Enhance hydrates formation with stainless steel fiber for high capacity methane storage
Zhixia Deng ,Shuanshi Fan,3,4, *,Yanhong Wang,4 ,Xuemei Lang,3 ,Gang Li
1 School of Chemistry and Chemical Engineering,South China University of Technology,Guangzhou 510640,China
2 Guangdong Engineering Technology Research Center of Advanced Insulating Coating,Zhuhai 519175,China
3 Institute of Modern Industrial Innovation,South China University of Technology,Zhuhai 519175,China
4 Key Laboratory of Fuel Cell Technology of Guangdong Province,Guangzhou 510640,China
Keywords:Methane Stainless steel fiber Gas storage capacity Hydrate Gas storage rate Heat transfer
ABSTRACT In order to realize efficient gas storage of hydrate,stainless steel fiber (SSF) was added to 0.03% (mass)sodium dodecyl sulfate(SDS)solution for gas storage experiment.SSF can not only improve the problem that hydration heat cannot be removed effectively in the hydration process,but also improve gas storage speed and gas storage by increasing hydrate nucleation sites.Under the experimental conditions(273.2 K,5-9 MPa),the peaks of temperature rise in SDS+SSF systems were found to become much smaller than those in SDS systems.The maximum gas storage rate and the maximum methane uptake of SSF+SDS system reached 9.89-24.90 cm3·g-1·min-1 and 178.65-200.89 cm3·(g H2O)-1,respectively.Compared with the surfactant SDS solution without SSF,they increased by 10.47%-33.22% and 9.16%-25.36%,respectively.The effect of SSF length on gas storage performance was studied.Due to the continuous thermal conduction network,longer SSF showed a higher gas storage capacity and methane uptake rate compared with shorter SSF.At the same time,compared with other metal fillers,SSF+SDS not only had excellent gas storage performance,but also the amount of SSF (0.1 g·ml-1) was only 7.6% of foamed aluminum,and the volume gas storage density was increased by 145.4%.The use of stainless steel fiber made the best use of the thermal conductivity of metal,reduced the amount of metal used,and improved the volume density and mass density of gas storage.
1.Introduction
Due to the increasing demand for natural gas energy and the importance of natural gas as the cleanest fossil fuel in environmental concerns,it is necessary to develop efficient storage and transportation technologies for natural gas [1].Compared with traditional natural gas storage methods such as liquefied natural gas (LNG) and compressed natural gas (CNG),the hydrate-based solidified natural gas (SNG) has the advantages of high efficiency,safety and economy [2,3].Therefore,SNG technology is a promising alternative method with good development prospects in the future.
However,the low gas-liquid heat transfer efficiency and slow hydration reaction in the actual gas storage process limit the development of this technology [4].The key to promote its industrial application is to be able to synthesize hydrate quickly and have a high water-hydrate conversion rate [5].In order to improve the gas storage efficiency of gas hydrate,it is necessary to strengthen the mass and heat transfer process of hydration reaction system[6].Therefore,many scholars have adopted a series of measures to promote hydrate nucleation and growth from the aspects of dynamic and static or the combination.
In order to promote the rapid formation of hydrate,dynamic strengthening technology accelerates gas-liquid migration and increases contact area [7,8].Although it may increase energy consumption additionally,dynamic strengthening technologies such as stirring [9],spraying [10],bubbling [11],jetting [12] and impinging [13] have been proved to greatly enhance hydrate nucleation and growth kinetics.With the continuous development of material technology,static gas storage technologies including surface activity modification [14-16],physical dispersion modification[17-18]and macroscopic thermal conductivity modification[19-21]have become research hotspots in the 21st century due to its unique charm in promoting efficient gas storage of hydrate.The surface activity modification method reduces the interphase tension of hydration reactants to make liquid phase dissolve more gas molecules,thus promoting the growth of gas hydrate [22].Under the background of energy saving and emission reduction,the static hydration strengthening technology through physical dispersion modification and macroscopic thermal conductivity modification has important reference value for the industrial application of hydrated gas storage technology [6].Due to the wide application prospect of these methods,it is necessary to develop an efficient material to promote hydrate formation.
Many materials that improve hydrate formation from the perspective of mass and heat transfer have been discussed,such as activated carbon [23-25],metal nanoparticles [26,27],silica sand[28,29],graphite [30-32],carbon nanotubes [33,34],MOF [35-37],dry water [38-40],metal foam [41,42].These materials have promoted the gas storage performance of hydrate and provided useful information for the scale-up of hydrate industry.However,its gas storage speed and gas storage capacity still have room for further strengthening.Moreover,although these materials promote the synthesis of hydrate,they occupy a certain volume,resulting in a reduced volumetric storage capacity.
