精原干细胞微环境研究进展
余志鑫,李鹏宇,李凯,缪时英,王琳芳,宋伟
综 述
精原干细胞微环境研究进展
余志鑫,李鹏宇,李凯,缪时英,王琳芳,宋伟
中国医学科学院基础医学研究所,北京协和医学院基础学院,医学分子生物学国家重点实验室,北京 100005
精原干细胞(spermatogonia stem cells, SSCs)是一类在睾丸中具有长期自我更新和分化潜能的生殖细胞(germ cells, GCs),即位于基底膜上的组织干细胞,其自我更新和分化受到周围微环境的调控。近年来对SSCs的研究取得了一系列重要进展,为临床治疗部分男性不育患者带来了曙光。其中,微环境对SSCs的调节功能的研究尤为重要,微环境负责整合不同类型的细胞成分、细胞外基质、细胞外调节分子及激素等对SSCs的作用,从而调节SSCs命运。关于SSCs微环境的研究已开始逐步成为干细胞研究的主要内容之一。本文主要对小鼠()SSCs微环境的细胞组成、调控因子以及特点等研究现状进行了综述,为深入研究SSCs微环境的结构和功能提供背景资料,希望在未来能够通过多种研究模式复用,发现更为丰富的细胞表型和微环境因子。
精原干细胞;微环境;自我更新;分化;激素调控
精原干细胞(spermatogonia stem cells, SSCs)位于曲细精管的基底膜上,对于精子发生(spermatogenesis)以及所有物种的繁殖都至关重要,并始终保持着自我更新和定向分化能力,一部分SSCs参与精子发生,而另一部分形成了SSC池。SSCs自我更新和分化之间的协调保证了SSCs在体内保持动态平衡,其减少或异常增殖都可能影响精子发生,从而导致男性不育。SSCs的自我更新和分化受到内在基因表达和外在环境的精确调控,因此对其调控机制的研究对于治疗男性不育具有重要意义。SSCs与体细胞以及体细胞分泌的多种因子之间相互作用构成了可持续产生精子的环境,称为微环境,也称为“niche”[1]。在此之前,支持细胞(sertoli cells, SCs)被认为是微环境的主要组成部分,近年来随着研究的深入,发现多种细胞类型和因子也参与微环境的组成,且SSCs的自我更新和分化与微环境中各类细胞的数量、生长因子及其下游信号通路、多种转录因子和表观调控因子等密切相关。微环境的研究为临床治疗男性不育症以及体外培养SSCs提供了新思路。本文总结了近几年关于SSCs微环境的组成和调节最新研究进展,以期为SSCs微环境的深入研究提供参考。
1 SSCs及其微环境
1.1 SSCs概述
未分化精原细胞(undifferentiated spermatogonia)约占成年小鼠()睾丸生殖细胞(germ cells, GCs)总数的0.3%,恒河猴()为4%,人类()为22%[2~4]。小鼠精子发生过程包括未分化精原细胞As (A single)、Apr (A paired)和Aal (A aligned),分化中的精原细胞(differentiating spermatogonia)A1依次形成A2、A3、A4、In型和B型精原细胞,再经过减数分裂形成初级精母细胞、次级精母细胞、圆形精子细胞,最终变形为成熟精子(图1)[5]。一般认为SSCs是As型精原细胞,且成年小鼠睾丸中大约有35,000个As细胞,而GFRα1阳性的As更接近真正意义上的SSCs[6~8]。在灵长类动物的基底膜上存在Ad(A dark)型和Ap(A pale)型未分化精原细胞以及B型分化型精原细胞,通常认为Ap是激活型的精原细胞,Ad是静止型精原细胞即SSCs[5,9]。SSCs的能力取决于它们产生两种后代的能力:一种复制母干细胞(自我更新),另一种获得特殊的形态和功能成为精子(分化)[10]。SSCs在微环境中保持自我更新和分化之间的平衡是通过SSCs细胞群体的不对称分裂,而不是通过恒定的单个细胞对称分裂所实现的[5,11]。在SSCs中除了确实进行自我更新的“实际干细胞”之外,还存在第二个群体—“潜在干细胞”,其在正常情况下不会自我更新,仅仅在损伤发生后启动其潜能[12]。
1.2 微环境定义
SSCs微环境被认为是一个开放的系统,不能单纯地从解剖学上定义,而应该从分子生物学上定义。微环境最初是为了解释造血干细胞移植后的行为[13,14]。而在精子发生中的微环境有狭义和广义之分:广义的微环境主要包括大部分的睾丸体细胞和其所分泌的因子和激素;狭义的微环境大多仅指与SSCs直接相邻的体细胞所构成的微环境[15,16]。在成年睾丸组织中,SSCs大多数位于曲细精管的基底膜上,几乎由SCs完全包绕,周围体细胞及其合成分泌的各种细胞因子共同构成了精子发生的微环境。这些生长因子和细胞外信号决定了SSCs的命运,最终在小鼠中经过35天形成成熟精子,在人类中经过64天形成成熟精子[17]。在小鼠中其生精上皮周期分为12个时期,其中最为特殊的是VII~VIII期,是精原细胞分化的时期[18~20]。本文中的微环境则指广义上的开放的微环境。
1.3 SSCs微环境的基本特征
在正常组织中,Yoshida等[21,22]研究表明SSCs优先定位在与间质组织和脉管系统相邻的管状区域,对于FGFs的竞争调节了小管基底层内SSCs种群的密度和大小。关于SSCs微环境的分布存在争议:如Oatley等[23]证明决定微环境分布的是SCs的数量而不是血管的位置;Brett Nixon等[24]则认为SSCs定位在缺血区,主要通过糖酵解供能,而祖细胞和分化生殖细胞优先定位在与脉管系统相邻的区域,以得到充足的氧气进行氧化磷酸化(oxidative phosphorylation, OXPHOS),产生丰富的ATP来推动快速扩增分裂;Mohyeldin等[25]认为低氧可维持多种干细胞表型、减少增殖和未分化状态。总之,关于SSCs所处的位置及代谢方式还有待进一步解决。
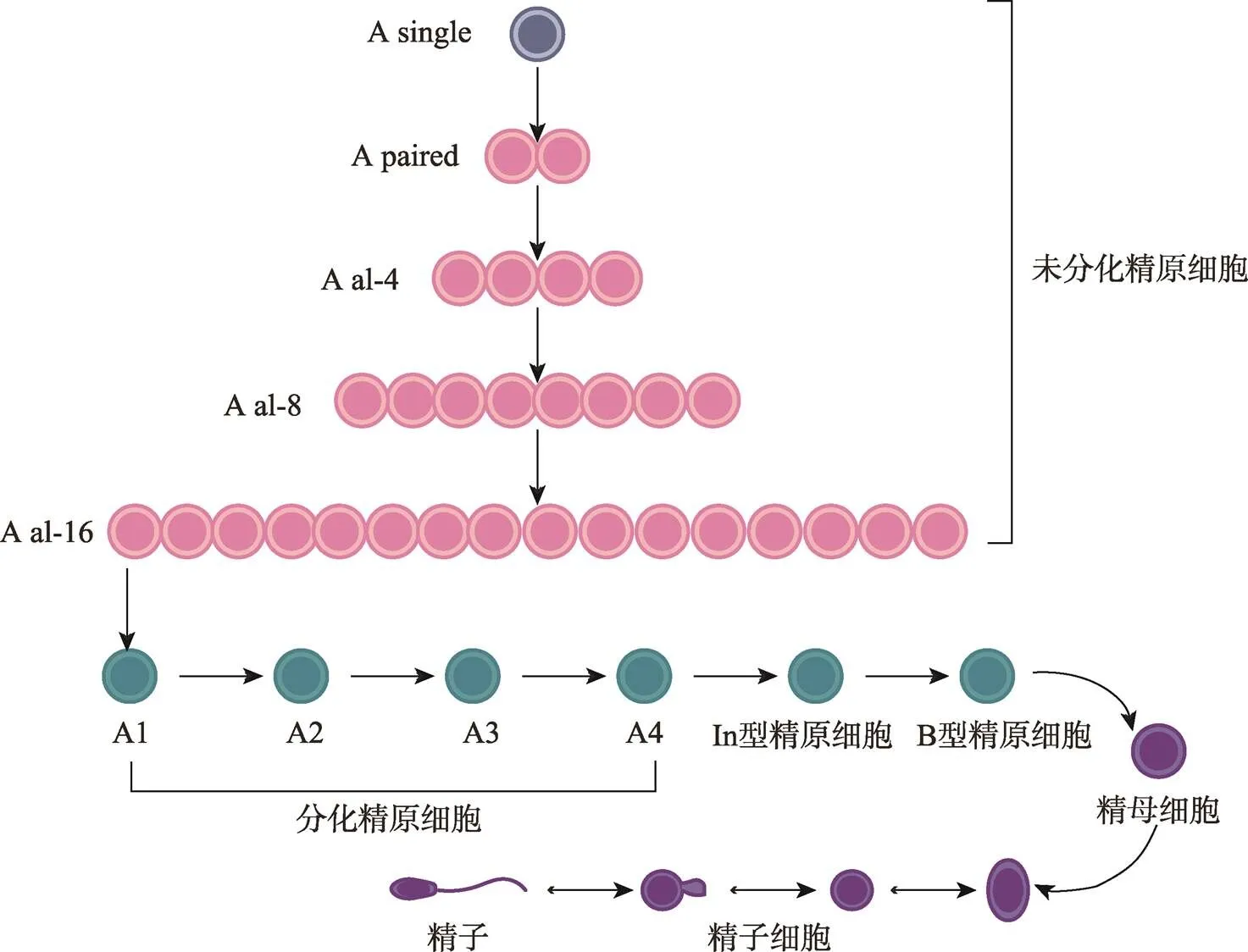
图1 小鼠精子发生过程示意图
小鼠精子发生过程:未分化精原细胞(A undiff)由A single、A paired、A al-4、A al-8和A al-16相互连接的细胞组成,逐渐分化为A1、A2、A3、A4、In型和B型,B型精原细胞经减数分裂成精母细胞,最终产生精子。
2 SSCs微环境的细胞组成
2.1 支持细胞
