纳米颗粒对ZM5镁合金微弧氧化涂层耐磨和耐蚀性能的影响
李健鹏,万红霞,涂小慧,李卫,郭静,宋东东
腐蚀与防护
纳米颗粒对ZM5镁合金微弧氧化涂层耐磨和耐蚀性能的影响
李健鹏1,万红霞2,涂小慧1,李卫1,郭静3,宋东东4
(1.暨南大学 先进耐磨蚀及功能材料研究院,广州 510632;2.中国石油大学(北京) 新能源与材料学院,北京 102249;3.中国特种设备检测研究院,北京 101300; 4.华北电力大学 电站能量传递转化与系统重点实验室,北京 102206)
进一步提高ZM5镁合金微弧氧化(MAO)涂层的耐磨和耐蚀性能。在镁合金表面制备了不含与含有SiC和CeO2纳米颗粒的3种MAO涂层。使用扫描电子显微镜(SEM)、能量色散X射线光谱仪(EDS)和X射线衍射仪(XRD),对MAO涂层的表面形貌和成分结构进行分析,通过摩擦试验测试了涂层的耐磨性能,通过极化曲线(Tafel)和电化学阻抗谱(EIS)测试了涂层的耐蚀性能。含有SiC纳米颗粒的MAO涂层厚度、硬度分别提升了19.40%、86.56%,含有CeO2纳米颗粒的MAO涂层厚度、硬度分别提升了3.74%、44.59%。含有SiC纳米颗粒的涂层孔隙率升高6.60%,而添加CeO2使涂层的孔隙率下降23.90%。摩擦试验表明,不含纳米颗粒的MAO涂层磨痕深度为36.4 µm,而含有纳米颗粒的涂层磨痕深度可以忽略不计。Tafel试验表明,CeO2纳米颗粒可以显著降低MAO涂层的腐蚀电流密度,从1.41×10‒9A/cm2降至5.72×10‒10A/cm2。同时延长了涂层的稳定钝化区间180 mV。EIS试验也表明,浸泡前后,含有CeO2纳米颗粒的涂层都具有最高的低频阻抗值。纳米颗粒可以填充MAO涂层中的孔隙和裂纹,增大涂层的厚度和硬度,因此有效地改善涂层的耐磨性能。但在MAO处理时,SiC纳米颗粒增大了涂层的稳定电流密度,提高了等离子体放电强度,导致纳米颗粒的填充作用不明显,使涂层孔隙率升高。同时,含有CeO2纳米颗粒的涂层具有较小的孔隙率,并且厚度较大。因此CeO2纳米颗粒还可以有效地改善涂层的耐蚀性能。
ZM5镁合金;微弧氧化涂层;纳米颗粒;耐磨性能;耐蚀性能
镁及其合金,如ZM5,具有低密度、高比强度和比刚度以及良好的导热性等特点,广泛应用于汽车、电子、航空航天和生物医学等领域[1-3]。然而,在服役过程中,ZM5耐蚀性能较差,其应用受到严重地限制[4]。表面处理是改善镁合金耐蚀性能的有效方式,包括有机涂层、化学镀层、化学转化涂层和微弧氧化涂层等[5-8]。由于简便、高效和环保等性能特点,MAO工艺在近几年得到了广泛的关注[9-14]。为了应对复杂的工作环境,MAO涂层仅仅具有较好的耐蚀性能已难以满足要求[1,8,15-17]。如何使MAO涂层同时具有优异的耐磨和耐蚀性能是目前研究的重点内容。
提高MAO涂层的耐磨和耐蚀性能的方法有很多[11,18-20]。在电解液中加入纳米颗粒是改善MAO涂层性能的有效方法。在涂层形成过程中,纳米颗粒可以增强基体表面的弧光放电,从而增加MAO涂层的厚度[21-23]。纳米颗粒也可以嵌入或者熔融进入涂层,改变涂层的成分和结构[21],从而改善涂层的耐磨和耐蚀性能。Yu等[24]通过对AZ31镁合金表面含有SiC颗粒的MAO涂层研究表明,SiC纳米颗粒可有效增大涂层厚度、改善涂层结构,从而提升涂层的耐磨性能。Zheng等[25]通过在纯镁表面MAO涂层中添加CeO2颗粒,降低了涂层的孔隙,从而提升了涂层的耐蚀性能,同时含有CeO2颗粒的涂层可以为基体提供长期稳定的保护。综上所述,纳米颗粒会优先填充MAO涂层中的孔隙和裂纹,改善涂层的成分和结构,从而改善耐磨或耐蚀性能。但缺乏对于耐磨和耐蚀性能的综合研究。在此基础上,本文研究SiC和CeO2纳米颗粒各自对MAO涂层的耐磨和耐蚀性能的影响,从而选择最合适的纳米颗粒,改善MAO涂层的耐磨和耐蚀性能。
本文在ZM5镁合金表面制备了不含、含有SiC和CeO2纳米颗粒的3种MAO涂层。通过SEM、EDS和XRD,对MAO涂层的表面形貌和成分结构进行表征。通过显微硬度试验,对其显微硬度进行表征。通过摩擦试验,对其耐磨性能进行研究。通过浸泡试验、电化学阻抗谱和极化曲线测试,对其耐蚀性能进行研究。
1 试验
1.1 涂层制备
ZM5镁合金的化学成分(以质量分数计)为:7.5%~9.0%Al,0.2%~0.8%Zn,0.15%~0.5%Mn,Si< 0.25%,Cu<0.1%,Fe<0.09%,Ni<0.01%,余量为Mg。试样规格为20 mm×20 mm×5 mm。在MAO处理前,经320#砂纸打磨基体的工作面,确保所有工作面都有相同的表面粗糙度。然后用去离子水、无水乙醇清洗试样并立即干燥。在MAO处理时,工作面积为20 mm×20 mm,其他部分用硅胶密封。
在恒定电压(400 V)下,使用微弧氧化电源(PN-III电源)制备MAO涂层。处理时间为20 min,脉冲频率为1 000 Hz,占空比为40%。在MAO过程中,试样作为阳极,不锈钢片作为阴极。电解液成分为15 g/L Na2SiO3+2 g/L NaOH+5 g/L NaF,pH为11.5。通过在电解液中加入5 g/L SiC纳米颗粒(颗粒尺寸为40 nm)或5 g/L CeO2纳米颗粒(颗粒尺寸为50 nm)制备含有纳米颗粒的MAO涂层。所有的化学品都是从Macklin购买的。在MAO处理之前,均匀搅拌电解液6 h。在MAO处理过程中,使用冷却系统将电解液的温度保持在(30±5) ℃。处理后,样品表面依次用蒸馏水、无水乙醇冲洗,然后立即用冷风吹干。表1为MAO涂层的编号。

表1 MAO涂层的编号
1.2 性能测试及组织观察
采用测厚仪(QNix-4500)测量厚度。采用显微硬度计(HXD-1000TMSC/LCD)测量硬度,载荷为0.5 N,加载时间为15 s,进行10次平行测试。用X射线衍射仪(XRD,Ultima Ⅳ)分析涂层物相,选用铜靶,电子加速电压为40 kV,电流为40 mA,扫描范围和步长分别为10°~80°和5 (°)/min。使用扫描电子显微镜(Phenom XL)研究MAO涂层的表面和横截面形貌以及元素成分。用Image J软件对SEM图像进行分析,得出孔隙率。
