Gestational heat stress alters skeletal muscle gene expression profiles and vascularity in fetal pigs in a sexually dimorphic manner
Weicheng Zhao ,MarkP.Green ,ChristinaD.Marth ,Fan Liu ,Hieu H.Le ,Gordon S.Lynch ,Alan W.Bell,BrianJ.Leury,Frank R.Dunshea,7and Jeremy J.Cottrell
Abstract Background: There is evidence that sow heat stress(HS) during gestation affects fetal development with implications for impaired muscle growth.We have previously demonstrated that maternal HS during early to midgestation compromised muscle fibre hyperplasia in developing fetal pigs.Thus,we hypothesised these phenotypic changes are associated with a change in expression of genes regulating fetal skeletal muscle development and metabolism.To test this,at d 60 of gestation,RNA sequencing and immunohistochemistry were performed on fetal longissimus dorsi (LD) muscle biopsies collected from pregnant gilts that had experienced either thermoneutral control (CON,20°C,n=7 gilts,18 LD samples)or controlled HS (cyclic 28 to 33°C,n=8 gilts,23 LD samples)conditions for 3 weeks.Results:A total of 282 genes were differentially expressed between the HS and CON groups in female LD muscles(false discovery rate (FDR)≤0.05),whereas no differentially expressed genes were detected in male LD muscles between the two groups (FDR>0.05).Gestational HS increased the expression of genes associated with transcription corepressor activity,adipogenesis cascades,negative regulation of angiogenesis and pro-inflammatory signalling in female LD muscles.Immunohistochemical analyses revealed a decreased muscle vascularity density in fetuses from HS group for both sexes compared to those from the CON group (P=0.004).Conclusions:These results reveal gilt HS during early to mid-gestation altered gene expression profiles in fetal LD muscles in a sexually dimorphic manner.The molecular responses,including transcription and angiogenesis repressions and enhanced adipogenesis cascades,were exclusively observed in females.However,the associated reductions in muscle vascularity were observed independently of sexes.Collectively this may indicate female fetal pigs are more adaptive to gestational HS in terms of gene expression changes,and/or there may be sexually dimorphic differences with respect to the timing of muscle molecular responses to gestational HS.
Keywords: Adipogenesis,Angiogenesis,Fetal pig,Gestation,Heat stress,Sexual dimorphism,Skeletal muscle,Sows
Background
Livestock species,such as the pig,are sensitive to elevated ambient temperatures,hence climate change presents a challenge to the pig industry through an increased incidence of heat stress(HS).Heat stress arises when animals are unable to maintain their core body temperatures within normal homeothermic limits,leading to an increase in thermoregulatory responses that compromise productivity [1].Due to the critical role of skeletal muscles in regulating energy metabolism [2],they are likely to be one of the main targets for HS.In pigs,skeletal muscles represent a large proportion of the body mass and are one of the most important economic traits.Heat stress can result in muscle oxidative stress[3,4],inflammation [5] and genome-wide epigenetic modifications that modulate stress responses and energy metabolism in pig skeletal muscles [6].Increased temperatures have been observed to reduce ribosomal biogenesis and elicit an increased heat shock protein response in cultured porcine satellite cells [7].These results show that pig skeletal muscles are sensitive to thermal stress,and when combined with reductions in feed intake and increased thermoregulatory efforts the overall impacts can compromise pig production efficiency [1].
In addition to the direct impact of postnatal HS on pig skeletal muscles,there is growing evidence that skeletal muscle growth of pig progeny can be affected by sow heat exposure during gestation [8].Controlled climate experiments have shown that gestational HS causes lasting effects on the progeny,with reductions in muscle accretion and increased adiposity observed at the finishing stage [9].Furthermore,sow HS during the first half of gestation contributed to increased subcutaneous fat thickness [10] and decreased loin muscle area in the offspring [11].A recent study showed that piglets born to sows mated and gestated during summer were lighter at birth,while the carcasses of those born-light progeny tended to deposit less lean and more fat tissue in the loin [12].Although the exact mechanisms have yet to be fully explored,placental insufficiency and impaired fetal muscle development may be contributing to this phenotype as postnatal productivity of livestock can be programmed by prenatal life [13].One of the primary thermoregulatory mechanisms of sows in response to HS might be a redistribution of blood flow to the periphery to augment radiant heat loss.This redistribution results in impaired blood flow to certain organs,including the placenta [14].We have shown that maternal HS between d 40 and 60 of gestation,corresponding to a period of robust placental growth,reduces placental efficiency and fetal muscle fibre number density at midgestation [15].In fetal pigs,early to mid-gestation is also characterised by the onset of fetal primary fibre myogenesis [16],making the developing fetus and the skeletal muscle in particular sensitive to maternal stressors during this critical stage.Therefore,in the current study we hypothesised that gilt HS during early to mid-gestation altered gene expression profiles underpinning skeletal muscle development and metabolism in the developing fetus.