SCs是唯一与GCs直接接触的体细胞,在其基部与周围SCs和生精细胞相连接,每个SC可支持30~50个不同发育阶段的GCs,为精子发生提供物理支持和稳定微环境[26]。SCs可通过分泌多种生长因子如胶质细胞源性神经营养因子(glial cell line- derived neurotrophic factor, GDNF)、维甲酸(retinoic acid, RA)、干细胞因子(stem cell factor, SCF)、成纤维细胞生长因子2(fibroblast growth factor-2, FGF2)以及激活素调节SSCs自我更新和分化;也可通过紧密连接(tight junctions, TJs)参与血睾屏障(blood- testis barrier, BTB)的形成,保护生精细胞免受自身免疫系统的干扰,以多种方式支持、培育和保护GCs;另外其还可以表达自杀配体相关因子(factor associated suicide ligand, FasL)和转化生长因子-β (transforming growth factor-β, TGF-β)等蛋白,有助于局部免疫豁免;SCs也可吞噬、消化精子发生过程中脱落的残余胞质[5,27,28]。SCs形成的BTB在精子发生的减数分裂中起到重要作用,Cldn11是BTB主要成份,其缺失后SSCs不能有效定植,精子发生阻滞停留在精母细胞阶段最终导致不育[29]。BTB是一种高度选择性的屏障,包括紧密连接、桥粒样连接和间隙连接,移植实验中移植1周后SSCs才能穿过BTB完全迁移到基底膜[30]。此外,SCs除了支持精子发生外,也可以保留正常的间质细胞群并支持正常的管周肌样细胞(peritubular myoid cells, PMCs)的功能[31]。
2.2 管周肌样细胞
曲细精管被PMCs包裹,与SCs共同形成生精上皮基膜。PMCs主要在间质细胞分泌的激素作用下收缩,也可以在GATA4的作用下抑制收缩[32]。除了提供结构支持和推动内容物流向睾丸网外,PMCs还分泌对SSCs重要的旁分泌因子,包括GDNF和巨噬细胞集落刺激因子-1 (macrophage colony stimulating factor-1, CSF-1)。以往的研究大多侧重于SCs所分泌的GDNF,但近几年的研究表明PMCs产生的GDNF对小鼠和人的SSCs维持也至关重要[6,33,34]。PMCs同时也参与雄激素受体(androgen receptor, AR)的表达,PMCs中AR的特异性敲除可导致睾丸重量显著减轻,无精子发生,所有类型GCs数量减少[35]。
2.3 睾丸内皮细胞
睾丸内皮细胞(testicular endothelial cells, TECs)是干细胞生物学中细胞因子的丰富来源,FGF2可促进TECs产生GDNF,且相比于SCs,TECs是GDNF的主要来源[36]。TECs除产生GDNF外,它也可以产生FGF2、基质细胞衍生因子-1 (stromal cell derived factor-1, SDF-1)、巨噬细胞炎症蛋白2 (macrophage inflammatory protein-2, MIP-2)、胰岛素样生长因子结合蛋白2 (insulin-like growth factor binding protein-2, IGFBP-2)以及一氧化氮(nitric oxide, NO),其中NO可抑制间质细胞(leydig cells, LCs)类固醇激素的产生[37]。最近研究表明,TECs能够在没有外源性GDNF的情况下在体外支持SSCs,并且其衍生因子显著促进细胞毒性损伤后生精上皮的重新填充[36]。此外,在大鼠()中,TECs可作为饲养层细胞,增强SSCs的增殖和自我更新的能力,同时保持其干性[38]。这些数据表明,TECs是SSCs生态位的关键组成部分。
2.4 睾丸间质细胞
睾丸LCs分泌的睾酮(testosterone)是睾丸中主要的旁分泌因子,雄激素与其AR结合后介导生精细胞的信号通路[39]。通过对发育中的人类睾丸细胞进行单细胞分析,发现LCs和SCs起源于同一个异质祖细胞亚群,这表明睾丸LCs和SCs具有同源性[40]。除睾酮外,LCs还可分泌众多激素如肽类激素抗利尿激素、催产素和前列腺素等对PMCs的收缩起重要作用[41,42]。LCs也可以分泌胰岛素样生长因子-1 (insulin-like growth factor-1, IGF-1)和CSF-1[43~45]。但其主要功能还是合成雄激素,男性体内95%的雄激素是由LCs合成及分泌。
2.5 睾丸巨噬细胞
在男性生殖器官中,睾丸和附睾是两个独特的免疫部位。睾丸巨噬细胞(testicular macrophages, TMs)大约占睾丸间质细胞的20%,在睾丸中分为间质巨噬细胞和管周巨噬细胞[46]。间质巨噬细胞来源于胚胎的卵黄囊细胞,但在出生后被骨髓来源的巨噬细胞取代;管周巨噬细胞在出生后出现且来源于骨髓中单核细胞[47,48]。近年来研究发现,睾丸中存在独立于单核细胞的TMs,即骨髓来源的单核细胞对于TMs池的补充无实质性贡献,但通过流式细胞分选等方法发现睾丸白细胞主要来源于单核吞噬细胞[49~52]。而TMs对SSCs的自我更新和分化的影响直到2015年才被关注,研究发现TMs可产生CSF-1并表达RA合成的酶,且可能参与调控SSCs的维持和分化[47,53]。
2.6 其他细胞群
除上述主要细胞群外,目前被广泛研究的还包括淋巴管内皮细胞(lymphatic endothelial cells, LECs)。LECs位于生精小管和睾丸间质的边缘,覆盖淋巴间隙的表面,靠近脉管系统的LECs可表达大量FGFs[21]。随着单细胞测序等技术的发展,逐渐有新的亚群被发现,关于睾丸细胞的分群也逐渐精细。例如:2018年Wang等[54]将人类睾丸细胞分为17个群,其中14个为GCs,另外3个为体细胞;同年,小鼠睾丸GCs被分为4个群,包括精原细胞、精母细胞、圆形精子细胞和长形精子细胞,体细胞被分为7个群,包括SCs、LCs、PMCs、TECs、先天性淋巴细胞(innate lymphoid, IL)、睾丸巨噬细胞以及未知细胞群[55];2022年,大鼠精原细胞被分为5个群,在原来的基础上新增了凝聚型精子(condensed spermatids, CSPT)[56]。
3 SSCs微环境的调控因子
在SSCs微环境中有多种因子调节其自我更新或分化,例如已知的GDNF、FGF2、CSF-1、白血病抑制因子(leukemia inhibitory factor, LIF)和IGF-1等调节SSCs的自我更新[5]。而RA、骨形态发生蛋白4 (bone morphogenetic protein4, BMP4)和SCF等可促进SSCs的分化(图2)[5]。促进自我更新和分化的细胞外微环境虽然并不在空间上产生分隔,但GDNF和RA等这些因素显示出不同时间的时间波动,随生精上皮周期的变化而变化,这预示着SSCs和分化细胞很可能暴露于相同的细胞外信号[17,57,58]。
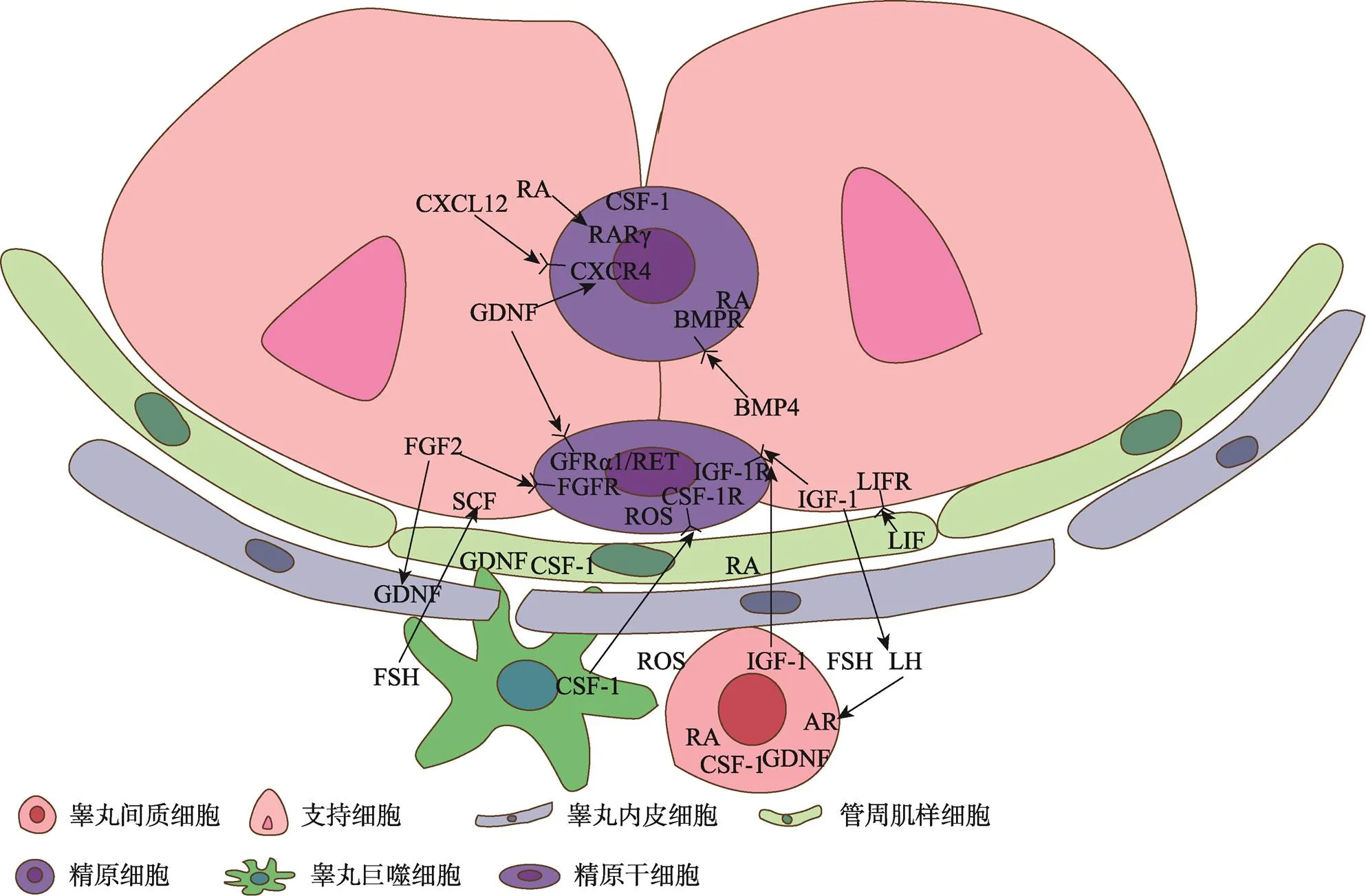
图2 SSCs微环境细胞组成及调控因子
SSCs微环境细胞组成及调控因子:SCs可产生GDNF、RA、SCF和FGF2等,PMCs可产生GDNF和CSF-1等,TECs可产生GDNF和FGF2等,LCs可产生IGF-1、RA和CSF-1等,这些因子与细胞相互作用共同构成微环境。
3.1 参与自我更新的调控因子
3.1.1 胶质细胞源性神经营养因子