使用摩擦试验机(RTEC MFT 5000)进行往复式摩擦试验,并用三维轮廓仪分析磨痕三维形貌。304不锈钢球作为摩擦副,载荷为2 N,频率为5 Hz,摩擦行程为5 mm,摩擦时间为30 min,进行3次平行测试,测试结果具有重复性。
使用电化学工作站(CHI-604C)进行电化学阻抗谱(EIS)测试和极化曲线测试,测试溶液为3.5%NaCl。采用标准的三电极体系,试样为工作电极,饱和甘汞电极为参比电极,铂电极为对电极。在电化学阻抗谱测试和极化曲线测试前,都先进行开路电位的测试,测试时间为30 min。电化学阻抗谱测试时,频率范围为10 mHz~100 kHz,振幅为20 mV。极化曲线测试时,扫描起始电位为相对于开路电位‒250 mV,扫描速度为0.5 mV/s。为保证结果的可靠性,电化学测试都进行了3次平行测试,测试结果具有重复性。
2 结果分析
2.1 电流密度-时间响应
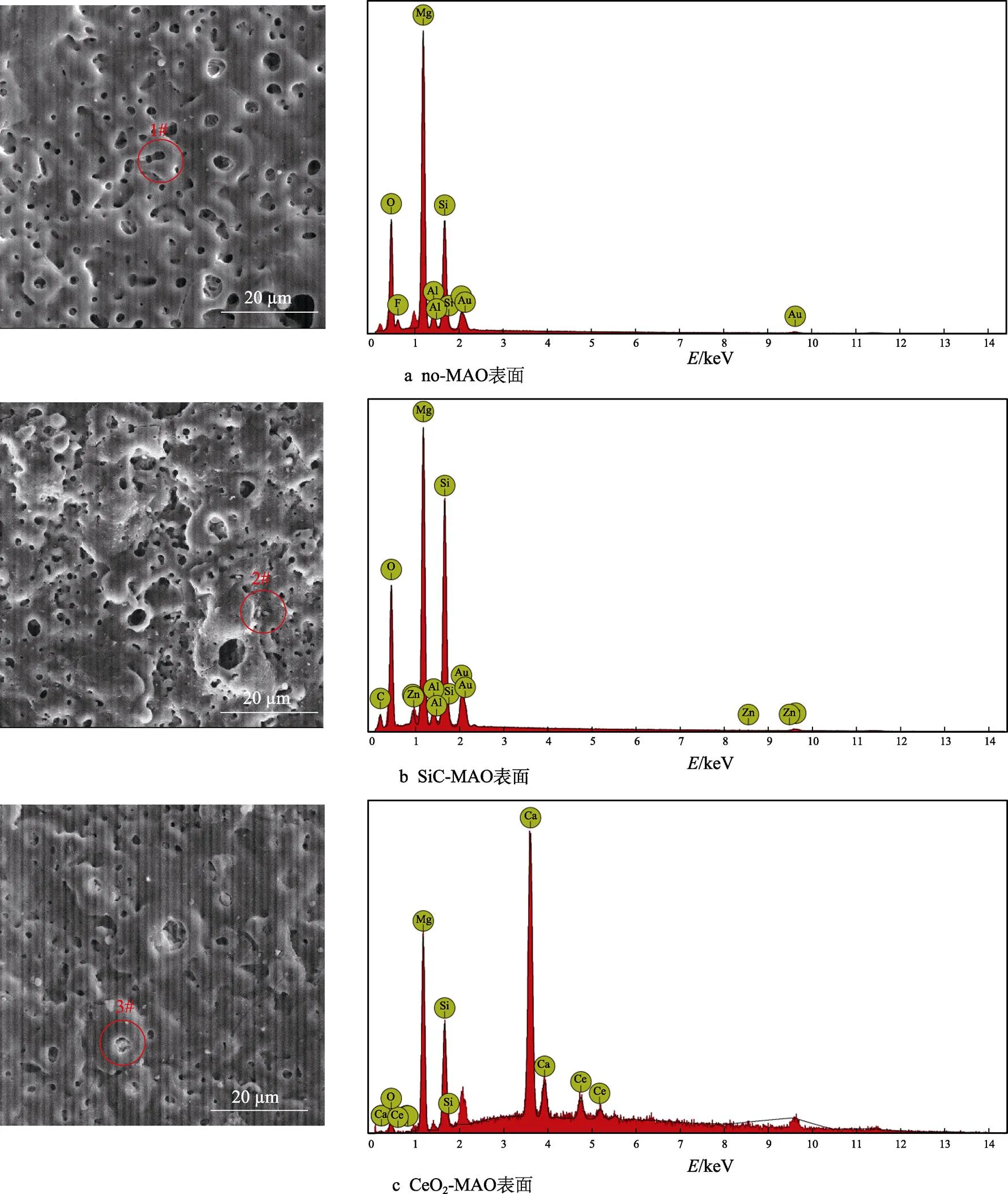
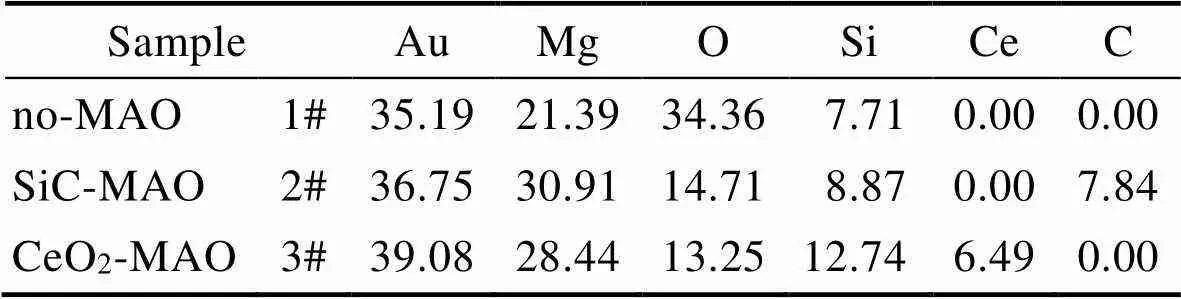
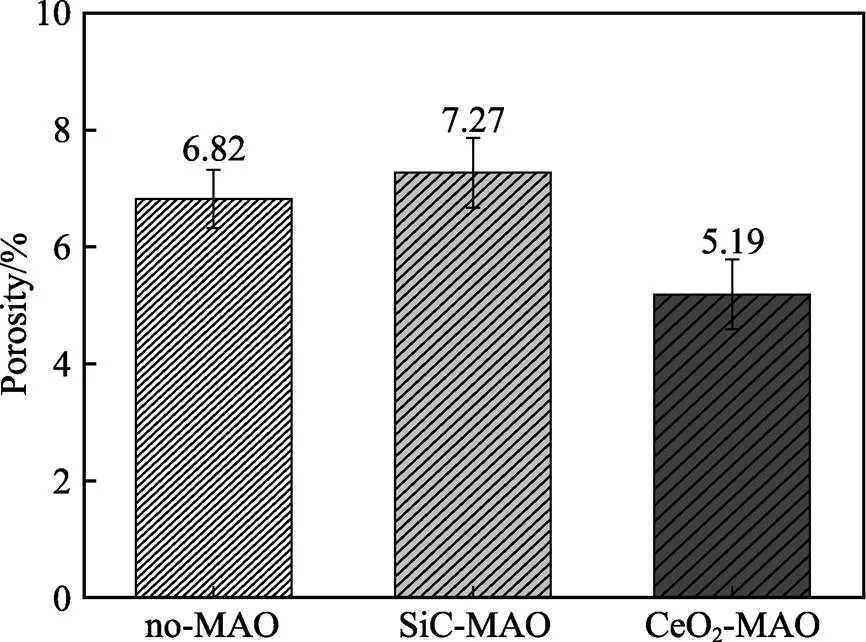
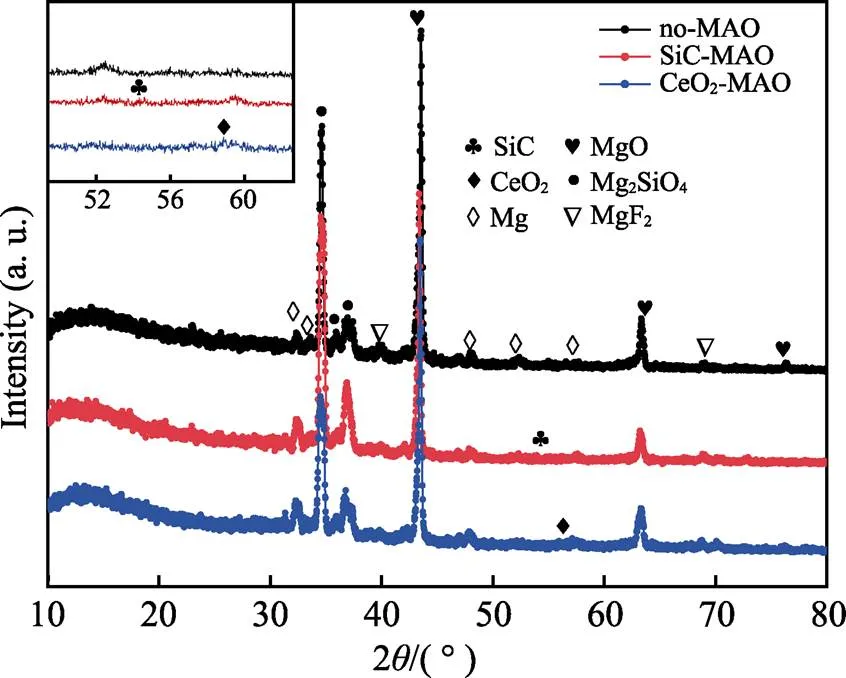
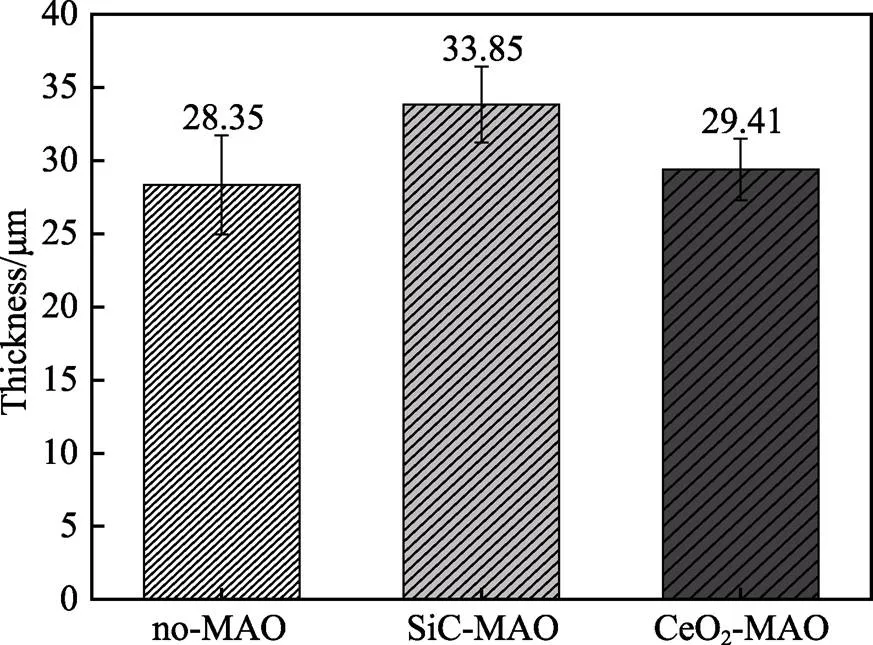
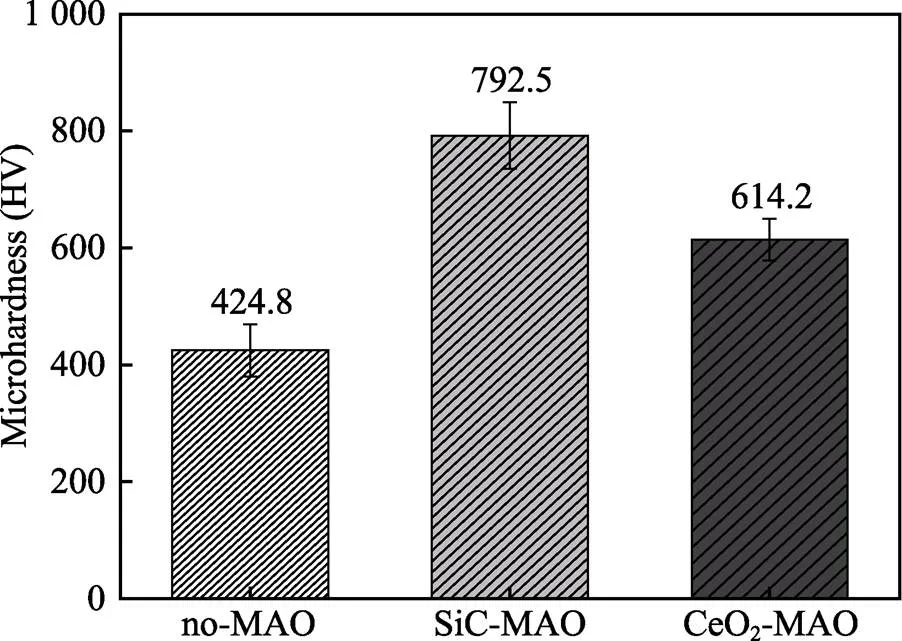
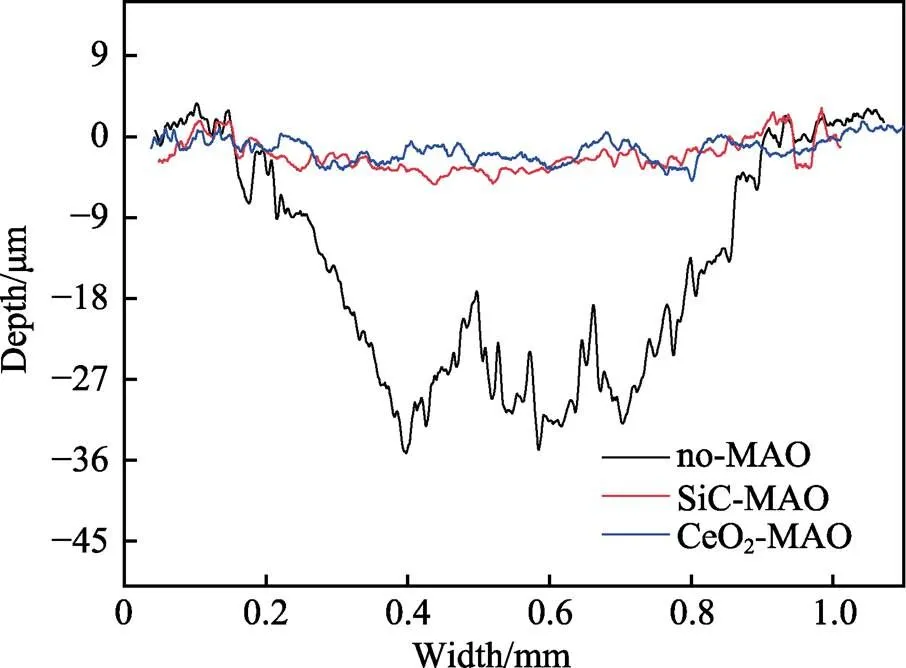
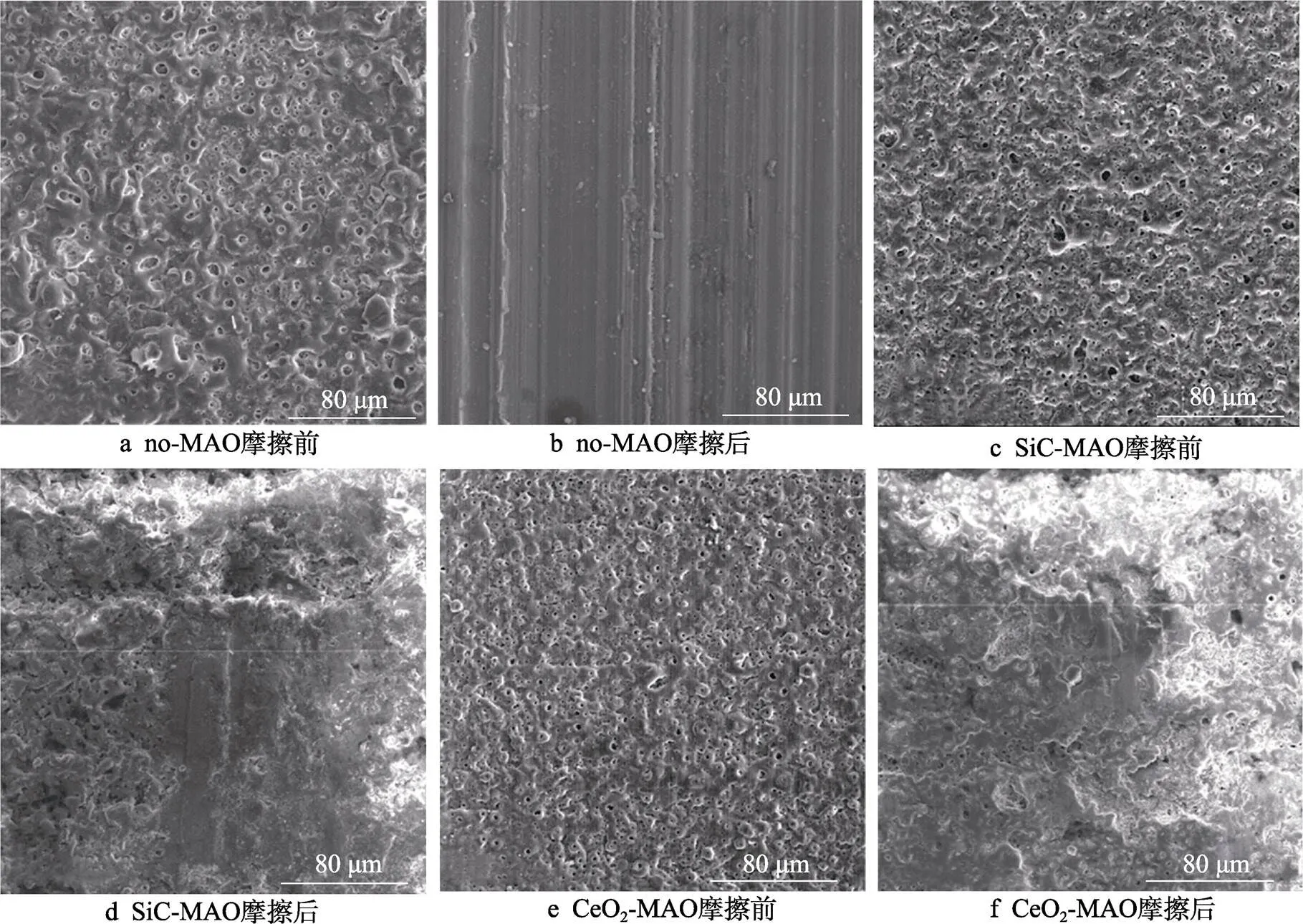
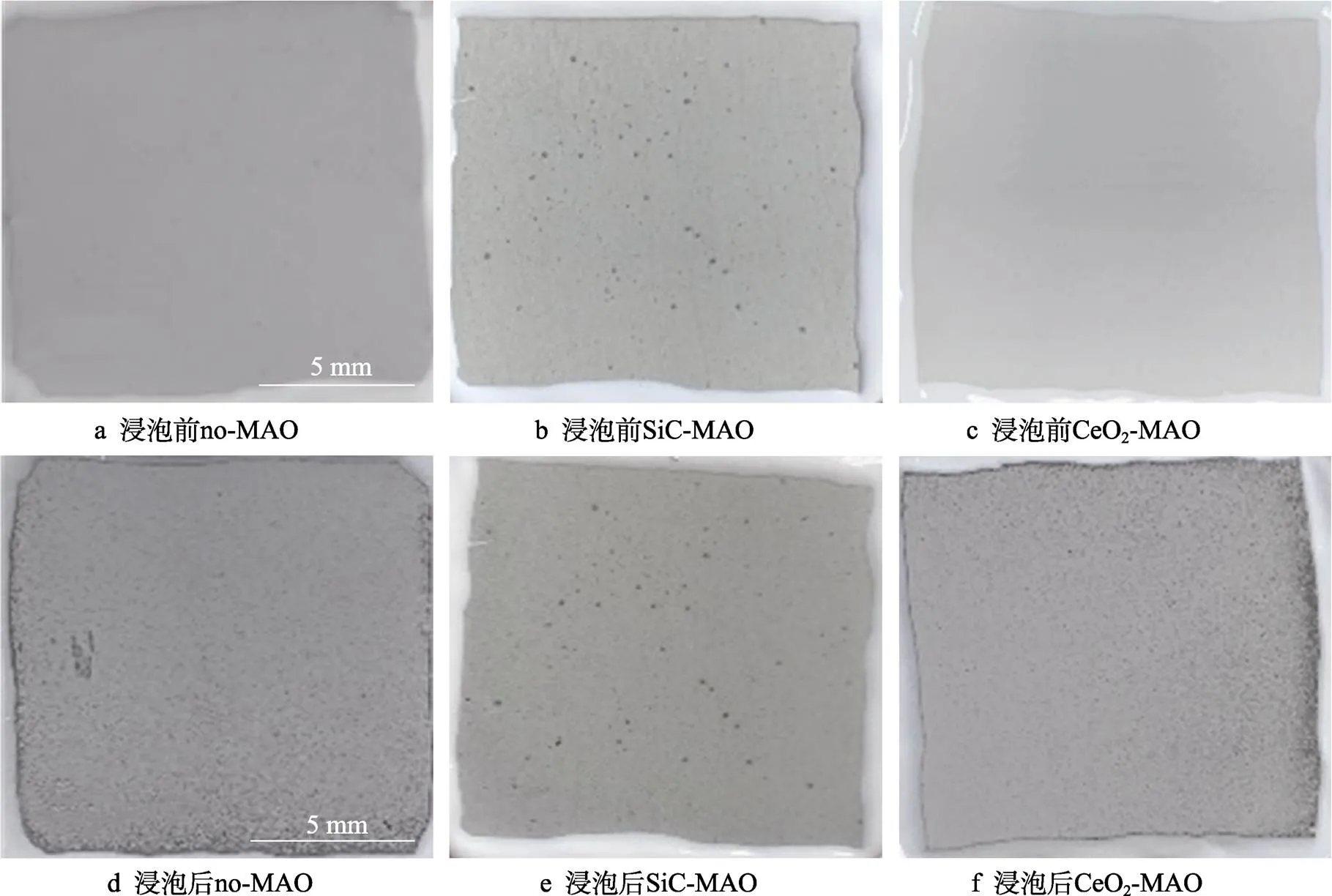
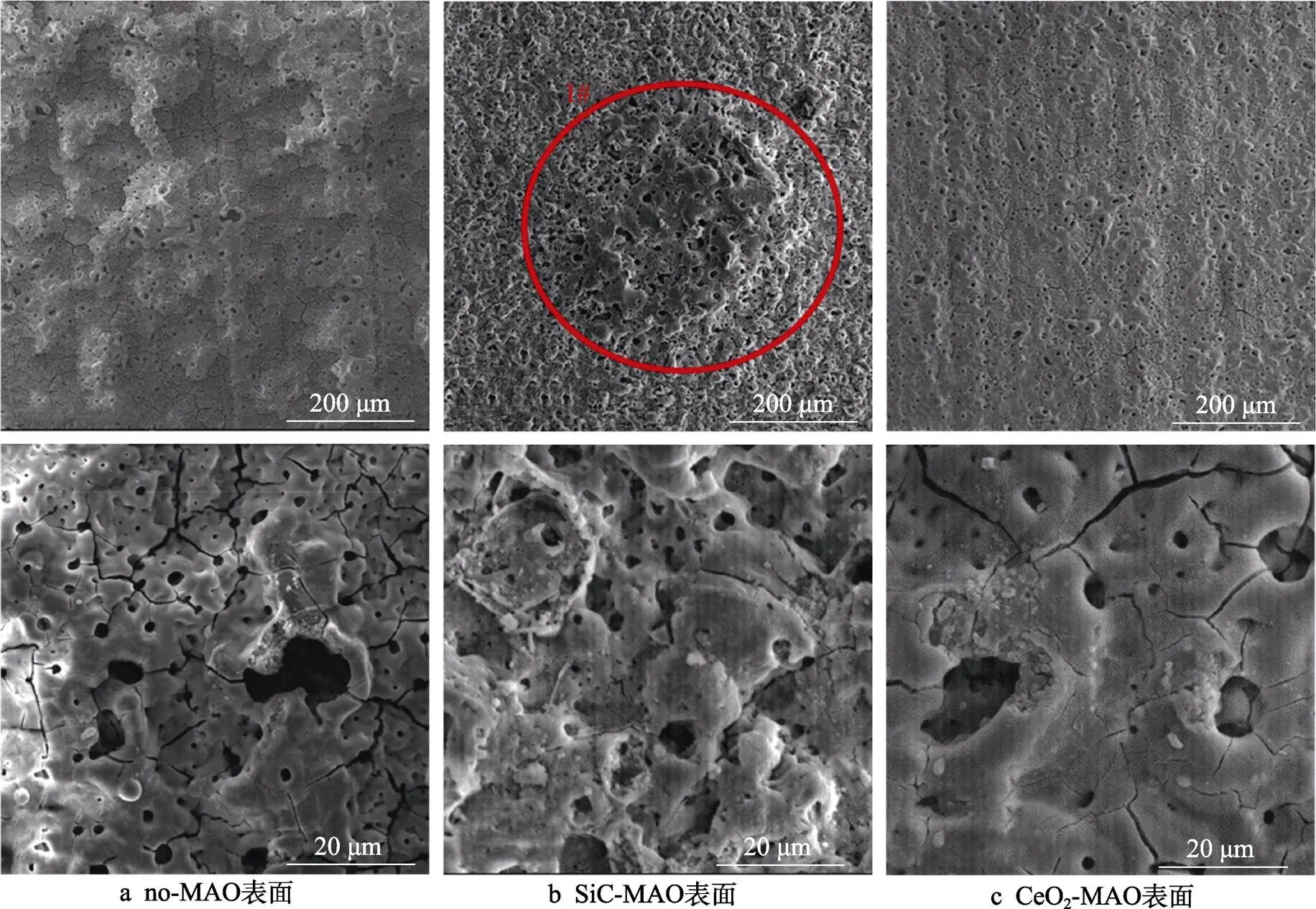
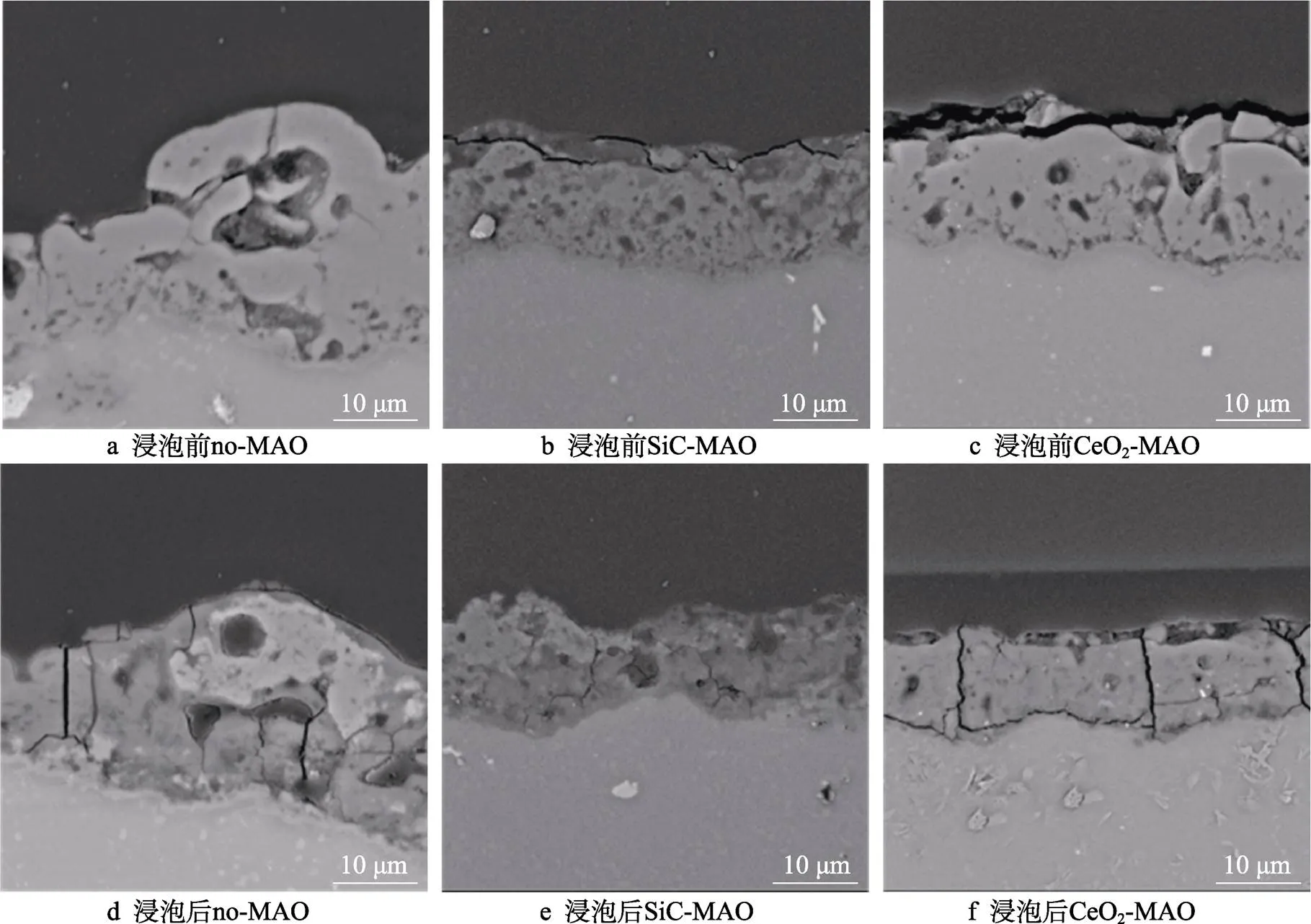
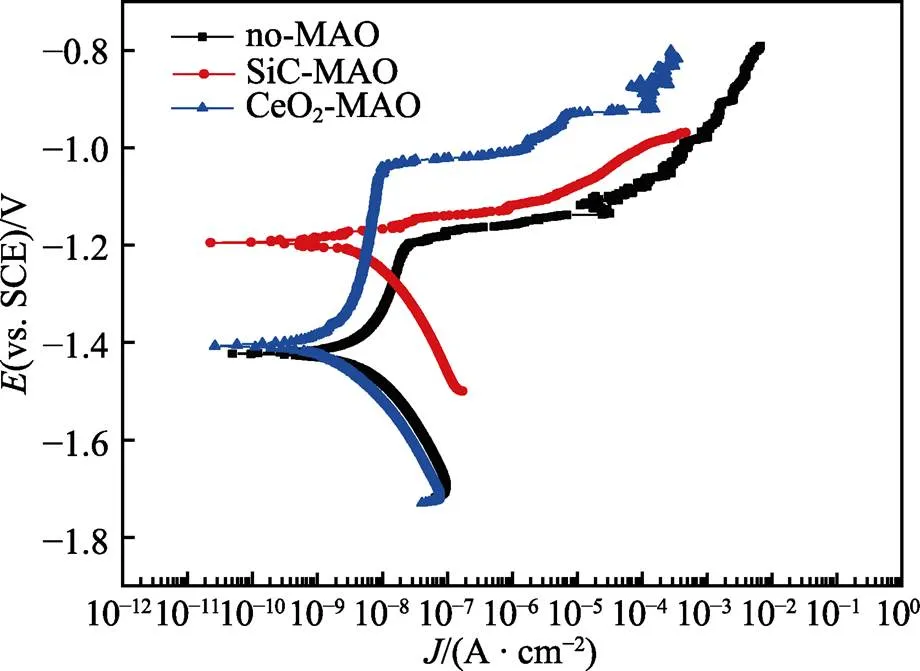
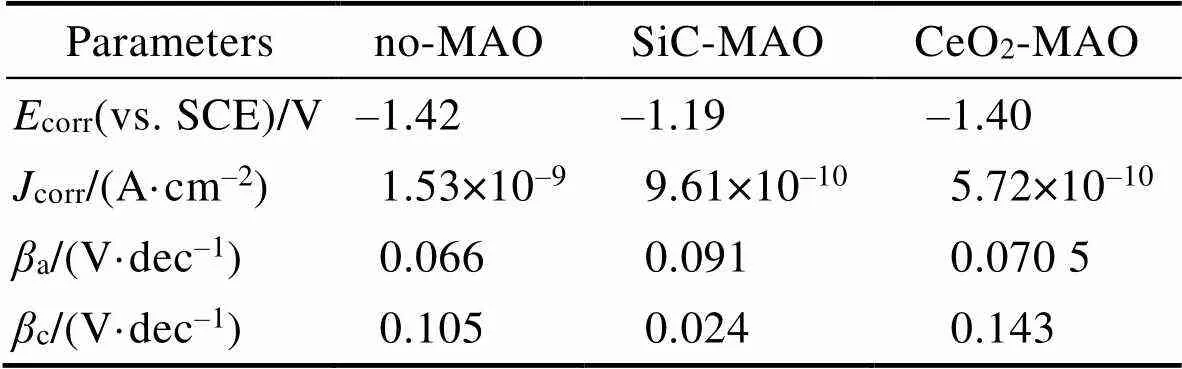
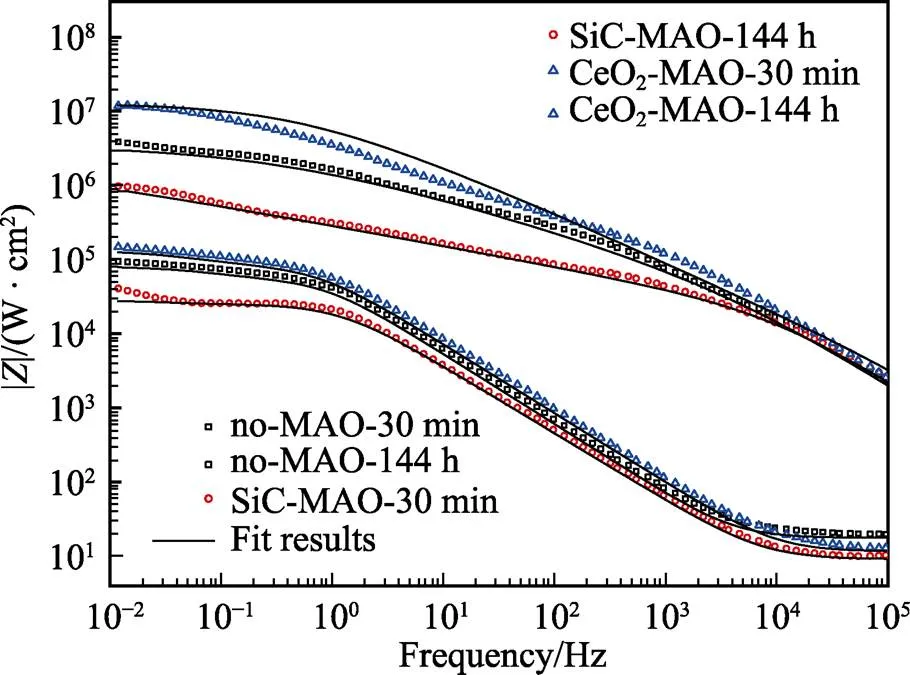
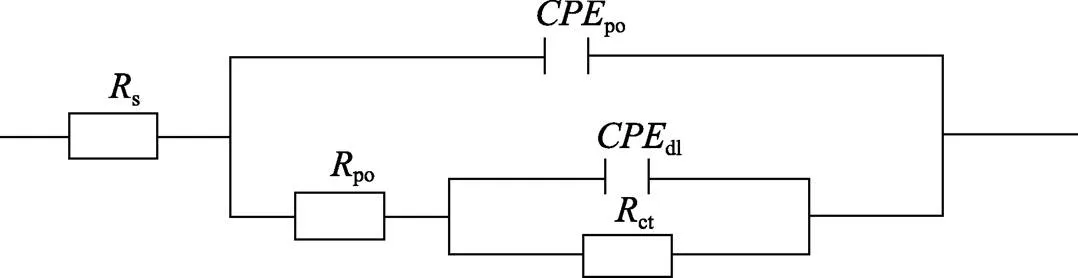
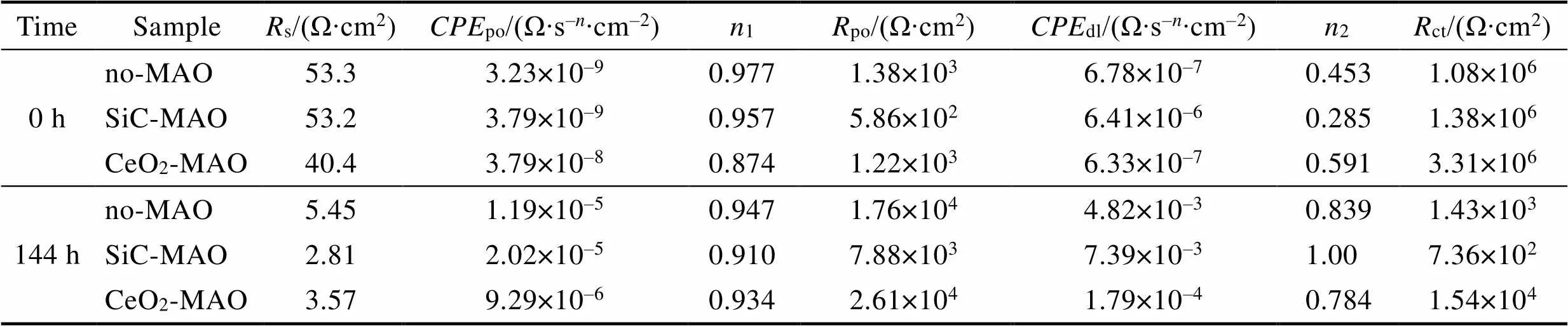
图1为在不同纳米颗粒的电解液中制备MAO涂层的电流密度-时间曲线。在初始阶段,3种样品的电流密度都快速下降。之后,3种样品的电流密度才趋于稳定状态。在进行MAO处理的过程中,SiC-MAO具有最高的稳定电流密度。在MAO涂层的生长过程中,纳米颗粒会优先填充涂层的孔隙和裂纹,进一步增强等离子体放电[21]。因此,纳米颗粒会增大MAO处理过程中的电流密度。在MAO过程中,尺寸更小的纳米粒子更容易填充涂层的缺陷[21]。本文使用的纳米颗粒尺寸为SiC 图1 镁合金微弧氧化过程中的电流密度-时间曲线 图2为含有不同纳米颗粒的MAO涂层的微观形貌。MAO涂层表面分布着类似“火山口”状的微孔结构,并且孔隙是随机分布的。孔隙的形成与MAO涂层的生长过程相关,即MAO涂层中的放电通道快速冷却,形成孔隙[26]。EDS检测了3种涂层表面中的孔隙,表2为图2a—c中红圈的EDS数据。1#不存在C元素和Ce元素。2#Si元素的原子数分数为8.87%,C元素的原子数分数为7.84%。Si元素与C元素的原子比几乎为1∶1,证明了SiC-MAO的孔隙中存在SiC纳米颗粒。3#Ce元素的原子数分数为6.49%,证明了CeO2-MAO孔隙中存在CeO2纳米颗粒。 图3为含有不同纳米颗粒的MAO涂层的孔隙率。SiC-MAO具有最高的孔隙率,CeO2-MAO具有最小的孔隙率。在MAO过程中,纳米粒子会以嵌入的方式进入MAO涂层,并填充涂层的孔隙和裂缝,从而降低涂层的孔隙率[27]。同时,纳米粒子也会促进等离子体放电强度,从而增大涂层的孔隙率[28]。根据图1可知,CeO2-MAO和no-MAO的稳定电流密度相同,并且纳米颗粒填充了涂层中的孔隙。因此,CeO2-MAO的孔隙率最小。根据图1可知,SiC-MAO具有最高的稳定电流密度,其MAO过程有更强烈的反应。因此,SiC-MAO的孔隙率最大。 图2 含有不同纳米颗粒的MAO涂层的表面形貌 表2 含有不同纳米颗粒的MAO涂层的化学组成 图3 含有不同纳米颗粒的MAO涂层的孔隙率 图4为不同纳米颗粒的MAO涂层的XRD图谱。结果表明,MAO涂层的主要成分为MgO、Mg2SiO4和MgF2。此外,在SiC-MAO中可以检测到SiC纳米颗粒,在CeO2-MAO中也可以检测到CeO2纳米颗粒。在MAO涂层的生长过程中,阳极(镁合金)表面产生Mg2+。在强电场作用下,Mg2+从试样表面向溶液中快速迁移,与溶液中的F‒、OH‒和SiO32‒发生化学反应,陆续生成MgO、Mg2SiO4和MgF2,见式(1)—(3)。同时,纳米颗粒也会进入MAO涂层。 Mg2++2F‒→MgF2(1) Mg2++2OH‒→MgO+H2O (2) 2Mg2++3SiO32‒+2OH‒→Mg2SiO4+H2O (3) 图5为含有不同纳米颗粒的MAO涂层的厚度。no-MAO涂层的厚度为28.35 µm。含有纳米颗粒的涂层厚度明显增加。其中SiC-MAO的厚度最大,为33.85 µm。在MAO过程中,纳米颗粒会嵌入涂层。此外,一部分纳米颗粒也会被高能量的放电火花熔化,然后进入涂层[22]。因此,纳米颗粒会以嵌入和熔融结合的方式进入MAO涂层,在涂层的孔隙和缺陷处优先沉积[21],促进涂层快速生长,从而增加了MAO涂层的厚度。而SiC纳米颗粒具有较小的尺寸,更容易进入涂层[21],更有利于增加涂层的厚度。图1也表明,SiC-MAO的稳定电流密度最大。电流密度增大会有利于MAO涂层的生长。因此,含有纳米颗粒的涂层具有更大的厚度,并且SiC-MAO的厚度最大。 图6为含有不同纳米颗粒的MAO涂层的显微硬度。镁合金基体的显微硬度为142.2HV。MAO涂层的显微硬度明显高于Mg合金基体。含有纳米颗粒的MAO涂层具有更高的硬度。其中,SiC-MAO的显微硬度最高,为792.5HV。涂层的显微硬度主要取决于涂层的结构和相成分。在MAO处理过程中,纳米颗粒会优先填充涂层的孔隙和裂纹,改善了MAO涂层的结构[21,27]。因此,与no-MAO相比,CeO2-MAO具有较高的硬度。