Therefore,compared with other metal fillers such as aluminum foam [43],the amount of stainless steel fiber (SSF) used in this experiment (0.1 g·ml-1) was only 7.6%,which reduced the space occupied by the material.In addition,SSF had a large rough metal surface due to its small diameter,and overlapping fibers can form a thermal conduction network to improve heat transmission.Sodium dodecyl sulfate(SDS)was used in this study to further promote hydrate formation,Since SDS was reported to be probably the most commercially suitable surfactant used in hydrate applications [44].Therefore,by studying the dynamic behavior of gas hydrate growth in SDS+SSF system,it is hoped that the system can make maximum use of the metal thermal conductivity of stainless steel fiber,reduce the use of metal,and improve the volume and mass gas storage density.
2.Experimental
2.1.Materials
Two lengths of stainless steel fiber(ϕ 6.5 μm×1300 mm and ϕ 6.5 μm × 3 mm)were provided by Hunan Huitong New Materials Co.,Ltd.The 99.99% pure methane was supplied by Yinglai Gas(Guangzhou) Co.,Ltd.Sodium dodecyl sulfate,purity 99.0%,purchased from Aladdin Biochemical Technology (Shanghai) Co.,Ltd.
2.2.Instruments
Fig.1 showed a schematic diagram of the experimental setup.The reactor made of 1Cr18Ni9Ti stainless steel(Jiangsu Haian Petroleum Research Instrument Factory) had an effective volume of 300 ml (50 mm inner diameter,8 mm wall thickness),can withstand the design pressure of 20 MPa.Two PT-100 thermal resistance detectors with accuracy of ±0.01 K (Shanghai Nanpu Instrument Factory) were inserted from the hatch on the reactor cover to measure the temperature of the gas and liquid phases respectively.A cold bath (THD-3015,Zhejiang Ningbo Tianheng Instrument Factory Co.,Ltd.) containing 95% (vol) glycol cooling liquid (freezing point lower than 223 K) was used to control the temperature of the reactor,with the control range of 243-373 K and the accuracy of ±0.1 K.The pressure sensor with accuracy of±0.01 MPa (Guangzhou Sennashi Instrument Co.,Ltd.) was connected to the hatch gas inlet pipeline to measure the internal gas pressure of the system.During the experiment,the relevant information every 10 seconds was recorded by a data recorder(Agilent 34901A,Agilent Technology(China)Co.,Ltd.)and displayed on the computer.
2.3.Experimental procedure
The structure of SSF in the open container was shown in Fig.2(a).SEM analysis showed that the diameter of SSF with rough surface was ϕ 6.5 μm(as shown in Fig.2(b)and(c)).First,3 g SSF and 0.03% (mass) SDS solution (30 ml) were added to the reactor cleaned with deionized water.Started the cooling system,set the temperature to 273.2 K,and immersed the reactor in the cold bath after the temperature stabilized.Blew with methane gas under low pressure to ensure that there was no residual air in the kettle.Then,by monitoring the temperature and pressure signals in the kettle through data acquisition,when the temperature reached 273.2 K,the methane with the preset experimental pressure would be injected into the reactor.If hydration occurred,the temperature would suddenly rise,while the pressure would drop.When the temperature was restored to 273.2 K and the gas pressure basically remained unchanged (less than 0.01 MPa) within 20 min,the hydration reaction process of the system was considered to be over.Three experiments under the same conditions were carried out to eliminate errors.Detailed treatment methods for changes in methane absorption and gas storage velocity during the experiment can be referred to our previous research [30].
3.Results and Discussion
3.1.Improving effect of SSF on gas storage performance
As low temperature and high pressure were favorable for hydration reaction[45],the huge hydration heat generated during the rapid formation of hydrate would increase the temperature to hinder the continuous formation of hydrate[46].Therefore,3 g SSF(6.5 μm in diameter and 138 cm in length) was added into the hydrate system to accelerate the heat of hydration transfer to the environment.At the same time,the larger rough surface provided by SSF provided nucleation and crystallization sites for hydration reactions.Therefore,the new hydration system can be expected to have efficient gas storage capacity.
Typical changes of liquid temperature and gas pressure over time in various systems including SDS+SSF,SDS and ionized water+SSF were shown in Fig.3.At the beginning of the experiment,methane pressure reached 9.0 MPa and the temperature dropped to 273.2 K in the kettle.In deionized water+SSF,the temperature and pressure did not change obviously,indicating that methane hydrate was hardly formed.Under static conditions,the formation of contiguous hydrate film at the gas-liquid interface would hinder the gas-liquid mass transfer,so that the hydrate cannot grow continuously [47].Therefore,the surfactant SDS was added to reduce the liquid surface tension and make the hydrate loose and porous to increase the gasliquid mass transfer which ensured the continuity of gas hydrate growth[48].With the addition of SDS,obvious pressure attenuation and temperature increase were observed in SDS+SSF and SDS systems,which proved that hydration reaction occurred.However,compared with SDS system,the maximum temperature rise of SDS+SSF system decreases by 39.7%and the pressure drop increases by 14.3%,indicating excellent gas storage capacity.This can be attributed to the sufficient mass transfer channels and excellent thermal conductivity of SDS+SSF system in hydrate induced nucleation and growth stage.