GDNF在睾丸中主要由SCs和TECs分泌,其次是已分化生殖细胞、PMCs和LCs。GDNF对精原细胞的增殖影响有剂量依赖:在过表达GDNF的小鼠中,未分化精原细胞聚集在生精小管中并停止分化,而在GDNF杂合小鼠中,精子发生伴随着精原细胞的丢失而逐渐停滞[59~61]。GDNF可以通过GFRα1/c-Ret两种受体促进SSCs的自我更新[62,63]。GFRα1和RET敲除也表现出与上述GDNF敲除 同样的现象,即由于精原细胞丢失精子发生逐渐停滞[64,65]。同样,PMCs中基因的破坏也能影响未分化精原细胞发育[6]。在GDNF、FGF2、EGF和LIF存在的培养基中培养SSCs,可生长2年,且在小鼠胚胎成纤维细胞(mouse embryo fibroblasts, MEFs)上呈葡萄状生长[17,66,67]。有研究发现,在精子发生的稳定阶段和白消安(busulfan)介导的精原细胞损伤后恢复阶段,尽管SSCs数量增加但GDNF是通过阻断分化而不是促进增殖来促进自我更新[57]。最近又有研究表明SSCs的数量因GDNF信号传导的瞬时抑制而减少,但在信号传导恢复后通过自我更新复制恢复[68]。
3.1.2 成纤维细胞生长因子2
FGF2由SCs分泌,对SSCs的自我更新起促进作用。FGF的靶基因包括、、和,受体包括FGFR1、FGFR2、FGFR3和FGFR4,主要靶细胞为未分化精原细胞[21]。有研究发现,GDNF和FGF2都增加了未分化精原细胞中GFRα1阳性细胞的亚群,但FGF2扩增亚群表达RARγ的频率是GDNF的1.9倍,即FGF2扩大了一个易于分化的亚群[61,69]。FGF2能刺激TECs表达GNDF,从而间接调控SSCs的数量恒定[36]。未分化型精原细胞可以在FGF2存在而无GDNF条件下在体外维持,但在该条件下的细胞显示出生长不良和干性降低。
3.1.3 巨噬细胞集落刺激因子1
CSF-1也称为MCSF-1,在小鼠睾丸中主要由TMs、LCs、PMCs和精原细胞产生,其受体CSF1R主要表达于GFRα1阳性和THY1阳性的精原细胞中[44,70]。近年来研究发现CSF1R在小鼠TMs、LCs、SCs和PMCs等细胞中表达,且CSF1R不仅是CSF的受体,也是IL-34的受体[70,71]。CSF-1蛋白在1周内的小鼠睾丸中表达最高,并随年龄显著降低,其可增强的配体作用,促进精子发生直至精子产生[72]。研究表明,CSF-1可以促进SSCs的增殖和自我更新[44,73]。β-雌二醇以剂量和时间依赖方式促进SCs分泌CSF-1进而促进人类胎儿SSCs的增殖并抑制SSCs凋亡,然而与雌激素受体抑制剂结合又可以完全逆转其对抑制SSCs凋亡的作用[74]。
3.1.4 趋化因子CXC模体12
趋化因子CXC模体12 (C-X-C motif chemokine 12,CXCL12)在人中由LCs分泌,在小鼠中由SCs分泌,并与SSCs上的CXCR4受体结合以促进SSCs的自我更新和维持,尤其是CXCL12-CXCR4信号通路可促进SSCs增殖并阻断RA诱导SSCs分化[15,75]。有研究表明CXCL12、GDNF和FGF2已形成了影响SSCs自我更新的调控网络,GDNF可上调CXCL12受体的表达。此外,CXCR4在胚胎发生期间参与原始生殖细胞(primordial germ cells, PGCs)归巢,在出生后的睾丸中也参与SSCs归巢至生态位,CXCL12转染SCs可提高归巢效率[76,77]。
3.1.5 白血病抑制因子
LIF主要由PMCs产生,其受体LIFR在小鼠的所有GCs、SCs、LCs和TMs中均表达,且SCs中的LIFR最为重要[78~80]。LIF在体外可增强新生小鼠睾丸培养中GCs集落的形成,目前报道最多的是在培养基中添加bFGF、GDNF、LIF和EGF使SSCs在体外迅速增殖[81~84]。在使用明胶和含有GDNF、FGF2、LIF和EGF的培养基中,6天后也产生了很大比例的雄性人类胎儿生殖细胞,这表明体外培养人GCs又前进了一步[85]。
3.1.6 活性氧
除上述调控因子外,微环境中还存在其他因素,如活性氧(reactive oxygen species, ROS)。一直以来普遍认为ROS对干细胞有害,近年来首次证明了ROS介导的正反馈在维持SSCs自我更新方面起着重要作用[86]。ROS在GDNF和FGF2存在状态下由SSCs中的NADPH氧化酶3 (NADPH oxidase 3, NOX3)产生,在稳定增殖阶段由SSCs中的NADPH氧化酶1(NADPH oxidase 1, NOX1)产生,两种NOX在不同条件下调节SSCs中ROS的产生[87]。添加过氧化氢(hydrogen peroxide, H2O2)使ROS含量升高时,可明显促进干细胞的增殖,而NOX1的缺乏又抑制SSCs的自我更新[88]。另外NOX1通过ROS-BCL6B-NOX1途径产生的ROS和氧气相互作用共同决定SSCs的自我更新效率[89]。这些研究都表明ROS在SSCs自我更新方面起了重要作用。
3.1.7 其他
除上述因子外,在微环境中还存在许多因素影响SSCs的自我更新。CARF (collaborator of ARF)可以通过Wnt信号通路促进SSCs的自我更新和增殖[90]。FGF9由睾丸中的体细胞产生,是SSCs增殖的重要调节因子,另外有研究表明,由LECs产生的FGFs的其他成员如FGF4、FGF5和FGF8都可以调节SSCs的数目[21,91]。最近又发现一种新的因子—白细胞介素-34 (interleukin-34, IL-34),它是一种新型的旁分泌/自分泌因子,可以在LCs、SCs和部分精原细胞中表达以调节精子发生[92]。上述因子都在SSCs自我更新中起着重要作用,其他新的因子还有待进一步研究发现。
3.2 参与分化的调控因子
3.2.1 维甲酸
RA是维生素A的中间代谢产物,主要在SCs、GCs、PMCs和LCs中表达, RALDH1和RALDH2分别是SCs和GCs中的关键RA合成酶[93,94]。SCs产生的RA及其靶基因在小鼠中可调节精原细胞分化的启动,周期性RA-信号传导与周期性GCs能力相互协调,以调节两种不同的细胞类型特异性反应:SSCs精子分化和减数分裂起始[61,95]。雌性GCs在性别决定后不久开始减数分裂,而雄性GCs进入静止状态,则是因为它们周围的SCs表达RA代谢酶,如CYP26B1,在RA作用于GCs之前有效降解RA[96]。精原细胞的分化依赖于生精上皮内部合成的RA,然而,在完全没有RA的情况下,精原细胞也可以启动减数分裂并表达[93,97]。在体外,RA与SCs配合或单独作用均能在不同程度上引起不同年龄段小鼠GCs的分化,且其诱导分化倾向与精原细胞链的长度有关[98]。近来有研究表明,RA与孕酮在体外协同作用于小鼠诱导多功能干细胞分化为雄性GCs[99]。
3.2.2 骨形态发生蛋白4