与CeO2纳米颗粒相比,SiC纳米颗粒还是一种高硬度的材料,可以显著提高MAO涂层的显微硬度[24,29]。因此,SiC-MAO的硬度最高。 图4 含有不同纳米颗粒的MAO涂层的XRD图谱 图5 含有不同纳米颗粒的MAO涂层的厚度 图6 含有不同纳米颗粒的MAO涂层的显微硬度 图7为MAO涂层的磨痕深度的二维剖面。在3种MAO涂层中,no-MAO具有最深的磨痕深度,而SiC-MAO和CeO2-MAO的磨痕深度可以忽略。说明纳米颗粒可以有效地改善涂层的耐磨性能。根据图8涂层摩擦前后的SEM形貌,在相同的摩擦条件下,no-MAO涂层已经被破坏,暴露出镁合金基体。裸露的镁合金磨损表面有明显的犁沟和凹槽。结果表明,no-MAO涂层已经产生了严重的磨损。SiC-MAO和CeO2-MAO摩擦前后的形貌表明,含有纳米颗粒的涂层表面仅有少量的磨损斑点,未见到暴露的镁合金基体。同时,有部分剥落的产物填入涂层表面的孔隙处,孔隙被堵塞。结果表明,在相同的摩擦条件下,含有纳米颗粒的涂层表面仅发生了轻微的磨损损伤。 根据图8涂层摩擦前的SEM形貌,MAO涂层表面存在许多孔隙和微裂纹。在外加负载下,裂纹极易在涂层的缺陷处产生,从而发生涂层剥落[22]。CeO2- MAO具有较小的孔隙率。因此,CeO2纳米颗粒可以改善涂层的致密性。根据图6可知,含有纳米颗粒的涂层具有更高的硬度。而涂层的结构和硬度与其耐磨性呈现正相关关系[29-30]。因此,在摩擦试验中,SiC纳米颗粒和CeO2纳米颗粒都会降低裂纹产生的概率,减少了剥落现象,减轻了涂层的磨损程度。同时,Pezzato等[31]证明,在摩擦过程中,纳米颗粒会分布在涂层的表面,参与摩擦过程。纳米颗粒可以显著地降低涂层与钢球之间的摩擦剪切应力。因此,纳米颗粒可以有效地降低涂层的磨损程度。综上所述,2种纳米颗粒都可以改善涂层的耐磨性能。 图7 含有不同纳米颗粒的MAO涂层的磨痕深度轮廓 图8 含有不同纳米颗粒的MAO涂层的磨痕形貌 2.5.1 浸泡试验 图9为浸泡在3.5%NaCl溶液中,MAO涂层腐蚀前后的宏观形貌。在浸泡前,no-MAO和CeO2-MAO的宏观表面光洁度一致,而SiC-MAO的宏观表面存在一些黑点。在浸泡144 h后,3种试样都存在黑色区域(即腐蚀严重区域)。图10为浸泡144 h后,MAO涂层的微观形貌。no-MAO的低倍表面存在明显的裂纹,高倍表面存在涂层破损的现象。图10b中1#为SiC-MAO宏观形貌上的一个黑点。在SiC-MAO高倍表面,可以观察到涂层存在破损,也存在裂纹。CeO2- MAO的低倍表面存在少量的裂纹,高倍表面也发生了涂层破损。综上所述,3种MAO涂层的表面仍具有较好的完整性。图11为浸泡前后MAO涂层的截面形貌。3种MAO涂层都由致密层和疏松层组成。腐蚀后的涂层截面都存在一些垂直的裂纹,但是涂层没有被严重损坏,基体也没有腐蚀。根据上述现象,在浸泡144 h后,3种涂层仍具有良好的耐蚀性能,涂层依然具备保护基体的作用。 图9 浸泡前后,含有不同纳米颗粒的MAO涂层的宏观形貌 图10 浸泡144 h后含有不同纳米颗粒的MAO涂层的表面形貌 图11 浸泡前后含有不同纳米颗粒的MAO涂层的截面形貌 2.5.2 电化学试验 图12为添加不同纳米颗粒的MAO涂层的极化曲线。表3为拟合得到的腐蚀电位、自腐蚀电流密度、阳极和阴极塔菲尔斜率。与no-MAO相比,CeO2-MAO的腐蚀电位略微正移,腐蚀电流密度从1.41× 10‒9A/cm2下降到5.72×10‒10A/cm2,阳极斜率和阴极斜率都增大。与no-MAO相比,SiC-MAO的腐蚀电位正移,腐蚀电流密度从1.41×10‒9A/cm2下降到9.61×10‒10A/cm2,阴极斜率增大,阳极斜率减小。CeO2-MAO的稳定钝化区间最大,为330 mV,no- MAO的稳定钝化区间为150 mV,而SiC-MAO没有出现稳定钝化区间。 涂层的显微结构会对其极化曲线产生影响[32]。MAO涂层为多孔结构,腐蚀介质(如Cl‒)会沿着孔隙渗入到涂层内部。而CeO2-MAO具有最低的孔隙率,较大的涂层厚度,这表明CeO2-MAO具有良好的涂层结构。纳米颗粒会在孔隙中沉积,起到填充孔隙的作用[21]。图2的结果显示,孔隙中存在CeO2纳米颗粒。因此,CeO2-MAO可以有效地抑制腐蚀介质的渗入,降低了涂层的腐蚀电流密度,并提高涂层的稳定钝化间范围,提高了180 mV。而SiC-MAO的孔隙率较大,导致涂层不能有效地抑制腐蚀介质渗入,腐蚀电流密度降低较少,也没有出现稳定钝化区间。因此,CeO2纳米颗粒可以有效地改善涂层的耐蚀性能,而SiC纳米颗粒没有改善作用。 图12 含有不同纳米颗粒的MAO涂层的极化曲线 表3 含有不同纳米颗粒的MAO涂层的腐蚀电流密度和腐蚀电位 为了进一步研究纳米颗粒对MAO涂层耐腐蚀性的影响,使用EIS技术监测浸泡过程中MAO涂层腐蚀行为的变化。图13为MAO涂层的EIS结果。随着浸泡时间的增加,3种涂层的低频阻抗模量都在下降。在浸泡144 h时,3种涂层的低频阻抗模量仍大于104Ω∙cm2。在浸泡30 min和144 h时,SiC-MAO的低频阻抗模量都比no-MAO低,CeO2-MAO的低频阻抗模量始终是最高的。对EIS曲线进行等效电路拟合,图14为对应的等效电路,表4为拟合结果。在图14中,s为溶液电阻,po和po分别为涂层多孔结构的电容和电阻,dl和ct分别为涂层/金属界面处的电容和电荷转移电阻。高的ct说明涂层具有优良的耐蚀性能[33]。根据表4可知,在30 min时,CeO2-MAO具有最高的ct,而SiC-MAO的ct与no- MAO的ct相近。而随着浸泡时间的延长,3种MAO涂层的ct都有所降低。在浸泡144 h后,CeO2-MAO仍保持最高的ct,而SiC-MAO具有最低的ct,表明CeO2纳米颗粒有效改善了涂层的耐蚀性能,而SiC纳米颗粒没有改善作用。Nadaraia等[32]说明了涂层的厚度越大,孔隙率越小,其耐蚀性能越好。CeO2-MAO的孔隙率比no-MAO小,其涂层的厚度也较大。因此,CeO2-MAO的涂层可以有效地抑制腐蚀介质进入涂层。根据图3可知,SiC-MAO的表面孔隙率比no-MAO大。因此,SiC纳米颗粒对涂层的耐蚀性能没有改善作用。综上所述,EIS结果也表明,在浸泡期间,CeO2纳米颗粒可以有效地改善涂层的耐蚀性能,而SiC纳米颗粒没有改善作用。 