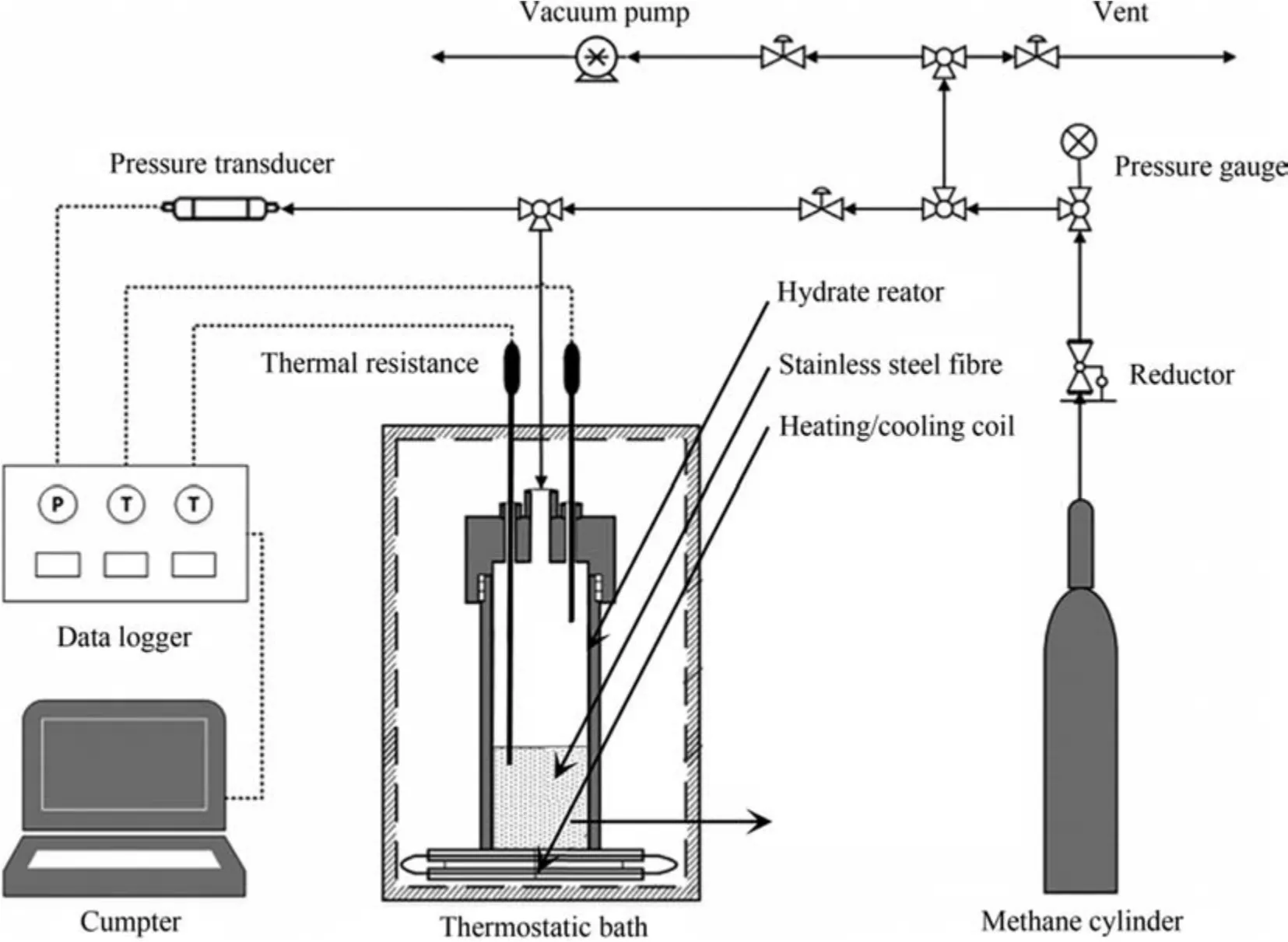
Fig.1.Schematic diagram of the experimental apparatus.(a) Appearance,(b) microcosmic surface of SSF.

Fig.2.Morphology of stainless steel fiber (a) in the kettle and (b),(c) SEM image.