BMP4属于TGF-β家族,主要在GCs中表达,其表达水平随着精子发生的进行而升高,其受体BMPR1A和BMPRII存在于精原细胞、精母细胞和圆形精子中,在精子发生过程中起重要作用[100]。在BMP4存在条件下SSCs更倾向分化,在大鼠中BMP4不但可以诱导SSCs分化,同时可以导致细胞的黏附性发生改变,如细胞黏附分子和肌动蛋白细胞骨架调节途径等[101]。此外BMP4可通过上调Sohlh2的表达,在SSCs的早期分化中发挥重要作用。BMP4及其多种受体存在于人SCs中,细胞增殖和Brdu掺入测定表明BMP4能促进SCs的DNA合成和复制[102]。另外BMP4也能与RA协同作用,诱导体内精原细胞分化[103]。
3.2.3 干细胞因子
SCF在哺乳动物睾丸中由SCs产生[104]。有研究表明,卵泡刺激素(follicle-stimulating hormone, FSH)可通过SCF/C-Kit信号通路调节SCF的表达,在FSH的刺激下,SCF会急剧升高[105]。通过与C-kit受体结合来触发其生物学功能,它们组成SCF/C-kit系统在雄性和雌性生殖道内发挥重要作用,近年来关于SCF/C-kit异常表达引起雄性生殖系统肿瘤和雌性生殖系统肿瘤的研究日益深入[106~108]。SCF通过转录上调、、、和基因改善SSCs的体外分化,并对表达C-kit的精原细胞的有丝分裂和存活的影响依赖于不同的信号通路[109~111]。有研究表明人重组SCF (recombinant human SCF, rhSCF)可促进蝾螈()精子的增殖,但不能促进减数分裂的开始[112]。
3.2.4 其他
除上述因子外,还存在诸多因子影响SSCs的分化。近年来发现的泛素羧基末端水解酶-L1 (ubiquitin carboxy-terminal hydrolase-l1, UCH-L1),其缺失可降低SSCs的分化能力,并影响SSCs稳态和新陈代谢的维持[113]。这些分化因子可能与其他因子,如自我更新因子和分化因子协同作用调节SSCs的自我更新和分化。
3.3 参与精子发生的转录因子调控
微环境中的一些转录因子也会对SSCs产生影响,例如影响SSCs自我更新相关的转录因子Plzf、Bcl6b、Etv5、ID4和Foxo1等,以及促进SSCs分化的相关转录因子如Sall4以及Sohlh1/2等。近年来对SSCs发生发展起作用的转录因子陆续被发现,如Dmrt1对于Ngn3阳性精原细胞转化为SSCs以恢复干细胞池是必需的,并且最新研究表明Dmrt1和Plzf的相互作用,通过激活Plzf的转录在稳态环境下维持SSCs[114,115]。Sohlh1/2能直接结合到和基因的启动子上调节精子发生,也可以直接结合到和启动子上抑制并激活的表达[116,117]。Plzf可以负向调控分化相关基因如和的启动子[118]。过度表达PAX7后,与自我更新相关的基因如和在SSCs中的表达上调[119]。近期研究发现,RHOX10可通过Dmrt1和Plzf驱动SSCs前体即前精原细胞(pro-spermatogonia, ProSG)分化[120]。随着转录组技术的发展,越来越多的对SSCs发生发展产生影响的转录因子被发现。
3.4 参与精子发生的激素调控
在SSCs微环境中除上述的调控因子外还存在一类影响精子发生的物质—激素,其中IGF-1在睾丸的SCs和LCs中表达,可以促进SSCs的增殖、自我更新以及调节多能性[43,121]。同时在体外培养睾丸组织时,在培养基中添加IGF-1可减少GCs凋亡和促进增殖[122]。其具体的促进自我更新和增殖的机制可解释为IGF-1可能通过PI3K/AKT信号转导介导IGF-1/IGF-1R调控OCT-4等多种转录因子和GCs多能性,LCs分泌的IGF-1也能以旁分泌的方式促进未成熟SCs的增殖[43,123]。促进精原细胞转变为初级精母细胞的具体机制可解释为下调导致IGF-1/IGF-1R上调,级联放大将ERK1/2和PI3K激活[124]。关于IGF结合蛋白,PMCs主要分泌IGFBP-2,SCs主要分泌IGFBP-3[125]。除上述因素外,精子发生也受控于以睾酮、FSH和黄体生成素(luteinizing hormone, LH)为主的外源性控制,LCs和SCs表面分别存在LH和FSH受体,LH可刺激LCs分泌睾酮,睾酮和FSH联合作用于SCs,从而影响精子发生。睾酮等雄激素是正常雄性生殖系统发育和功能所必需的类固醇激素,在维持精子数目、BTB完整性、减数分裂的完成等方面起重要作用[39,126]。另外激素可与细胞因子共同作用,如EGF和FGF2作为细胞因子培养SSCs形成克隆数较少,然而当添加FSH后SSCs形成的菌落数量大幅度增加[23]。
4 结语与展望
SSCs是终身精子发生的来源,而SSCs的行为以及命运受到微环境整体因素相互作用的综合调控。目前关于微环境的研究主要基于单细胞转录组测序技术(single-cell RNA sequencing, scRNA-seq),新的技术正将单细胞谱分析扩展到转录组之外,如单细胞空间转录组、单细胞翻译组、单细胞蛋白质组和单细胞代谢组等技术,未来这些策略将会复用在一起联合分析来自同一个细胞的多种组学模式,有助于人们定义和发现丰富的细胞表型和微环境因子。目前,对于SSCs微环境研究的小鼠模型大多都集中在W/Wv鼠以及白消安处理的小鼠模型等,近年来也有新的小鼠模型不断出现,如苯扎氯氨(benzalkonium chloride, BC)特异性去除SCs的细胞模型等。新的小鼠模型的出现有助于人们对微环境的进一步研究[127]。对于GCs的转染技术也在不断优化,例如综合考虑转染试剂、质粒用量和细胞数量等因素优化了鸡() PGCs转染条件[128]。随着SSCs微环境研究的逐渐深入,越来越多对精子发生有影响的因素逐渐被发现,关于微环境的研究也会越来越完善。SSCs自身基因的表达也不可忽视,如近期Wang等[129]发现在果蝇()睾丸中基因是一个生殖系统优势表达的基因,能影响GCs的自我更新和分化。人类SSCs与啮齿动物SSCs共享一些生殖表型但并非完全相同,且人类SSCs的长期培养尚未实现,大多数关于SSCs微环境的知识都来自啮齿类动物,关于人类SSCs还需不断探索。相信在不久的将来,SSCs微环境的研究也将为临床因微环境缺陷引起的不育症治疗带来新的希望,以及开发安全有效的男性避孕方法,同时对于体外培养成年SSCs带来新思路。此外SSCs在病理和生理条件下的细胞和分子变化仍有待发现,这将为SSCs提供有价值的生物学信息,还可能揭示雄性不育与癌症关联的机制。关于SSCs微环境的研究尚存在一些伦理、遗传以及炎症等方面的问题,相信随着研究的不断深入,这些问题也终将被解决。今后,SSCs微环境研究也会有更加广阔的应用前景。
[1] Li LH, Xie T. Stem cell niche: structure and function., 2005, 21: 605–631.