图13 含有不同纳米颗粒的MAO涂层的电化学阻抗谱 图14 含有不同纳米颗粒的MAO涂层的等效电路 表4 含有不同纳米颗粒的EIS拟合结果 1)纳米颗粒可以提高涂层的厚度和硬度。含有SiC纳米颗粒的MAO涂层的厚度、硬度分别提升19.40%、86.56%,含有CeO2纳米颗粒的MAO涂层的厚度、硬度分别提升3.74%、44.59%。含有SiC纳米颗粒的涂层孔隙率升高6.60%,而添加CeO2使涂层的孔隙率下降23.90%。在MAO处理时,纳米颗粒会优先填充MAO涂层中的孔隙和裂纹,增强涂层生长时的等离子体放电强度,提升MAO涂层的生长速度,使得含有纳米颗粒的涂层具有较大的厚度和较高的硬度。SiC-MAO具有最大的稳定电流密度,其等离子体放电强度最强,导致其孔隙率较大。CeO2- MAO的稳定电流密度较低,并且纳米颗粒起到填充孔隙的作用,孔隙率较低。 2)SiC纳米颗粒可以改善涂层的耐磨性能,但无法提升涂层的耐蚀性能。摩擦试验中,SiC-MAO的磨痕深度比no-MAO浅。磨痕微观形貌也表明,no-MAO的涂层被破坏,而SiC-MAO表面涂层完好。然而电化学测试表明,SiC-MAO的极化曲线没有出现类似钝化的稳定钝化区间,并且其电化学阻抗谱的低频阻抗模量值也低于no-MAO。 3)CeO2纳米颗粒可以同时改善涂层的耐磨和耐蚀性能。摩擦试验中,CeO2-MAO的磨痕深度比no-MAO浅。磨痕微观形貌也表明,CeO2-MAO表面涂层在摩擦试验后保持完好。电化学测试表明,CeO2-MAO的极化曲线中腐蚀电流密度比no-MAO小1个数量级,稳定钝化区间也延长了180 mV,其电化学阻抗谱中的低频阻抗模量值也高于no-MAO,并且CeO2-MAO一直保持最高的ct值。 [1] HUSSEIN R O. A Study of the Interactive Effects of Hybrid Current Modes on the Tribological Properties of a PEO (Plasma Electrolytic Oxidation) Coated AM60B Mg- Alloy[J]. Surface and Coatings Technology, 2013, 215: 421-430. [2] SAMADIANFARD R. Sol-Gel Coating Filled with SDS- Stabilized Fullerene Nanoparticles for Active Corrosion Protection of the Magnesium Alloy[J]. Surface and Coatings Technology, 2021, 419: 127292. [3] ZHANG Jiao-jiao, WEI Jin-fei, LI Bu-cheng, et al. Long- Term Corrosion Protection for Magnesium Alloy by Two- Layer Self-Healing Superamphiphobic Coatings Based on Shape Memory Polymers and Attapulgite[J]. Journal of Colloid and Interface Science, 2021, 594: 836-847. [4] CHEN Li-man, WANG Gui-xiang, HE Yan-dong, et al. The Influence of Overpotential on Electrodeposited Aluminum Coating Onto Zincated ZM5 Magnesium Alloy in Ionic Liquid[J]. Materials Letters, 2020, 258: 126814. [5] HU Rong, SU Yong-yao, LIU Hong-dong. Deposition Behaviour of Nickel Phosphorus Coating on Magnesium Alloy in a Weak Corrosive Electroless Nickel Plating Bath[J]. Journal of Alloys and Compounds, 2016, 658: 555-560. [6] DUAN Guo-qing, YANG Li-xin, LIAO Shang-ju, et al. Designing for the Chemical Conversion Coating with High Corrosion Resistance and Low Electrical Contact Resistance on AZ91D Magnesium Alloy[J]. Corrosion Science, 2018, 135: 197-206. [7] HU Rong-gang, ZHANG Su, BU Jun-fu, et al. Recent Progress in Corrosion Protection of Magnesium Alloys by Organic Coatings[J]. Progress in Organic Coatings, 2012, 73(2-3): 129-141. [8] KASEEM M, FATIMAH S, NASHRAH N, et al. Recent Progress in Surface Modification of Metals Coated by Plasma Electrolytic Oxidation: Principle, Structure, and Performance[J]. Progress in Materials Science, 2021, 117: 100735. [9] LIU Chang, LU Xiao-peng, LI Yan, et al. Influence of Post-Treatment Process on Corrosion and Wear Properties of PEO Coatings on AM50 Mg Alloy[J]. Journal of Alloys and Compounds, 2021, 870: 159462. [10] 董凯辉, 宋影伟, 韩恩厚. 