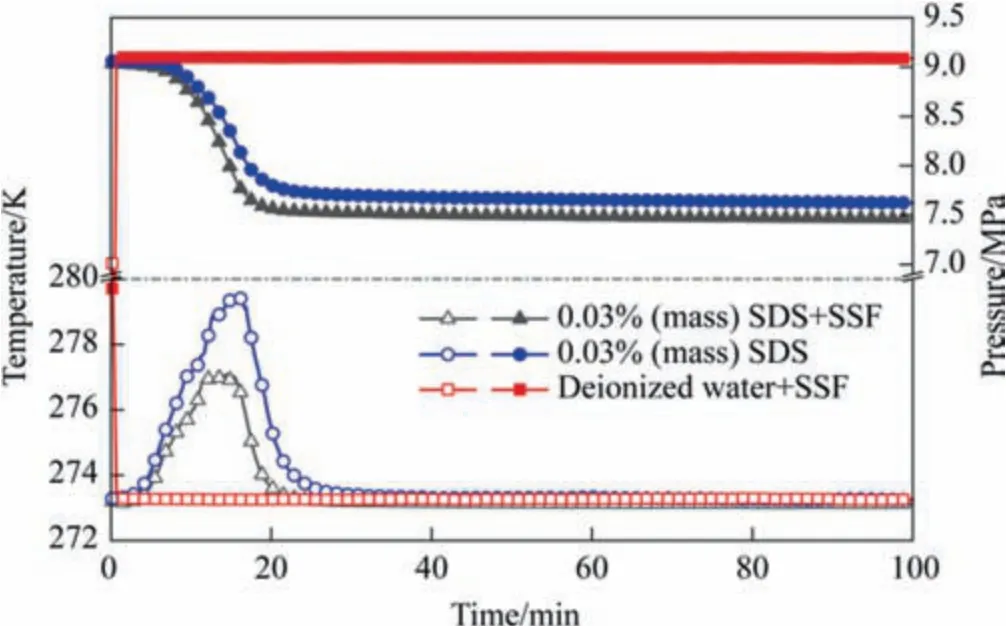
Fig.3.Variation of methane pressure and liquid temperature during hydration in the presence of SSF and SDS (P=9 MPa, T=273.2 K).
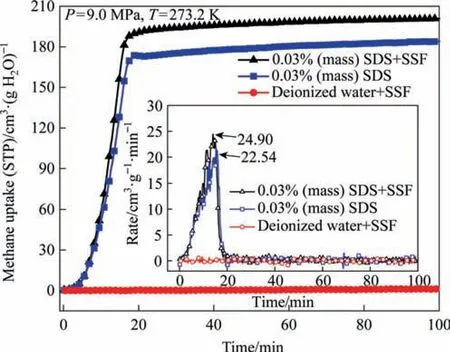
Fig.4.Kinetics of methane hydrate formation in the presence of SSF and SDS(P=9.0 MPa, T=273.2 K).
The formation dynamics of gas hydrate in SDS+SSF,SDS and deionized water+SSF system at 9.0 MPa was calculated by the temperature and pressure change,as shown in Fig.4.Methane consumption rate was the instantaneous gas storage capacity derived over time,and the extreme value represented the fastest gas storage rate that can be achieved under this condition.It was noted that gas hydrate hardly formed in pure water due to the blockage of gas-liquid mass transfer channel.In the presence of SSF only,deionized water hardly stored methane.Due to no heat generated by the absence of hydrate formation,SSF with high thermal conductivity failed to promote gas storage.In SDS solution and SDS+SSF system,the gas consumption curve was almost a vertical line,indicating that hydrate was rapidly formed.Compared with SDS solution,the methane consumption of SDS+SSF system increased by 9.16% with shorter time to reach the plateau (maximum).Therefore,SDS+SSF system had the best hydration effect,which made the methane storage reach 200.89 cm3·g-1·min-1within 20 min,and the hydrate saturation was as high as 93%.It was not easy to achieve such high saturation in hydrate rapid gas storage.Because the accumulation of hydrate heat may cause hydrate decomposition,and too fast hydrate formation rate may lead to the formation of empty cages,which meant that the hydrate continued to grow without the guest molecule entering the hydrate cage.Therefore,in addition to making the mass transfer between methane and water smoother by SDS,SSF also played a vital role in enhancing the heat dissipation of hydration process.
3.2.Influence of SSF under different pressure
In order to reduce the harsh conditions of hydration reaction and to study the influence of SSF on methane hydrate formation and growth dynamics in detail,SSF was added to SDS system at a pressure range of 5.0 to 8.0 MPa.Compared to water with a conductivity of 0.61 W·m-1·K-1,Stainless steel fiber with a thermal conductivity of 17 W·m-1·K-1were sufficient to direct hydration heat to the environment,resulting in a 35%-42% decrease in temperature rise during hydrate process (supporting information Figs.S1-S4 in Supplementary Material).Therefore,as shown in Fig.5,the maximum gas consumption of SDS+SSF system was superior to SDS system under each pressure,and the promoting effect of SSF was more obvious under milder conditions.At the lowest experimental pressure of 5.0 MPa,SDS system hardly formed hydrate within 100 min of the experiment,on the contrary,SDS+SSF system had a good gas storage capacity of 178.65 cm3-·g-1·min-1.This showed that the existence of SSF not only improved the storage capacity of methane and the hydrate storage rate under higher pressure,but also enabled the hydration process to proceed under lower pressure,which helped to reduce the energy consumption of hydrate formation.Meanwhile,compared with the existence of induction time in SDS system,SDS+SSF system was shorter or almost non-existent.These resulted indicate that SSF accelerated hydrate growth by enhancing hydration heat transfer,as well as promoted hydrate nucleation by providing larger rough metal surface.