[2] Cai YH, Wang JJ, Zou K. The progresses of spermatogonial stem cells sorting using fluorescence-activated cell sorting., 2020, 16(1): 94–102.
[3] Shinohara T, Orwig KE, Avarbock MR, Brinster RL. Remodeling of the postnatal mouse testis is accompanied by dramatic changes in stem cell number and niche accessibility., 2001, 98(11): 6186–6191.
[4] Fayomi AP, Orwig KE. Spermatogonial stem cells and spermatogenesis in mice, monkeys and men., 2018, 29: 207–214.
[5] Oatley JM, Brinster RL. The germline stem cell niche unit in mammalian testes., 2012, 92(2): 577–595.
[6] Chen LY, Willis WD, Eddy EM. Targeting the gdnf gene in peritubular myoid cells disrupts undifferentiated spermatogonial cell development., 2016, 113(7): 1829–1834.
[7] Lord T, Oatley JM. A revised A(single) model to explain stem cell dynamics in the mouse male germline., 2017, 154(2): R55–R64.
[8] de Rooij DG. The nature and dynamics of spermatogonial stem cells., 2017, 144(17): 3022–3030.
[9] Ehmcke J, Schlatt S. A revised model for spermatogonial expansion in man: lessons from non-human primates., 2006, 132(5): 673–680.
[10] Wu ZR, Luby-Phelps K, Bugde A, Molyneux LA, Denard B, Li WH, Süel GM, Garbers DL. Capacity for stochastic self-renewal and differentiation in mammalian spermatogonial stem cells., 2009, 187(4): 513–524.
[11] Hara K, Nakagawa T, Enomoto H, Suzuki M, Yamamoto M, Simons BD, Yoshida S. Mouse spermatogenic stem cells continually interconvert between equipotent singly isolated and syncytial states., 2014, 14(5): 658–672.
[12] Nakagawa T, Nabeshima YI, Yoshida S. Functional identification of the actual and potential stem cell compartments in mouse spermatogenesis., 2007, 12(2): 195–206.
[13] Wong MD, Jin ZG, Xie T. Molecular mechanisms of germline stem cell regulation., 2005, 39: 173–195.
[14] Schofield R. The relationship between the spleen colony- forming cell and the haemopoietic stem cell., 1978, 4(1-2): 7–25.
[15] Guo JT, Grow EJ, Mlcochova H, Maher GJ, Lindskog C, Nie XC, Guo YX, Takei Y, Yun JN, Cai L, Kim R, Carrell DT, Goriely A, Hotaling JM, Cairns BR. The adult human testis transcriptional cell atlas., 2018, 28(12): 1141–1157.
[16] Mäkelä JA, Hobbs RM. Molecular regulation of spermatogonial stem cell renewal and differentiation., 2019, 158(5): R169–R187.
[17] Yoshida S. Mouse spermatogenesis reflects the unity and diversity of tissue stem cell niche systems., 2020, 12(12): a036186.
[18] Oakberg EF. Duration of spermatogenesis in the mouse and timing of stages of the cycle of the seminiferous epithelium., 1956, 99(3): 507–516.
[19] Leblond CP, Clermont Y. Definition of the stages of the cycle of the seminiferous epithelium in the rat., 1952, 55(4): 548–573.
[20] Hess RA. Quantitative and qualitative characteristics of the stages and transitions in the cycle of the rat seminiferous epithelium: light microscopic observations of perfusion-fixed and plastic-embedded testes., 1990, 43(3): 525–542.
[21] Kitadate Y, Jörg DJ, Tokue M, Maruyama A, Ichikawa R, Tsuchiya S, Segi-Nishida E, Nakagawa T, Uchida A, Kimura-Yoshida C, Mizuno S, Sugiyama F, Azami T, Ema M, Noda C, Kobayashi S, Matsuo I, Kanai Y, Nagasawa T, Sugimoto Y, Takahashi S, Simons BD, Yoshida S. Competition for mitogens regulates spermatogenic stem cell homeostasis in an open niche., 2019, 24(1): 79–92.e6.
[22] Yoshida S, Sukeno M, Nabeshima YI. A vasculature- associated niche for undifferentiated spermatogonia in the mouse testis., 2007, 317(5845): 1722–1726.
[23] Oatley MJ, Racicot KE, Oatley JM. Sertoli cells dictate spermatogonial stem cell niches in the mouse testis., 2011, 84(4): 639–645.
[24] Lord T, Nixon B. Metabolic changes accompanying spermatogonial stem cell differentiation., 2020, 52(4): 399–411.
[25] Mohyeldin A, Garzón-Muvdi T, Quiñones-Hinojosa A. Oxygen in stem cell biology: a critical component of the stem cell niche., 2010, 7(2): 150–161.
[26] Cheng CY, Wong EWP, Yan HHN, Mruk DD. Regulation of spermatogenesis in the microenvironment of the seminiferous epithelium: new insights and advances., 2010, 315(1-2): 49–56.
[27] Watanabe S, Kanatsu-Shinohara M, Ogonuki N, Matoba S, Ogura A, Shinohara T.genetic manipulation of spermatogonial stem cells and their microenvironment by Adeno-associated viruses., 2018, 10(5): 1551–1564.
[28] Garcia TX, Farmaha JK, Kow S, Hofmann MC. RBPJ in mouse sertoli cells is required for proper regulation of the testis stem cell niche., 2014, 141(23): 4468–4478.
[29] Kanatsu-Shinohara M, Ogonuki N, Matoba S, Ogura A, Shinohara T. Autologous transplantation of spermatogonial stem cells restores fertility in congenitally infertile mice., 2020, 117(14): 7837–7844.
[30] Nishimura H, L'Hernault SW. Spermatogenesis., 2017, 27(18): R988–R994.
[31] Rebourcet D, O'Shaughnessy PJ, Monteiro A, Milne L, Cruickshanks L, Jeffrey N, Guillou F, Freeman TC, Mitchell RT, Smith LB. Sertoli cells maintain leydig cell number and peritubular myoid cell activity in the adult mouse testis., 2014, 9(8): e105687.
[32] Wang YQ, Batool A, Chen SR, Liu YX. GATA4 is a negative regulator of contractility in mouse testicular peritubular myoid cells., 2018, 156(4): 343–351.
[33] Chen LY, Brown PR, Willis WB, Eddy EM. Peritubular myoid cells participate in male mouse spermatogonial stem cell maintenance., 2014, 155(12): 4964–4974.
[34] Spinnler K, Köhn FM, Schwarzer U, Mayerhofer A. Glial cell line-derived neurotrophic factor is constituteively produced by human testicular peritubular cells and may contribute to the spermatogonial stem cell niche in man., 2010, 25(9): 2181–2187.
[35] Zhou R, Wu J, Liu B, Jiang YQ, Chen W, Li J, He QY, He ZP. The roles and mechanisms of leydig cells and myoid cells in regulating spermatogenesis., 2019, 76(14): 2681–2695.
[36] Bhang DH, Kim BJ, Kim BG, Schadler K, Baek KH, Kim YH, Hsiao W, Ding BS, Rafii S, Weiss MJ, Chou ST, Kolon TF, Ginsberg JP, Ryu BY, Ryeom S. Testicular endothelial cells are a critical population in the germline stem cell niche., 2018, 9(1): 4379.
[37] Del Punta K, Charreau EH, Pignataro OP. Nitric oxide inhibits leydig cell steroidogenesis., 1996, 137(12): 5337–5343.
[38] Kim YH, Oh MG, Bhang DH, Kim BJ, Jung SE, Kim SM, Dohr G, Kim SU, Ryeom S, Ryu BY. Testicular endothelial cells promote self-renewal of spermatogonial stem cells in rats., 2019, 101(2): 360–367.
[39] O'Hara L, Smith LB. Androgen receptor roles in spermatogenesis and infertility., 2015, 29(4): 595–605.
[40] Guo JT, Sosa E, Chitiashvili T, Nie XC, Rojas EJ, Oliver E, DonorConnect, Plath K, Hotaling JM, Stukenborg JB, Clark AT, Cairns BR. Single-cell analysis of the developing human testis reveals somatic niche cell specification and fetal germline stem cell establishment., 2021, 28(4): 764–778.e4.