Methods
Animal ethics
The animal procedures were reviewed and approved by the Animal Ethics Committee of The University of Melbourne (Ethics Id: 1714365.2).The experimental protocols followed the Australian Code for the Care and Use of Animals for Scientific Purposes (8th edition;National Health and Medical Research Council,2013).
Animals and experimental design
Fetallongissimus dorsi(LD) muscle samples were obtained from animals used in a previously published study[15].Briefly,fifteen primiparous sows (gilts) (Large White × Landrace) were sourced from a commercial farm (Huntly Piggery,Victoria,Australia).The gilts were artificially inseminated (d 0) using semen collected from a single sire followed by pregnancy confirmation on d 28 of pregnancy.The pregnant gilts were then transported to the climate-controlled facility,University of Melbourne,and individually housed in floor pens (220 cm×120 cm) for acclimation.The climate conditions set for acclimation were constant 20°C and~50% relative humidity.At d 40 of gestation,the pregnant gilts were assigned to either the thermoneutral control group(CON;n=7;constant 20°C;~50% relative humidity) or the cyclic heat stress group (HS;n=8;33°C between 09:00 and 17:00 h and 28°C between 17:00 and 9:00 h;~50% relative humidity) and housed in the respective conditions for 3 weeks until d 60 of gestation.The pregnant gilts were fed twice daily with a commercially available gestational diet at 2.0 kg per day (equivalent to 1.3×metabolizable energy (ME)) as per commercial procedures.Thus,all pregnant gilts had equivalent nutrient intakes as well as ad libitum access to water throughout the experimental period.
Tissue collection and morphometric analysis
At the end of the climate-controlled period at d 60 of gestation,the pregnant gilts were sedated and humanely euthanised via intracardiac injections of Lethabarb(pentobarbitone sodium;162.5 mg/kg liveweight;Virbac Animal Health,NSW,Australia) followed by collection of their uteri.In each gilt (litter),one uterine horn was randomly chosen from which a total of three developing fetuses located at the cervical,middle and distal uterine position,respectively,were obtained (n=45 fetuses).The fetuses were sexed and a biopsy of LD muscle was collected from each fetus and snap frozen in liquid nitrogen.The frozen muscle samples were pulverised by a tissue pulverizer under liquid nitrogen and stored at −80°C until further processing.
Total RNA extraction,cDNA library preparation and sequencing
Muscle total RNA was extracted using ReliaPrep RNA tissue Miniprep System (Cat# Z6111;Promega,Madison,WI,USA) as per the manufacturer’s instructions.RNA integrity and concentration were assessed using the RNA Nano 6000 Assay Kit of the Bioanalyzer 2100 system (Agilent Technologies,CA,USA) according to the manufacturer’s instructions.Total RNA purity was verified using a nanodrop spectrophotometer (Thermo Scientific,Walton,MA,USA).The muscle samples used for downstream processing had an average RNA integrity number (RIN) of 9.57±0.18 (mean±SD) and a 28/18S ratio of 1.65±0.09 (mean±SD).Four samples (from two females and two males) were excluded from the experiment due to poor RNA quality.The sample distributions were as follows: 10 female and 8 male muscle samples in the CON group;and 17 female and 6 male muscle samples in the HS group.The sequencing library was prepared using the NEBNext® Ultra™ Directional RNA Library Prep Kit for Illumina® (New England Biolabs,Ipswich,MA,USA) as per the manufacturer’s instructions.The cDNA library was sequenced on an Illumina NovaSeq6000 sequencing platform (Annoroad Gene Technology Corporation,Beijing,China),and 150 base-pair,paired-end reads were generated from each sample.