钛合金耐磨微弧氧化制备技术的研究进展[J]. 表面技术, 2021, 50(7): 57-65. DONG Kai-hui, SONG Ying-wei, HAN En-hou. Research Progress on the Preparation of Wear-Resistant Micro-Arc Oxidation Coatings on Titanium Alloys[J]. Surface Technology, 2021, 50(7): 57-65. [11] 孙乐, 马颖, 李奇辉, 等. 纯镁表面等离子体电解渗硼与微弧氧化复合膜的制备及耐蚀性[J]. 表面技术, 2021, 50(6): 64-76. SUN Le, MA Ying, LI Qi-hui, et al. Fabrication and Corrosion Resistance of Hybrid Coatings on Pure Magnesium by Combining Plasma Electrolytic Boronizing with Micro-Arc Oxidation[J]. Surface Technology, 2021, 50(6): 64-76. [12] XIA Qiu-xing, LI Xiang, YAO Zhong-ping, et al. Investigations on the Thermal Control Properties and Corrosion Resistance of MAO Coatings Prepared on Mg-5Y-7Gd- 1Nd-0.5Zr Alloy[J]. Surface and Coatings Technology, 2021, 409: 126874. [13] MO Qiu-feng, QIN Ge-mei, LING Kui, et al. Layer-by- Layer Self-Assembled Polyurea Layers Onto MAO Surface for Enhancing Corrosion Protection to Aluminum Alloy 6063[J]. Surface and Coatings Technology, 2021, 405: 126653. [14] 胡波, 李德江, 李子昕, 等. 铸造镁合金热裂行为的研究进展[J]. 精密成形工程, 2020, 12(5): 1-19. HU Bo, LI De-jiang, LI Zi-xin, et al. Research Progress on Hot Tearing Behavior of Cast Magnesium Alloys[J]. Journal of Netshape Forming Engineering, 2020, 12(5): 1- 19. [15] CAI Jing-shun, CAO Fa-he, CHANG Lin-rong, et al. The Preparation and Corrosion Behaviors of MAO Coating on AZ91D with Rare Earth Conversion Precursor Film[J]. Applied Surface Science, 2011, 257(8): 3804-3811. [16] HUSSEIN R O, ZHANG P, NIE X, et al. The Effect of Current Mode and Discharge Type on the Corrosion Resistance of Plasma Electrolytic Oxidation (PEO) Coated Magnesium Alloy AJ62[J]. Surface and Coatings Technology, 2011, 206(7): 1990-1997. [17] KEYVANI A, ZAMANI M, BAHAMIRIAN M, et al. Role of Incorporation of ZnO Nanoparticles on Corrosion Behavior of Ceramic Coatings Developed on AZ31 Magnesium Alloy by Plasma Electrolytic Oxidation Technique[J]. Surfaces and Interfaces, 2021, 22: 100728. [18] DU Qing, WEI Da-qing, WANG Ya-ming, et al. The Effect of Applied Voltages on the Structure, Apatite-Inducing Ability and Antibacterial Ability of Micro Arc Oxidation Coating Formed on Titanium Surface[J]. Bioactive Materials, 2018, 3(4): 426-433. [19] CHANG Lin-rong, CAO Fa-he, CAI Jing-shun, et al. Influence of Electric Parameters on MAO of AZ91D Magnesium Alloy Using Alternative Square-Wave Power Source[J]. Transactions of Nonferrous Metals Society of China, 2011, 21(2): 307-316. [20] 国泰榕, 卢小鹏, 李岩, 等. 磷酸盐后处理对Mg-Gd-Y合金微弧氧化涂层耐蚀性能的影响[J]. 