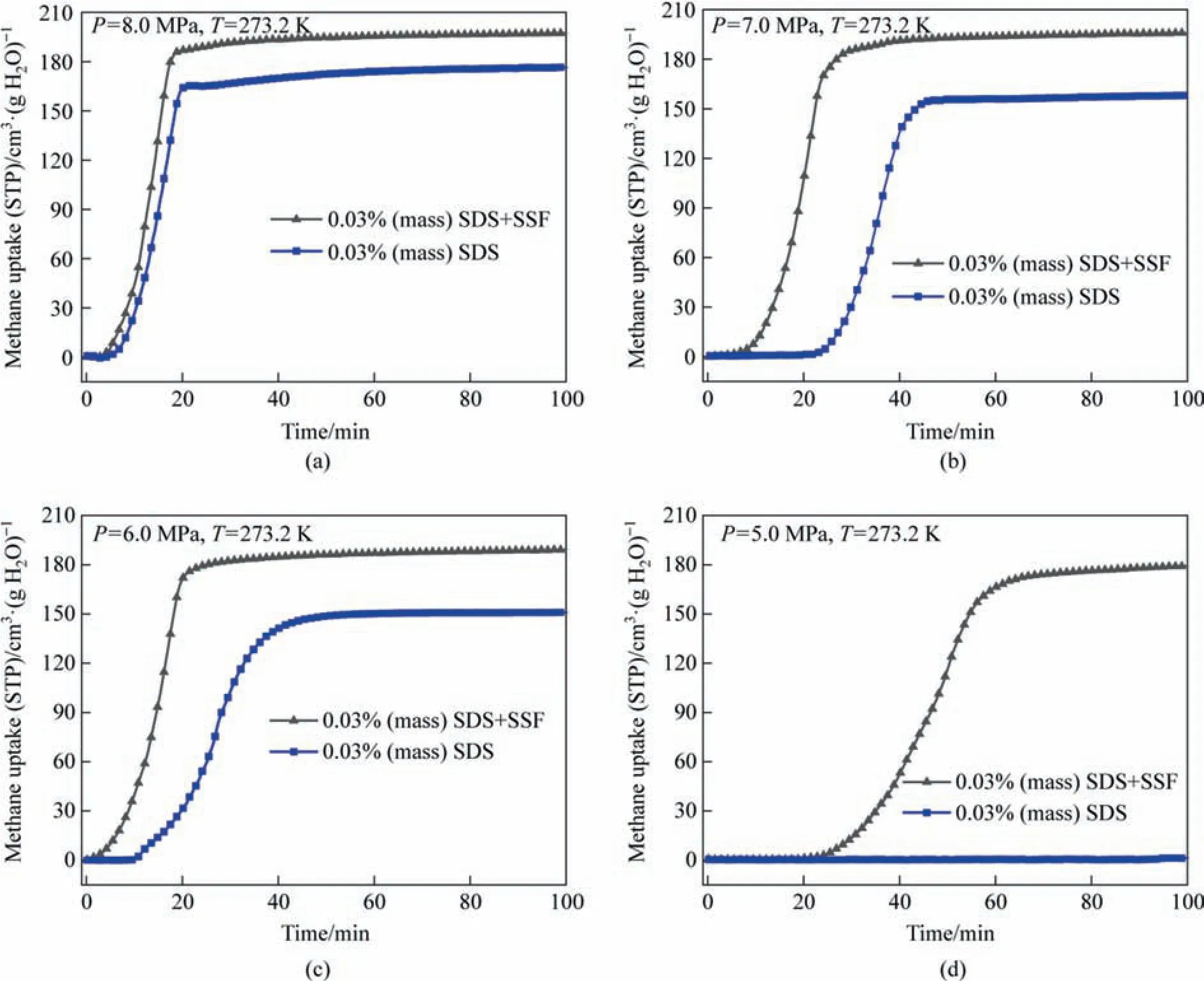
Fig.5.Effect of SSF on methane uptake during hydrate formation based on SDS solution under various pressures (P=5.0-8.0 MPa, T=273.2 K).
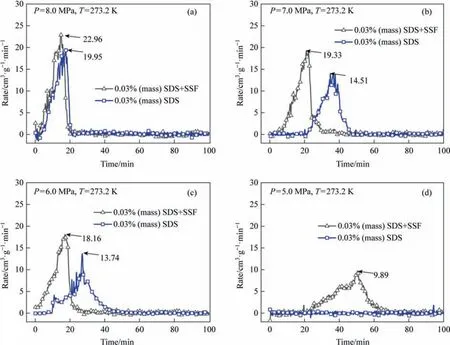
Fig.6.Methane uptake rate in the presence of SSF and SDS (P=5.0-8.0 MPa, T=273.2 K).