Sequencing data processing
The sequencing data processing followed the pipeline as previously described [17].The quality of sequencing reads was verified using FastQC software version 0.11.9[18].The Illumina adaptors and low-quality bases were removed before further processing.The clean reads for downstream processing and analyses had a Phred score above 30,equivalent to a nucleobase accuracy above 99.9%.The pig reference genome (sus scrofa11.1) DNA sequence (FASTA) and the annotation GTF files were obtained from Ensembl database.Clean reads were mapped against the pig reference genome using Hisat2 version 2.1.0 [19].The samples had an average alignment rate (%) of 96.9±0.3 (mean±SD).The aligned reads were counted per gene using HTSeq software (version 0.11.3) with reverse strand interpretation [20].
Differential gene expression,and gene ontology (GO) and pathway enrichment analyses
Differential gene expression analyses were performed in edgeR version 3.30.0 [21] in R software version 4.0.0[22].Genes with low read counts were filtered and library sizes were normalised using the trimmed mean of M-values (TMM) method [23].Gestational temperature treatment and fetal sex were included in the model.Fetuses within the same litter was adjusted for repeated measures.Differential expression analyses were performed for six pairs of comparisons within the model(HS vs.CON across males and females;HS vs.CON in females;HS vs.CON in males;males vs.females across HS and CON;males vs.females in HS;and males vs.females in CON).To control false positive findings,the Benjamini-Hochberg false discovery rate (FDR) correction was performed.Genes with an adjustedPvalue(FDR)≤0.05 were considered as differentially expressed for each pairwise comparison.Gene ontology statistical overrepresentation tests were performed against the upand downregulated genes separately using g:Profiler annotated for pigs [24].Gene set enrichment analysis [25]was performed in fgsea R package version 1.14.0 [26].Briefly,the complete gene list (13,267 genes) obtained from edgeR [21] was ranked based on log2fold change(FC) in an ascending order (the most upregulated gene to the most downregulated gene by fold change),and the rank metric for each gene was calculated as signed(log2(FC))×−log10(Pvalue).The pre-ranked gene list was analysed against the Kyoto Encyclopedia of Genes and Genomes (KEGG) and REACTOME gene sets (C2),and transcription factor targets (C3) downloaded from the Molecular Signatures Database (MSigDB) v7.2 [25].Gene ontology or pathway terms with an adjustedPvalue (FDR)≤0.05 were considered enriched or overrepresented.
Immunohistochemistry
To further quantify the impact of gestational HS on fetal muscle vascularity,immunohistochemistry (IHC) was performed to test the differences in LD muscle blood vessel density by quantifying the relative abundance of the platelet endothelial cell adhesion molecule (CD31),a biomarker of vascular endothelial cells [27].Longissimus dorsisamples were obtained from two fetuses per litter(CON:n=14 LD samples (7 males and 7 females),HS:n=16 LD samples (7 males and 9 females).The LD samples were fixed and paraffin-embedded as per methods previously described [15].The embedded tissue cross-sectional areas were cut at a thickness of 5 μm by a rotary microtome (Leica biosystems,Victoria,Australia).The sections were fully dried at 37°C overnight and de-paraffinised using xylene solutions.The sections were rehydrated through graded ethanol solutions (100%,90% and 70%) for 2 min each.Heatmediated antigen retrieval was performed using citrate buffer (pH=6).The subsequent IHC experiment was performed using the Novolink Max Plymer Detection System (Cat# RE7280-K,Leica biosystems,Victoria,Australia) as per the manufacturer’s instructions.The sections were incubated with anti-CD31 rabbit primary antibody (1:100 dilution,Cat# ab28364,Abcam,Cambridge,MA,USA) diluted in IHC diluent (Cat# RE7133-CE,Leica,Nussloch,Germany) overnight at 4°C.This primary antibody was validated for pig tissue IHC,as previously described [28].Finally,the sections were counterstained with hematoxylin and dehydrated with 100% ethanol and xylene solutions.Images were taken using a microscope (Olympus,Tokyo,Japan) at 100×total magnification.For each section (sample),10 microscopic fields were randomly chosen and analysed.The positive CD31 staining areas were identified and calculated using ImageJ software (NIH,USA).The averaged blood vessel density across the 10 microscopic fields was calculated and expressed as area percentages of positive CD31 staining per square micrometre of the microscopic section.
