;Guo SP,None;Sun XH,None.
 International Journal of Ophthalmology2023年1期
International Journal of Ophthalmology2023年1期
- International Journal of Ophthalmology的其它文章
- Instructions for Authors
- Morphological and functional changes in the macular area in diabetic macular edema after a single intravitreal injection of aflibercept
- Macular vascularisation changes analysed using OCT angiography after successful rhegmatogenous retinal detachment repair
- Comparison of success rate and intraocular pressure spikes between selective laser trabeculoplasty and micropulse laser trabeculoplasty in African American and Hispanic patients
- Efficacy of custom-made soft keratoconus lenses on corneal aberrations and photic phenomena in patients with keratoconus: a corneal topography imaging based study
- Clinical observation of recombinant human nerve growth factor in the treatment of neurotrophic keratitis