Fig.6 showed the effect of SSF on promoting hydrate growth rate of SDS system under different pressures.Among them,the peak value represented the maximum gas storage speed that can be achieved under such conditions.Constant pressure gas storage may maintain this gas storage rate for continuous hydration experiments.The maximum gas storage rates of SSF+SDS system and SDS system were 22.95 cm3·g-1·min-1and 19.95 cm3·g-1·min-1,respectively,within 18 min at a higher pressure of 8 MPa.Under the condition of high enough hydration reaction driving force,SSF did not significantly improve the maximum gas storage rate.However,when SSF was not present in SDS solution at lower pressure of 5 MPa,even though SDS can promote mass transfer,hydrate may not be synthesized due to insufficient driving force of hydration formation or fewer nucleation sites.For each hydration process under the same pressure,adding SSF to SDS solution can advance the nucleation time of hydrate by 0.1-20 min,and achieve a faster growth rate.For each hydration process,adding SSF into SDS solution at 6-7 MPa pressure can advance the nucleation time of hydrate by 10-20 min and shorten the complete growth time by 20-22 min.The results showed that the synergy between SDS and SSF can promote hydrate formation more effectively than SDS surfactant alone.
The experimental conditions and results were summarized in Table 1.For intuitive comparison,the experimental data were further analyzed.As shown in Fig.7,the maximum methane consumption peaks increased by 25.36%,23.16%,11.86% and 9.16%with the addition of SSF at 6.0-9.0 MPa.At relatively higher pressure,hydrate had a large gas storage capacity.However,due to the promoting effect of SSF,hydrate saturation had reached 87.6%and 90.5% at 6 and 7 MPa,respectively.The gas storage capacity of SDS+SSF system almost reached the theoretical level of sI type methane hydrate at 9 MPa.In addition,from the point of view of maximum gas storage speed,under the pressure of 6.0-9.0 MPa,these systems with SSF improved by 32.17%,33.22%,15.09% and 10.47% respectively.At 6 and 7 MPa,SDS+SSF system already had high gas storage performance,and the gas hydrate reached high saturation.Therefore,the effect of different fiber structures on gas storage performance under this pressure would be studied.

Table 1 Experimental conditions and methane uptake in the presence and absence of SSF (ϕ 6.5 μm × 1300 mm)
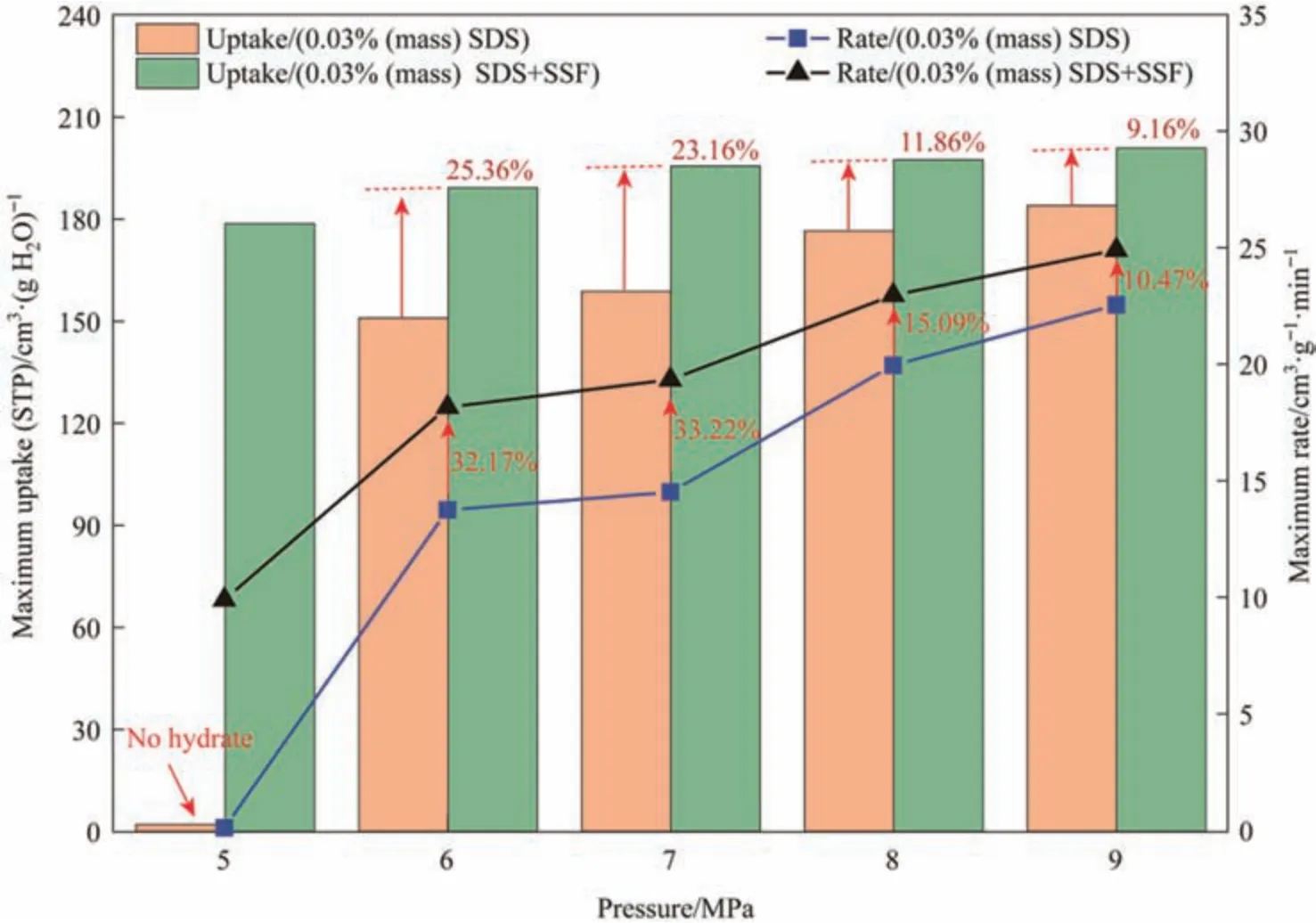
Fig.7.Maximum methane uptake and rate of hydrate in the presence of SSF and SDS (P=5.0-9.0 MPa, T=273.2 K).