Statistical analyses for the immunohistochemistry data
Data normality was verified by Shapiro–Wilk’s test before they were analysed by linear mixed models (REML)(Genstat 18th ed.,VSN International,Hemel Hempstead,UK).The fixed terms were gestational temperature treatment,fetal sex and their interactions with dam ID used as a random factor.APvalue ≤0.05 was considered significantly different.
Results
Gilt thermal stress and fetal morphology
The impacts of gestational HS on gilt thermal stress responses,and placental and fetal morphology have been reported elsewhere [15].Those data were reproduced here in order to relate them to the transcriptome data generated from the current study.Gilt averaged respiration rates (CON=23±4 breaths per min vs.HS=101±4 breaths per min,P<0.001),skin temperatures(CON=30.3±0.2°C vs.HS=36.6±0.2°C,P<0.001)and rectal temperatures (CON=37.5±0.08°C vs.HS=38.3±0.07°C,P<0.001) were increased by heat exposure.Individual fetal weight was not affected by HS at fetal d 60 of age (P>0.05),but males were heavier than females (male=96.3±3.7 g vs.female=91.4±3.7 g,P=0.019).Individual placental weight was increased by gestational HS (CON=90±8 g vs.HS=110±7 g,P=0.041).There was a main effect of gestational temperature on fetal:placental weight ratio (CON=1.16±0.08 vs.HS=0.91±0.07,P=0.013).A trend for an interaction between gestational temperature and fetal sex was observed suggesting that reduction in fetal:placental weight ratio by HS exposure in utero was most pronounced in female fetuses (P=0.081).Fetal muscle fibre number density (CON=858±41 vs.HS=722±38 fibres per mm2area of field,P=0.032) and nuclei number density (CON=1680±40 vs.HS=1423±38 nuclei per mm2area of field,P<0.001) were reduced in fetuses from the HS group.No interaction effect was observed between gestational temperature treatment and gender for the measured muscle parameters (P>0.1).
Differentially expressed genes and gene ontology and pathway enrichment analyses
A total of 31,907 genes were annotated in pig fetal LD muscles,of which 13,267 genes were expressed at fetal d 60 age after removing low count genes.There were 282 differentially expressed genes (DEG) between female LD muscles in the HS and CON groups(FDR ≤0.05,Fig.1),whereas no DEG was detected in male LD muscles (FDR>0.05,Table 1).Among the 282 genes expressed at different levels in the HS vs.CON groups in females,29 genes were downregulated,and 253 genes were upregulated by gestational HS.Table 2 lists a subset of the DEG for the comparison of HS vs.CON in female LD muscles,including the top five most up-and downregulated genes by fold change,and genes involved in transcription,angiogenesis,fibrogenesis and metabolism that are a particular focus herein.When combining females and males,no DEG was detected between HS and CON groups (FDR>0.05).A total of 13 genes (12 of which were linked to sex chromosomes) were differentially expressed (FDR ≤0.05) between LD muscles in females and males,regardless of gestational temperature treatment.A total of 16 genes (13 of which linked to sex chromosomes) were differentially expressed between females and males in the CON group,and 13 genes (11 of which linked to sex chromosomes) were differentially expressed between females and males in the HS group (Table 1).The full list of the DEG for each pair of comparison can be accessed via Additional file 1: Table S1.