表面技术, 2021, 50(9): 278-285, 310. GUO Tai-rong, LU Xiao-peng, LI Yan, et al. Effect of Phosphate Post-Treatment on Corrosion Resistance of Micro-Arc Oxidation Coating on Mg-Gd-Y Alloy[J]. Surface Technology, 2021, 50(9): 278-285, 310. [21] ZHANG Pei, ZUO Yu, NIE Guo-chao. The Pore Structure and Properties of Microarc Oxidation Films on 2024 Aluminum Alloy Prepared in Electrolytes with Oxide Nanoparticles[J]. Journal of Alloys and Compounds, 2020, 816: 152520. [22] LI Zheng-yang, CAI Zhen-bing, CUI Xue-jun, et al. Influence of Nanoparticle Additions on Structure and Fretting Corrosion Behavior of Micro-Arc Oxidation Coatings on Zirconium Alloy[J]. Surface and Coatings Technology, 2021, 410: 126949. [23] MOHAMAD S, LIZA S, YAAKOB Y. Strengthening of the Mechanical and Tribological Properties of Composite Oxide Film Formed on Aluminum Alloy with the Addition of Graphite[J]. Surface and Coatings Technology, 2020, 403: 126435. [24] YU Lu, CAO Jin-hui, CHENG Ying-liang. An Improvement of the Wear and Corrosion Resistances of AZ31 Magnesium Alloy by Plasma Electrolytic Oxidation in a Silicate-Hexametaphosphate Electrolyte with the Suspension of SiC Nanoparticles[J]. Surface and Coatings Technology, 2015, 276: 266-278. [25] ZHENG Zhong-ren, ZHAO Ming-chun, TAN Li-li, et al. Corrosion Behavior of a Self-Sealing Coating Containing CeO2Particles on Pure Mg Produced by Micro-Arc Oxidation[J]. Surface and Coatings Technology, 2020, 386: 125456. [26] WANG Xin-yan, LU Xiao-peng, JU Peng-fei, et al. Influence of ZnO on Thermal Control Property and Corrosion Resistance of Plasma Electrolytic Oxidation Coatings on Mg Alloy[J]. Surface and Coatings Technology, 2021, 409: 126905. [27] REHMAN Z, UZAIR M, LIM H, et al. Structural and Electrochemical Properties of the Catalytic CeO2Nanoparticles-Based PEO Ceramic Coatings on AZ91 Mg Alloy[J]. Journal of Alloys and Compounds, 2017, 726: 284-294. [28] ZHANG Pei, ZUO Yu, NIE Guo-chao. The Pore Structure and Properties of Microarc Oxidation Films on 2024 Aluminum Alloy Prepared in Electrolytes with Oxide Nanoparticles[J]. Journal of Alloys and Compounds, 2020, 816: 152520. [29] NASIRI V H, EBRAHIMI-KAHRIZSANGI R, KASIRI- ASGARANI M, et al. Structural, Tribological and Electrochemical Behavior of SiC Nanocomposite Oxide Coatings Fabricated by Plasma Electrolytic Oxidation (PEO) on AZ31 Magnesium Alloy[J]. Journal of Alloys and Compounds, 2016, 683: 241-255. [30] ATAPOUR M, BLAWERT C, ZHELUDKEVICH M L. The Wear Characteristics of CeO2Containing Nanocomposite Coating Made by Aluminate-Based PEO on AM 50 Magnesium Alloy[J]. Surface and Coatings Technology, 2019, 357: 626-637. [31] PEZZATO L, LORENZETTI L, TONELLI L, et al. Effect of SiC and Borosilicate Glass Particles on the Corrosion and Tribological Behavior of AZ91D Magnesium Alloy after PEO