Fig.8.Effect of different SSF on methane uptake and rate of hydrate (P=6.0-7.0 MPa, T=273.2 K).

Table 2 Comparison of efficiency of SSF and some metal materials in promoting hydrate formation
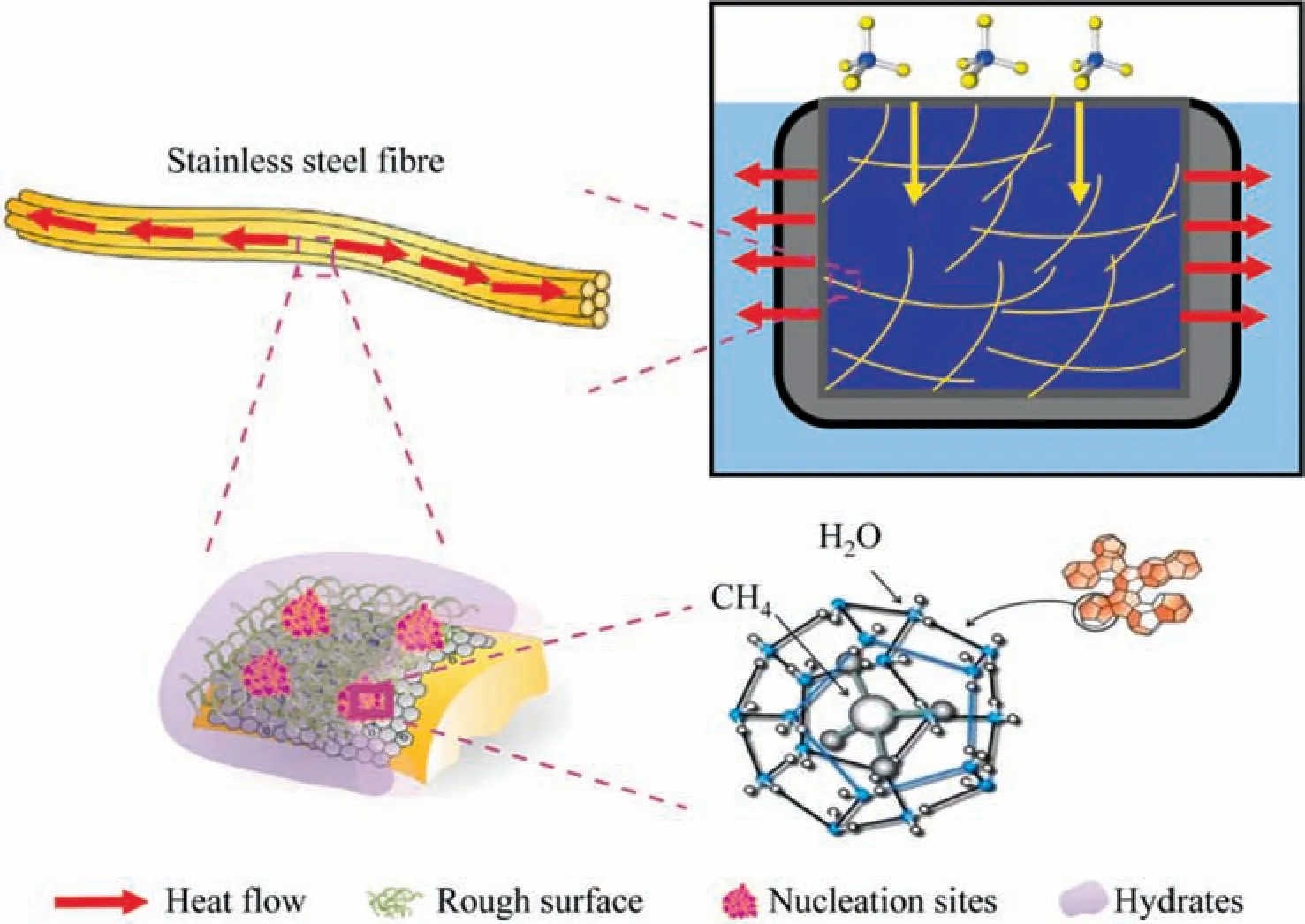
Fig.9.Schematic microscopic interpretation of methane hydrate formation in the SSF system.