[41] Tripiciano A, Filippini A, Ballarini F, Palombi F. Contractile response of peritubular myoid cells to prostaglandin F2alpha., 1998, 138(1-2): 143–150.
[42] Nicholson HD, Hardy MP. Luteinizing hormone differentially regulates the secretion of testicular oxytocin and testosterone by purified adult rat leydig cells., 1992, 130(2): 671–677.
[43] Huang YH, Chin CC, Ho HN, Chou CK, Shen CN, Kuo HC, Wu TJ, Wu YC, Hung YC, Chang CC, Ling TY. Pluripotency of mouse spermatogonial stem cells maintained by IGF-1-dependent pathway., 2009, 23(7): 2076–2087.
[44] Oatley JM, Oatley MJ, Avarbock MR, Tobias JW, Brinster RL. Colony stimulating factor 1 is an extrinsic stimulator of mouse spermatogonial stem cell self- renewal., 2009, 136(7): 1191–1199.
[45] Wang S, Wang XX, Wu YJ, Han CS. IGF-1R signaling is essential for the proliferation of cultured mouse spermatogonial stem cells by promoting the G2/M progression of the cell cycle., 2015, 24(4): 471–483.
[46] Hume DA, Halpin D, Charlton H, Gordon S. The mononuclear phagocyte system of the mouse defined by immunohistochemical localization of antigen F4/80: macrophages of endocrine organs., 1984, 81(13): 4174–4177.
[47] DeFalco T, Potter SJ, Williams AV, Waller B, Kan MJ, Capel B. Macrophages contribute to the spermatogonial niche in the adult testis., 2015, 12(7): 1107–1119.
[48] Fehervari Z. Testicular macrophage origin., 2017, 18(10): 1067.
[49] Lokka E, Lintukorpi L, Cisneros-Montalvo S, Mäkelä JA, Tyystjärvi S, Ojasalo V, Gerke H, Toppari J, Rantakari P, Salmi M. Generation, localization and functions of macrophages during the development of testis., 2020, 11(1): 4375.
[50] Wang M, Yang YL, Cansever D, Wang YM, Kantores C, Messiaen S, Moison D, Livera G, Chakarov S, Weinberger T, Stremmel C, Fijak M, Klein B, Pleuger C, Lian ZX, Ma WT, Liu QZ, Klee K, Händler K, Ulas T, Schlitzer A, Schultze JL, Becher B, Greter M, Liu ZY, Ginhoux F, Epelman S, Schulz C, Meinhardt A, Bhushan S. Two populations of self-maintaining monocyte- independent macrophages exist in adult epididymis and testis., 2021, 118(1): e2013686117.
[51] Indumathy S, Pueschl D, Klein B, Fietz D, Bergmann M, Schuppe HC, Da Silva N, Loveland BE, Hickey MJ, Hedger MP, Loveland KL. Testicular immune cell populations and macrophage polarisation in adult male mice and the influence of altered activin A levels., 2020, 142: 103204.
[52] Mossadegh-Keller N, Gentek R, Gimenez G, Bigot S, Mailfert S, Sieweke MH. Developmental origin and maintenance of distinct testicular macrophage populations., 2017, 214(10): 2829–2841.
[53] Potter SJ, DeFalco T. Role of the testis interstitial compartment in spermatogonial stem cell function., 2017, 153(4): R151–R162.
[54] Wang M, Liu XX, Chang G, Chen YD, An G, Yan LY, Gao S, Xu YW, Cui YL, Dong J, Chen YH, Fan XY, Hu YQ, Song K, Zhu XH, Gao Y, Yao ZK, Bian SH, Hou Y, Lu JH, Wang R, Fan Y, Lian Y, Tang WH, Wang YP, Liu JQ, Zhao LM, Wang LY, Liu ZT, Yuan RP, Shi YJ, Hu BQ, Ren XL, Tang FC, Zhao XY, Qiao J. Single-cell RNA sequencing analysis reveals sequential cell fate transition during human spermatogenesis., 2018, 23(4): 599–614.e4.
[55] Green CD, Ma QY, Manske GL, Shami AN, Zheng XN, Marini S, Moritz L, Sultan C, Gurczynski SJ, Moore BB, Tallquist MD, Li JZ, Hammoud SS. A comprehensive roadmap of murine spermatogenesis defined by single-cell RNA-seq., 2018, 46(5): 651–667.e10.
[56] Zhao JX, Lu P, Wan C, Huang YP, Cui MM, Yang XY, Hu YQ, Zheng Y, Dong J, Wang M, Zhang S, Liu ZT, Bian SH, Wang XM, Wang R, Ren SF, Wang DZ, Yao ZK, Chang G, Tang FC, Zhao XY. Cell-fate transition and determination analysis of mouse male germ cells throughout development., 2021, 12(1): 6839.
[57] Sharma M, Braun RE. Cyclical expression of GDNF is required for spermatogonial stem cell homeostasis., 2018, 145(5): dev151555.
[58] Tokue M, Ikami K, Mizuno S, Takagi C, Miyagi A, Takada R, Noda C, Kitadate Y, Hara K, Mizuguchi H, Sato T, Taketo MM, Sugiyama F, Ogawa T, Kobayashi S, Ueno N, Takahashi S, Takada S, Yoshida S. SHISA6 confers resistance to differentiation-promoting Wnt/β-catenin signaling in mouse spermatogenic stem cells., 2017, 8(3): 561–575.
[59] Meng X, Lindahl M, Hyvönen ME, Parvinen M, de Rooij DG, Hess MW, Raatikainen-Ahokas A, Sainio K, Rauvala H, Lakso M, Pichel JG, Westphal H, Saarma M, Sariola H. Regulation of cell fate decision of undifferentiated spermatogonia by GDNF., 2000, 287(5457): 1489–1493.
[60] Uchida A, Kishi K, Aiyama Y, Miura K, Takase HM, Suzuki H, Kanai-Azuma M, Iwamori T, Kurohmaru M, Tsunekawa N, Kanai Y. In vivo dynamics of GFRα1- positive spermatogonia stimulated by GDNF signals using a bead transplantation assay., 2016, 476(4): 546–552.
[61] Masaki K, Sakai M, Kuroki S, Jo JI, Hoshina K, Fujimori Y, Oka K, Amano T, Yamanaka T, Tachibana M, Tabata Y, Shiozawa T, Ishizuka O, Hochi S, Takashima S. FGF2 has distinct molecular functions from GDNF in the mouse germline niche., 2018, 10(6): 1782–1792.
[62] Zhou Z, Shirakawa T, Ohbo K, Sada A, Wu Q, Hasegawa K, Saba R, Saga Y. RNA binding protein Nanos2 organizes post-transcriptional buffering system to retain primitive state of mouse spermatogonial stem cells., 2015, 34(1): 96–107.
[63] Sariola H, Saarma M. Novel functions and signalling pathways for GDNF., 2003, 116(Pt 19): 3855–3862.
[64] Jain S, Naughton CK, Yang M, Strickland A, Vij K, Encinas M, Golden J, Gupta A, Heuckeroth R, Johnson EM, Milbrandt J. Mice expressing a dominant-negative ret mutation phenocopy human hirschsprung disease and delineate a direct role of ret in spermatogenesis., 2004, 131(21): 5503–5513.
[65] Naughton CK, Jain S, Strickland AM, Gupta A, Milbrandt J. Glial cell-line derived neurotrophic factor-mediated RET signaling regulates spermatogonial stem cell fate., 2006, 74(2): 314–321.
[66] Kanatsu-Shinohara M, Shinohara T. Spermatogonial stem cell self-renewal and development., 2013, 29: 163–187.
[67] Sadri-Ardekani H, Mizrak SC, van Daalen SKM, Korver CM, Roepers-Gajadien HL, Koruji M, Hovingh S, de Reijke TM, de la Rosette JJMCH, van der Veen F, de Rooij DG, Repping S, van Pelt AMM. Propagation of human spermatogonial stem cells., 2009, 302(19): 2127–2134.
[68] Parker N, Laychur A, Sukwani M, Orwig KE, Oatley JM, Zhang C, Rutaganira FU, Shokat K, Wright WW. Spermatogonial stem cell numbers are reduced by transient inhibition of GDNF signaling but restored by self-renewing replication when signaling resumes., 2021, 16(3): 597–609.
[69] Zhang Y, Wang S, Wang XX, Liao SY, Wu YJ, Han CS. Endogenously produced FGF2 is essential for the survival and proliferation of cultured mouse spermatogonial stem cells., 2012, 22(4): 773–776.
[70] Sawaied A, Arazi E, AbuElhija A, Lunenfeld E, Huleihel M. The presence of colony-stimulating factor-1 and its receptor in different cells of the testis; it involved in the development of spermatogenesis., 2021, 22(5): 2325.