Gene ontology and pathway overrepresentation analyses
Gene ontology overrepresentation analysis was performed for the up-and downregulated genes for each pairwise comparison against the GO domains of biological process and molecular function.Among the upregulated genes for the comparison of HS vs.CON in females,negative regulation of transcription -DNA templated (GO:0045892,FDR=0.030),negative regulation of angiogenesis (GO:0016525,FDR=0.030),Notch signalling pathway (GO:0007219,FDR=0.036)and negative regulation of cellular response to growth factor stimulus (GO:0090288,FDR=0.030) were among the enriched biological process GO terms(Fig.2A).In the domain of molecular function (Fig.2B),transcription corepressor activity (GO:0003714,FDR=0.032) and profilin binding (GO:0005522,FDR=0.040) were significantly enriched by the upregulated genes (HS compared to CON in females).Among the downregulated genes (HS compared to CON in females),response to ketone (GO:1901654,FDR=0.004) and response to endoplasmic reticulum stress (GO:0 034976;FDR=0.040) were among the enriched GO terms in the domain of biological process (Fig.3).None of the significantly downregulated genes (HS compared to CON in females)contributed to significantly enriched GO terms for the domain of molecular function (FDR>0.05).
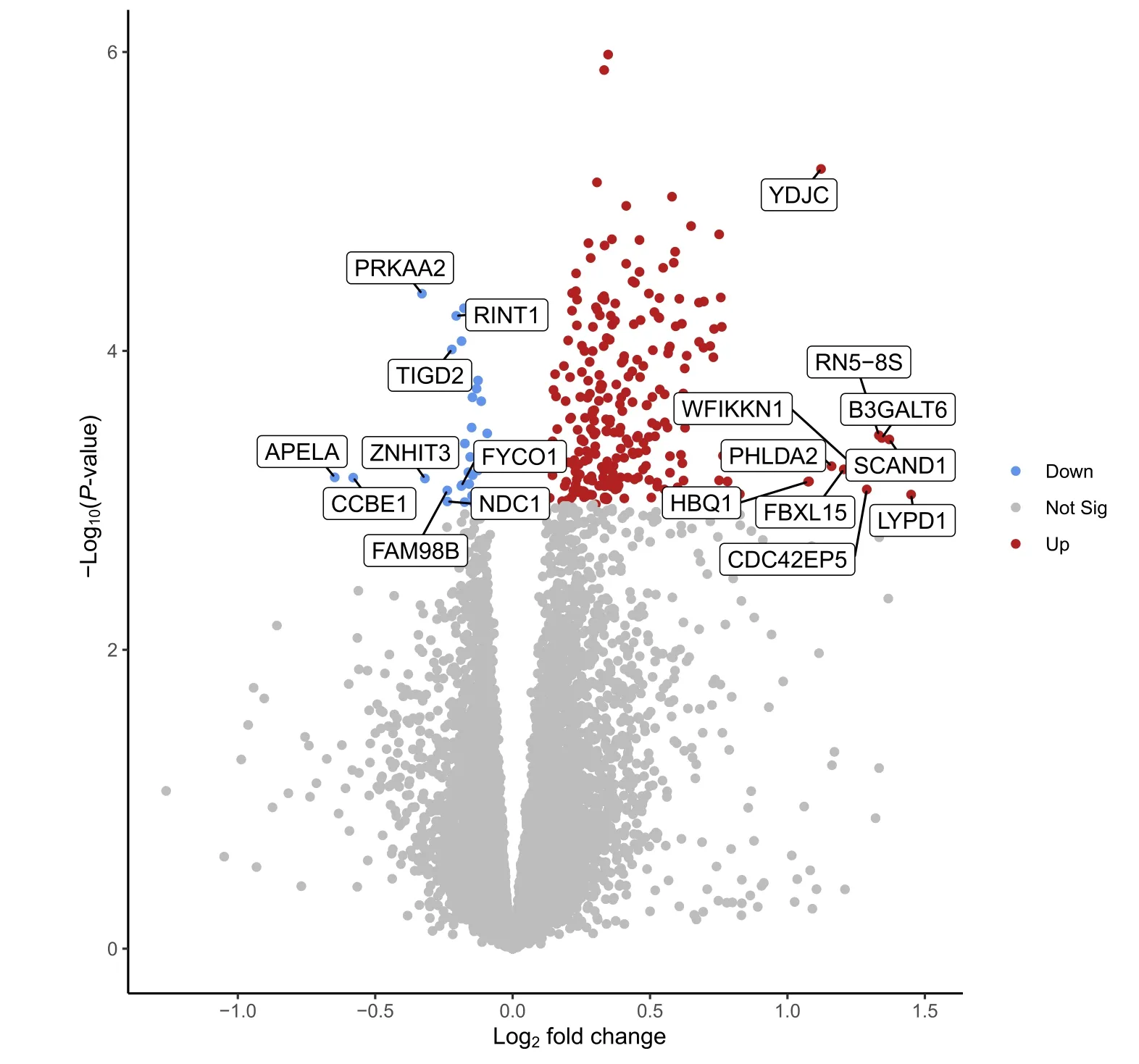
Fig.1 Volcano plot of the differentially expressed genes(DEG)for the comparison of heat stress(HS,n=17)vs.control(CON,n=10)in female fetal longissimus dorsi(LD) muscles at fetal d 60 age.Blue,red and grey dots denote downregulated (FDR(false discovery rate) ≤0.05),upregulated (FDR ≤0.05)and nondifferentially expressed(FDR>0.05)genes in the HS group,respectively,compared to the CON group.The most up-and down-regulated genes by fold change are labelled
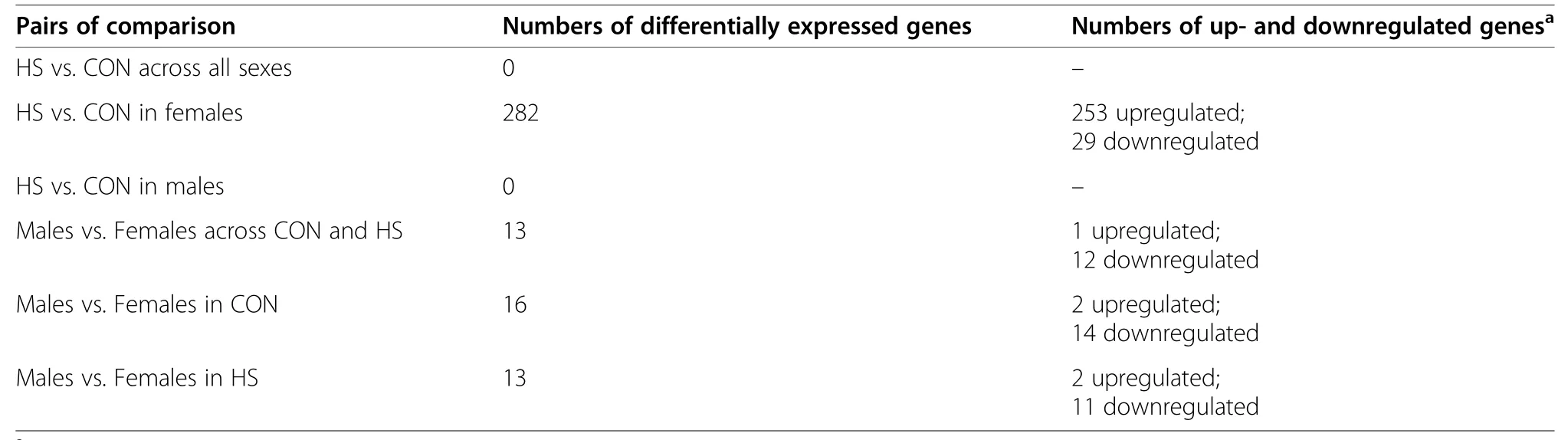
Table 1 The number of differentially expressed genes in each pair of comparison