3.3.Effects of different structure SSF
In order to compare the strength of SSF in enhancing gas storage performance of hydrate by mass transfer and heat transfer,different types of SSF(ϕ 6.5 μm,0.3 cm length)were used to conduct gas storage experiments.According to temperature and pressure(Figs.S2 and S3 in Supplementary Material),the variation of gas storage capacity and gas storage speed over time was shown in Fig.8,there was little difference in gas storage performance between the two kinds of fibers.Under the pressure of 273.2 K and 6.0 MPa,the methane absorption amount and maximum absorption rate of the system with long fiber can reach 189.25 cm3-·(g H2O)-1and 18.16 cm3·g-1·min-1,respectively,which were 8.1%and 5.7%higher than that short fiber.Although long fibers provided slightly fewer nucleation sites,due to a continuous thermal network,they had a higher methane uptake and a slightly larger absorption rate,as shown in supporting information Table S1.Therefore,in addition to providing nucleation sites,the enhancement of heat transfer performance of the system by SSF should be the main factor to improve the gas storage effect of hydrate.The methane absorption of hydrate was improved by establishing a continuous three-dimensional network to guide the heat of hydrate formation to the environment.
In order to visually compare the promotion effect of SSF,we counted some metal materials that promoted hydrate formation reported in the past decade,as shown in Table 2.According to these reports,promoting materials improved methane absorption capacity and faster hydrate formation dynamics,but there were still engineering challenges in industrial gas storage applications.In addition,the cost and processing of materials also hindered the large-scale use of these materials for methane storage.Although 10% (mass) SSF was used in the experiment,it only accounted for about 1.5%of the water volume and had little impact on the gas storage space of hydrate,which only reduced the gas storage capacity by about 3.3 cm3·(g H2O)-1at most.Compared with other metal fillers [41,49-51],SSF+SDS not only had excellent gas storage performance with 90.5% saturation,but also the amount of SSF (0.1 g·ml-1) was only 7.6% of foamed aluminum,and the gas storage density was increased by 145.4% [43].The use of stainless steel fiber wire made the best use of the thermal conductivity of metal,while reduced the use of metal,improved the volume and mass gas storage density.
Therefore,as shown in Fig.9,in order to improve the gas storage performance of hydrate,SSF was added to SDS solution with a concentration of 0.03%(mass).It was expected to improve the problem that hydration heat cannot be removed effectively in the hydration process,and to increase the methane absorption amount and efficiency by adding nucleation sites of hydrate.The fibers used in the experiment had a diameter of 6.5 microns and a large specific surface area,which can provide more nucleation sites.In addition,the excellent thermal conductivity of the fiber was conducive to heat emission of hydrate,thus improving heat transfer and methane absorption of hydrate.The model of promoting gas hydrate storage through SSF would provide favorable help for the advancement of SNG process.
4.Conclusions
Slow hydrate reaction,low conversion rate and insufficient gas storage limited the development of hydrate-based technology.In order to promote hydrate formation efficiently,SSF was added to SDS solution.The results show that SSF accelerated hydrate growth by promoting hydration heat transfer,in addition to providing nucleation sites for hydrate.The temperature rise peak of SDS+SSF system was 35%-42% lower than SDS system at 6-9 MPa.Under the experimental conditions,the maximum methane absorption capacity and the maximum methane absorption rate increased by 9.16%-25.36% and 10.47%-33.22% after adding SSF into SDS solution.Compared with other promoting materials,SSF can achieve 90.5% hydrate saturation under similar conditions and had a higher volumetric gas storage density due to less usage.Therefore,the continuous heat conduction network formed by SSF can improve the methane absorption capacity of hydrate,making the gas storage capacity up to 195.56 cm3·(g H2O)-1,and the gas storage rate up to 19.33 cm3·g-1·min-1at 273.2 K and 7 MPa.It is hoped that this study can provide a new idea for improving the methane absorption capacity of hydrate with metal filler and provided useful information for SNG process with SSF.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We are thankful to the National Natural Science Foundation of China (21736005 and 51876069),Special project for marine economy development of Guangdong (six marine industries) (GDNRC[2022]46),Key Research &Development Program of Guangzhou City (No.202206050002,202206050001).
Supplementary Material
Supplementary material to this article can be found online at https://doi.org/10.1016/j.cjche.2022.07.028.
 Chinese Journal of Chemical Engineering2022年10期
Chinese Journal of Chemical Engineering2022年10期
- Chinese Journal of Chemical Engineering的其它文章
- Understanding the effects of electrode meso-macropore structure and solvent polarity on electric double layer capacitors based on a continuum model
- Refrigeration system synthesis based on de-redundant model by particle swarm optimization algorithm
- Adaptive multiscale convolutional neural network model for chemical process fault diagnosis
- Injectable self-healing nanocellulose hydrogels crosslinked by aluminum: Cellulose nanocrystals vs.cellulose nanofibrils
- Establishment of nucleation and growth model of silica nanostructured particles and comparison with experimental data
- Molecular dynamics simulations of ovalbumin adsorption at squalene/water interface