[71] Otsuka R, Wada H, Seino KI. IL-34, the rationale for its expression in physiological and pathological conditions., 2021, 54: 101517.
[72] Sato T, Yokonishi T, Komeya M, Katagiri K, Kubota Y, Matoba S, Ogonuki N, Ogura A, Yoshida S, Ogawa T. Testis tissue explantation cures spermatogenic failure in c-Kit ligand mutant mice., 2012, 109(42): 16934–16938.
[73] Kokkinaki M, Lee TL, He ZP, Jiang JJ, Golestaneh N, Hofmann MC, Chan WY, Dym M. The molecular signature of spermatogonial stem/progenitor cells in the 6-day-old mouse testis., 2009, 80(4): 707–717.
[74] Tao K, Sun Y, Chao YC, Xing L, Leng LZ, Zhou D, Zhu WB, Fan LQ. β-estradiol promotes the growth of primary human fetal spermatogonial stem cells via the induction of stem cell factor in sertoli cells., 2021, 38(9): 2481–2490.
[75] Yang QE, Kim D, Kaucher A, Oatley MJ, Oatley JM. CXCL12-CXCR4 signaling is required for the maintenance of mouse spermatogonial stem cells., 2013, 126(Pt 4): 1009–1020.
[76] Kanatsu-Shinohara M, Inoue K, Takashima S, Takehashi M, Ogonuki N, Morimoto H, Nagasawa T, Ogura A, Shinohara T. Reconstitution of mouse spermatogonial stem cell niches in culture., 2012, 11(4): 567–578.
[77] Han R, Shang KG. Germ cells in the murine embryonic development., 2002, 24(1): 77–81.
韩嵘, 尚克刚. 小鼠生殖细胞的胚胎发育. 遗传, 2002, 24(1): 77–81.
[78] Jenab S, Morris PL. Testicular leukemia inhibitory factor (LIF) and LIF receptor mediate phosphorylation of signal transducers and activators of transcription (STAT)-3 and STAT-1 and induce c-fos transcription and activator protein-1 activation in rat sertoli but not germ cells., 1998, 139(4): 1883–1890.
[79] Curley M, Milne L, Smith S, Atanassova N, Rebourcet D, Darbey A, Hadoke PWF, Wells S, Smith LB. Leukemia inhibitory factor-receptor is dispensable for prenatal testis development but is required in sertoli cells for normal spermatogenesis in mice., 2018, 8(1): 11532.
[80] Piquet-Pellorce C, Dorval-Coiffec I, Pham MD, Jégou B. Leukemia inhibitory factor expression and regulation within the testis., 2000, 141(3): 1136–1141.
[81] Kim YH, Kang HG, Kim BJ, Jung SE, Karmakar PC, Kim SM, Hwang S, Ryu BY. Enrichment andculture of spermatogonial stem cells from pre- pubertal monkey testes., 2017, 14(5): 557–566.
[82] Medrano JV, Rombaut C, Simon C, Pellicer A, Goossens E. Human spermatogonial stem cells display limited proliferationunder mouse spermatogonial stem cell culture conditions., 2016, 106(6): 1539–1549.e8.
[83] Aponte PM, Soda T, Teerds KJ, Mizrak SC, van de Kant HJ, de Rooij DG. Propagation of bovine spermatogonial stem cells., 2008, 136(5): 543–557.
[84] Kanatsu-Shinohara M, Inoue K, Ogonuki N, Miki H, Yoshida S, Toyokuni S, Lee J, Ogura A, Shinohara T. Leukemia inhibitory factor enhances formation of germ cell colonies in neonatal mouse testis culture., 2007, 76(1): 55–62.
[85] Martin-Inaraja M, Ferreira M, Taelman J, Eguizabal C, Chuva De Sousa Lopes SM. Improvingculture of human male fetal germ cells., 2021, 10(8): 2033.
[86] Morimoto H, Kanastu-Shinohara M, Ogonuki N, Kamimura S, Ogura A, Yabe-Nishimura C, Mori Y, Morimoto T, Watanabe S, Otsu K, Yamamoto T, Shinohara T. ROS amplification drives mouse spermatogonial stem cell self-renewal., 2019, 2(2): e201900374.
[87] Morimoto H, Kanatsu-Shinohara M, Shinohara T. ROS- generating oxidase Nox3 regulates the self-renewal of mouse spermatogonial stem cells., 2015, 92(6): 147.
[88] Morimoto H, Iwata K, Ogonuki N, Inoue K, Atsuo O, Kanatsu-Shinohara M, Morimoto T, Yabe-Nishimura C, Shinohara T. ROS are required for mouse spermatogonialstem cell self-renewal., 2013, 12(6): 774–786.
[89] Morimoto H, Yamamoto T, Miyazaki T, Ogonuki N, Ogura A, Tanaka T, Kanatsu-Shinohara M, Yabe-Nishimura C, Zhang HL, Pommier Y, Trumpp A, Shinohara T. An interplay of NOX1-derived ROS and oxygen determines the spermatogonial stem cell self-renewal efficiency under hypoxia., 2021, 35(3-4): 250–260.
[90] Cui WH, He XL, Zhai XH, Zhang H, Zhang YW, Jin F, Song XM, Wu DQ, Shi QH, Li L. CARF promotes spermatogonial self-renewal and proliferation through wnt signaling pathway., 2020, 6(1): 85.
[91] Yang F, Whelan EC, Guan XB, Deng BQ, Wang S, Sun JC, Avarbock MR, Wu X, Brinster RL. FGF9 promotes mouse spermatogonial stem cell proliferation mediated by p38 MAPK signalling., 2021, 54(1): e12933.
[92] Sawaied A, Lunenfeld E, Huleihel M. Interleukin-34, a novel paracrine/autocrine factor in mouse testis, and its possible role in the development of spermatogonial cells., 2020, 21(21): 8143.
[93] Teletin M, Vernet N, Yu JS, Klopfenstein M, Jones JW, Féret B, Kane MA, Ghyselinck NB, Mark M. Two functionally redundant sources of retinoic acid secure spermatogonia differentiation in the seminiferous epithelium., 2019, 146(1): dev170225.
[94] Kent T, Arnold SL, Fasnacht R, Rowsey R, Mitchell D, Hogarth CA, Isoherranen N, Griswold MD. ALDH enzyme expression is independent of the spermatogenic cycle, and their inhibition causes misregulation of murine spermatogenic processes., 2016, 94(1): 12.
[95] Endo T, Romer KA, Anderson EL, Baltus AE, de Rooij DG, Page DC. Periodic retinoic acid-STRA8 signaling intersects with periodic germ-cell competencies to regulate spermatogenesis., 2015, 112(18): E2347–E2356.
[96] Wang S, Wang XX, Ma LF, Lin XW, Zhang DQ, Li Z, Wu YJ, Zheng CW, Feng X, Liao SY, Feng YM, Chen J, Hu XJ, Wang M, Han CS. Retinoic acid is sufficient for theinduction of mouse spermatocytes., 2016, 7(1): 80–94.
[97] Endo T, Freinkman E, de Rooij DG, Page DC. Periodic production of retinoic acid by meiotic and somatic cells coordinates four transitions in mouse spermatogenesis., 2017, 114(47): E10132– E10141.
[98] Wang S, Wang X, Ma L, Lin X, Zhang D, Li Z, Wu Y, Zheng C, Feng X, Liao S, Feng Y, Chen J, Hu X, Wang M, Han C. Retinoic acid is sufficient for theinduction of mouse spermatocytes., 2016, 7(1): 80–94.
[99] Mahabadi JA, Tameh AA, Talaei SA, Karimian M, Rahiminia T, Enderami SE, Gheibi Hayat SM, Nikzad H. Retinoic acid and/or progesterone differentiate mouse induced pluripotent stem cells into male germ cells., 2020, 121(3): 2159–2169.
[100] Nagano M, Ryu BY, Brinster CJ, Avarbock MR, Brinster RL. Maintenance of mouse male germ line stem cells., 2003, 68(6): 2207–2214.
[101] Carlomagno G, van Bragt MPA, Korver CM, Repping S, de Rooij DG, van Pelt AMM. BMP4-induced differentiation of a rat spermatogonial stem cell line causes changes in its cell adhesion properties., 2010, 83(5): 742–749.
[102] Hai YN, Sun M, Niu MH, Yuan QQ, Guo Y, Li Z, He ZP. BMP4 promotes human sertoli cell proliferation via smad1/5 and ID2/3 pathway and its abnormality is associated with azoospermia., 2015, 19(105): 311–325.
[103] Yang YG, Feng YM, Feng X, Liao SY, Wang XX, Gan HY, Wang LX, Lin XW, Han CS. BMP4 cooperates with retinoic acid to induce the expression of differentiation markers in cultured mouse spermatogonia., 2016, 2016: 9536192.
[104] Mauduit C, Hamamah S, Benahmed M. Stem cell factor/c-kit system in spermatogenesis., 1999, 5(5): 535–545.