Process[J]. Surface and Coatings Technology, 2021, 428: 127901. [32] NADARAIA K V, SUCHKOV S N, IMSHINETSKIY I M, et al. Some New Aspects of the Study of Dependence of Properties of PEO Coatings on the Parameters of Current in Potentiodynamic Mode[J]. Surface and Coatings Technology, 2021, 426: 127744. [33] TOORANI M, ALIOFKHAZRAEI M, MAHDAVIAN M, et al. Superior Corrosion Protection and Adhesion Strength of Epoxy Coating Applied on AZ31 Magnesium Alloy Pre-Treated by PEO/Silane with Inorganic and Organic Corrosion Inhibitors[J]. Corrosion Science, 2021, 178: 109065. Effect of Nanoparticles on the Wear and Corrosion Resistance of MAO Coatings on ZM5 Mg Alloy 1,2,1,1,3,4 (1. Institute of Advanced Wear & Corrosion Resistant and Functional Materials, Jinan University, Guangzhou 510632, China; 2. Department of Materials Science and Engineering, China University of Petroleum, Beijing, Beijing 102249, China; 3. China Special Equipment Inspectionand Research Institute, Beijing 101300, China; 4. Key Laboratory of Energy Transfer and System of Power Station of Ministry of Education, North China Electric Power University, Beijing 102206, China) Micro-arc oxidation (MAO) is an advanced surface modification technology, which can improve material properties, such as corrosion resistance and wear resistance. To further improve the wear and corrosion resistance of the MAO coatings on ZM5 magnesium (Mg) alloy, the MAO coatings with different nanoparticles (no, SiC and CeO2) were prepared on the ZM5 Mg alloy. ZM5 Mg alloy samples were cut in the size of 20 mm×20 mm×5 mm. Prior to the MAO process, the substrate was polished with silicon carbide paper in 320 grit. Then the samples were cleaned with deionized water, anhydrous ethanol and dried immediately. The working size of the sample was 20 mm×20 mm, and the other parts were coated with silicone. The power supply (PN-III power source) was used to prepare MAO coating under a constant voltage mode (400±5) V for 20 min. The pulse frequency was 1 000 Hz and the duty ratio was 40%. The electrolyte solution was 2 g/L sodium hydroxide (NaOH), 15 g/L sodium silicate (Na2SiO3), and 5 g/L sodium fluoride (NaF). The MAO coatings with different nanoparticles were prepared by adding 5 g/L SiC nanoparticles or 5 g/L CeO2nanoparticles to the electrolyte. During the MAO treatment, the electrolyte temperature was maintained at (30±5) ℃ through the cooling system. After the treatment, the surfaces of the samples were sequentially rinsed with distilled water, anhydrous ethanol and then dried by cool airflow immediately. The surface morphology of MAO coatings was analyzed by SEM (Phenom XL). The composition was analyzed by EDS and XRD (Ultima Ⅳ). The wear resistance was studied by friction tests (rTEC MFT 5000). And the corrosion resistance was tested by Tafel and EIS(CHI-604C). The thickness of MAO coatings with SiC and CeO2nanoparticles increased by 19.40% and 3.74%, respectively. And the microhardness of MAO coatings with SiC and CeO2nanoparticles increased by 86.56% and 44.59%, respectively. The porosity of MAO coatings with SiC nanoparticles increased by 6.60% but with CeO2nanoparticles decreased by 23.90%. The result of the friction tests showed that the MAO coatings without nanoparticles had an abrasion depth of 36.4 µm, while the MAO coatings with SiC and CeO2nanoparticles had a negligible abrasion depth. The result of Tafel showed that the corrosion current density of MAO coatings with CeO2nanoparticles significantly reduced from 1.41×10‒9A/cm2to 5.72×10‒10A/cm2and the passivation zone extended by 180 mV. The result of EIS also showed that the coatings with CeO2nanoparticles had the highest impedance value at low frequency in immersion. During the MAO treatment, the nanoparticles can fill the pores and cracks in the MAO coatings and enhance the growth rate of the MAO coating, resulting in an increase in the thickness and microhardness of the coatings. Thus, SiC and CeO2nanoparticles improved the wear resistance of the MAO coating. During the MAO process, the SiC nanoparticles increased the stable current density, resulting in insignificant filling of the nanoparticles. Therefore, the SiC nanoparticles increased the porosity of the coatings. In contrast, CeO2nanoparticles reduced the porosity. Thus, CeO2nanoparticles improved the corrosion resistance of the MAO coating. ZM5 magnesium alloy; MAO coating; nanoparticles; wear resistance; corrosion resistance TG174.4 A 1001-3660(2022)12-0131-11 10.16490/j.cnki.issn.1001-3660.2022.12.013 2021–10–23; 2022–03–09 2021-10-23; 2022-03-09 国家自然科学基金(51701055);国家市场监督管理总局科技计划项目(2019MK134) National Natural Science Foundation of China (51701055); Science and Technology Project of State Administration for Market Regulation (2019MK134) 李健鹏(1997—),男,硕士研究生,主要研究方向为材料腐蚀与防护。 LI Jian-peng (1997-), Male, Postgraduate, Research focus: corrosion and protection of materials. 万红霞(1986—),女,博士,讲师,主要研究方向为石油管道腐蚀与防护。 WAN Hong-xia (1986-), Female, Doctor, Lecturer, Research focus: oil pipeline corrosion and protection. 李健鹏, 万红霞, 涂小慧, 等. 纳米颗粒对ZM5镁合金微弧氧化涂层耐磨和耐蚀性能的影响[J]. 表面技术, 2022, 51(12): 131-141. LI Jian-peng, WAN Hong-xia, TU Xiao-hui, et al. Effect of Nanoparticles on the Wear and Corrosion Resistance of MAO Coatings on ZM5 Mg Alloy[J]. Surface Technology, 2022, 51(12): 131-141. 责任编辑:万长清
2.2 涂层的微观形貌及化学成分
2.3 涂层厚度及硬度
2.4 涂层的耐磨性能
2.5 涂层的耐蚀性能
3 结论