[105] Ohmura M, Ogawa T, Ono M, Dezawa M, Hosaka M, Kubota Y, Sawada H. Increment of murine spermatogonial cell number by gonadotropin-releasing hormone analogue is independent of stem cell factor c-kit signal., 2003, 68(6): 2304–2313.
[106] Figueira MI, Cardoso HJ, Correia S, Maia CJ, Socorro S. The stem cell factor (SCF)/c-KIT system in carcinogenesis of reproductive tissues: what does the hormonal regulation tell us?, 2017, 405: 10–21.
[107] Cardoso HJ, Figueira MI, Socorro S. The stem cell factor (SCF)/c-KIT signalling in testis and prostate cancer., 2017, 11(4): 297–307.
[108] Chui K, Trivedi A, Cheng CY, Cherbavaz DB, Dazin PF, Huynh ALT, Mitchell JB, Rabinovich GA, Noble- Haeusslein LJ, John CM. Characterization and functionality of proliferative human sertoli cells., 2011, 20(5): 619–635.
[109] Nasimi M, Jorsaraei SGA, Fattahi E, Tabari MG, Neyshaburi EZ. SCF improvesdifferentiation of SSCs through transcriptionally up-regulating PRTM1, STRA8, c-KIT, PIWIL2, and OCT4 genes., 2021, 28(4): 963–972.
[110] Dolci S, Pellegrini M, Di Agostino S, Geremia R, Rossi P. Signaling through extracellular signal-regulated kinase is required for spermatogonial proliferative response to stem cell factor., 2001, 276(43): 40225–40233.
[111] Hakovirta H, Yan W, Kaleva M, Zhang F, Vänttinen K, Morris PL, Söder M, Parvinen M, Toppari J. Function of stem cell factor as a survival factor of spermatogonia and localization of messenger ribonucleic acid in the rat seminiferous epithelium., 1999, 140(3): 1492–1498.
[112] Abé K, Jin Y, Yamamoto T, Abé S. Human recombinant stem cell factor promotes spermatogonial proliferation, but not meiosis initiation in organ culture of newt testis fragments., 2002, 294(3): 695–699.
[113] Alpaugh WF, Voigt AL, Dardari R, Su L, Al Khatib I, Shin W, Goldsmith TM, Coyle KM, Tang LA, Shutt TE, Klein C, Biernaskie J, Dobrinski I. Loss of ubiquitin carboxy- terminal hydrolase l1 impairs long-term differentiation competence and metabolic regulation in murine spermatogonial stem cells., 2021, 10(9): 2265.
[114] Wei YD, Yang DH, Du XM, Yu XW, Zhang MF, Tang FR, Ma FL, Li N, Bai CL, Li GP, Hua JL. Interaction between DMRT1 and PLZF protein regulates self-renewal and proliferation in male germline stem cells., 2021, 476(2): 1123–1134.
[115] Zhang T, Oatley J, Bardwell VJ, Zarkower D. DMRT1 is required for mouse spermatogonial stem cell maintenance and replenishment., 2016, 12(9): e1006293.
[116] Barrios F, Filipponi D, Campolo F, Gori M, Bramucci F, Pellegrini M, Ottolenghi S, Rossi P, Jannini EA, Dolci S. SOHLH1 and SOHLH2 control Kit expression during postnatal male germ cell development., 2012, 125(Pt 6): 1455–1464.
[117] Suzuki H, Ahn HW, Chu TJ, Bowden W, Gassei K, Orwig K, Rajkovic A. SOHLH1 and SOHLH2 coordinate spermatogonial differentiation., 2012, 361(2): 301–312.
[118] Song WX, Shi XL, Xia Q, Yuan M, Liu JX, Hao KY, Qian YJ, Zhao XD, Zou K. PLZF suppresses differentiation of mouse spermatogonial progenitor cells via binding of differentiation associated genes., 2020, 235(3): 3033–3042.
[119] Du XM, Wu SY, Wei YD, Yu XW, Ma FL, Zhai YX, Yang DH, Zhang MF, Liu WQ, Zhu HJ, Wu J, Liao MZ, Li N, Bai CL, Li GP, Hua JL. PAX7 promotes CD49f-positive dairy goat spermatogonial stem cells’ self-renewal., 2021, 236(2): 1481–1493.
[120] Tan K, Song HW, Wilkinson MF. RHOX10 drives mouse spermatogonial stem cell establishment through a transcription factor signaling cascade., 2021, 36(3): 109423.
[121] Binsila BK, Selvaraju S, Ghosh SK, Ramya L, Arangasamy A, Ranjithkumaran R, Bhatta R. EGF, GDNF, and IGF-1 influence the proliferation and stemness of ovine spermatogonial stem cells., 2020, 37(10): 2615–2630.
[122] Yao JF, Zuo HY, Gao J, Wang MM, Wang D, Li XD. The effects of IGF-1 on mouse spermatogenesis using an organ culture method., 2017, 491(3): 840–847.
[123] Neirijnck Y, Kühne F, Mayère C, Pavlova E, Sararols P, Foti M, Atanassova N, Nef S. Tumor suppressor PTEN regulates negatively sertoli cell proliferation, testis size, and sperm production., 2019, 160(2): 387–398.
[124] Shen GQ, Wu RP, Liu B, Dong WH, Tu Z, Yang JR, Xu Z, Pan TJ. Upstream and downstream mechanisms for the promoting effects of IGF-1 on differentiation of spermatogonia to primary spermatocytes., 2014, 101(1-2): 49–55.
[125] Smith EP, Dickson BA, Chernausek SD. Insulin-like growth factor binding protein-3 secretion from cultured rat sertoli cells: dual regulation by follicle stimulating hormone and insulin-like growth factor-I., 1990, 127(6): 2744–2751.
[126] Oduwole OO, Peltoketo H, Poliandri A, Vengadabady L, Chrusciel M, Doroszko M, Samanta L, Owen L, Keevil B, Rahman NA, Huhtaniemi IT. Constitutively active follicle-stimulating hormone receptor enables androgen-independent spermatogenesis., 2018, 128(5): 1787–1792.
[127] Yokonishi T, McKey J, Ide S, Capel B. Sertoli cell ablation and replacement of the spermatogonial niche in mouse., 2020, 11(1): 40.
[128] Zou X, He YH, He JY, Wang Y, Shu DM, Luo CL. Optimization of transfection conditions of chicken primordial germ cells., 2021, 43(3): 280–288.
邹娴, 何燕华, 何静怡, 王艳, 舒鼎铭, 罗成龙. 鸡原始生殖细胞转染条件优化. 遗传, 2021, 43(3): 280–288.
[129] Chen WY, Yan YD, Luan XJ, Wang M, Fang J. Functional analysis of CG8005 gene intestis., 2020, 42(11): 1122–1132.
陈万银, 颜一丹, 栾晓瑾, 王敏, 方杰. CG8005基因在果蝇睾丸生殖细胞中的功能分析. 遗传, 2020, 42(11): 1122–1132.
Progress on spermatogonial stem cell microenvironment
Zhixin Yu, Pengyu Li, Kai Li, Shiying Miao, Linfang Wang, Wei Song
Spermatogonial stem cells (SSCs) are germ cells (GCs) with long-term self-renewal and differentiation potential in testis, namely tissue stem cells located on the basement membrane, whose self-renewal and differentiation are regulated by the surrounding microenvironment. In recent years, the research of SSCs has made a series of important progress, which brings the hope for the clinical treatment of some male infertility patients. Among them, the microenvironment is particularly important in regulating SSCs. The microenvironment is responsible for integrating the effects of different types of cell components, extracellular matrix, extracellular regulatory molecules and hormones on SSCs, thus regulating the fate of SSCs. The research on SSCs microenvironment has gradually become one of the main contents of stem cell research. In this review, we mainly summarize the cell composition, regulatory factors and characteristics of mouse SSCs microenvironment, thereby providing background information for in-depth study on the structure and function of SSCs microenvironment, and opportunity to find more abundant cell phenotypes and microenvironmental factors through multiple research models in the future.
spermatogonia stem cells; microenvironment; self-renewal; differentiation; hormone regulation
2022-05-05;
2022-07-30;
2022-09-01
国家重点研发计划项目(编号:2018YFC1003500)和国家自然科学基金项目(编号:31970794,32000586)资助[Supported by the National Key Research and Development Program (No. 2018YFC1003500) and the National Natural Science Foundation of China (Nos. 31970794, 32000586)]
余志鑫,在读硕士研究生,专业方向:生物化学与分子生物学。E-mail: 15619830345@163.com
宋伟,博士,研究员,研究方向:雄性生殖及相关重大疾病(不育与肿瘤)的分子机理与转化应用研究。E-mail: songwei@ibms.pumc.edu.cn
10.16288/j.yczz.22-136
(责任编委: 史庆华)