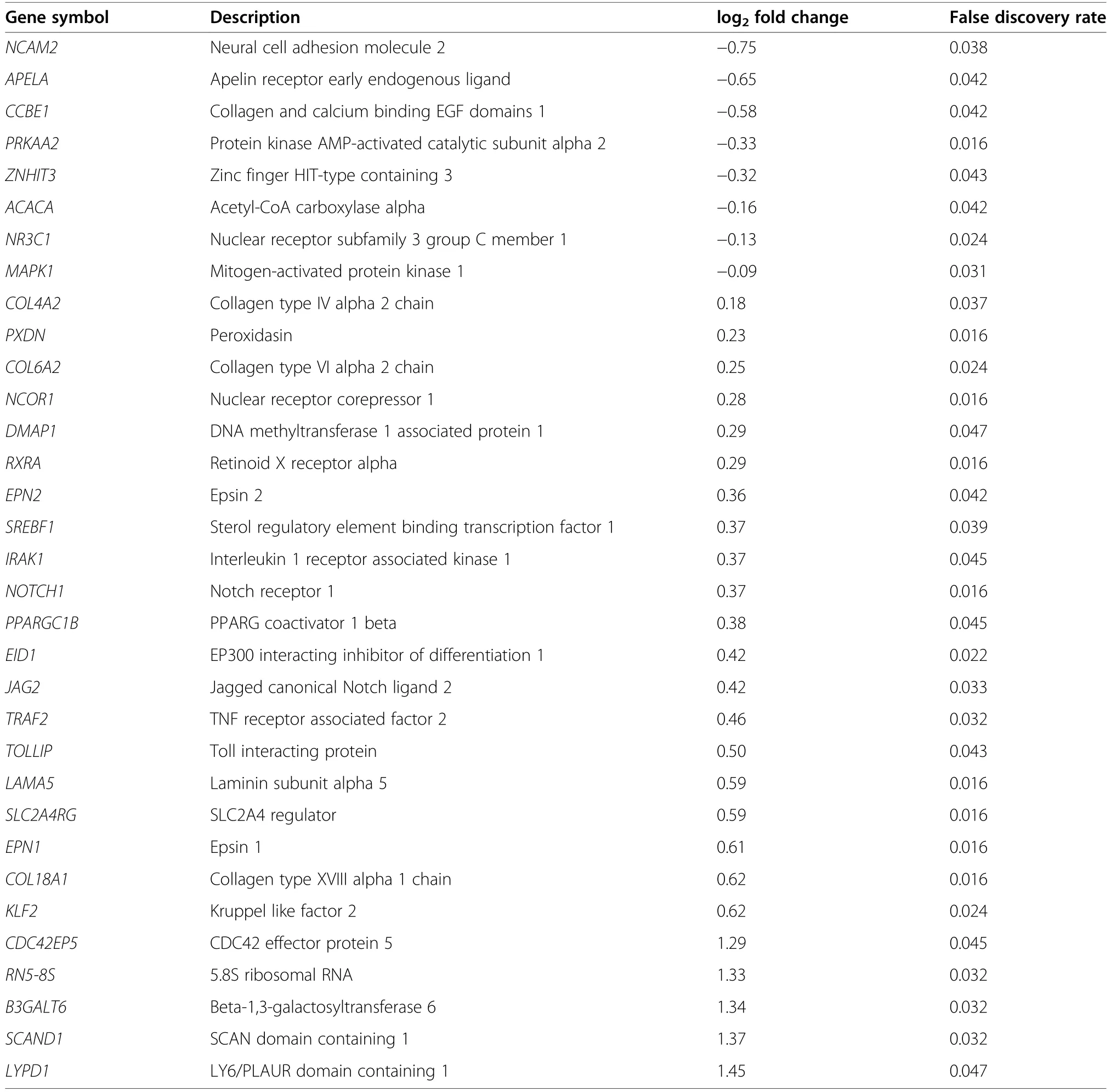
Table 2 A subset of differentially expressed genes that includes the top five most up-and downregulated genes by fold change,and genes involved in transcription,angiogenesis,fibrogenesis and metabolism that are a particular focus herein for the comparison of heat stress(HS,n=17)vs.control(CON,n=10)groups in female fetal longissimus dorsi(LD) muscles at fetal d 60 age
In the comparison between male and female fetuses,translation initiation (GO:0006413) was significantly enriched for the group of CON (FDR=0.037),HS(FDR=0.015),and CON and HS combined (FDR=0.024).In addition,X-linked dominant inheritance(HP:0001423) was enriched for the group of CON(FDR=0.011),HS (FDR=0.011),and CON and HS combined (FDR=0.031).The full list of significantly enriched gene ontology terms,pathways,FDRs and their associated genes can be accessed via Additional file 2: Table S2.
Gene set enrichment analyses(GSEA)
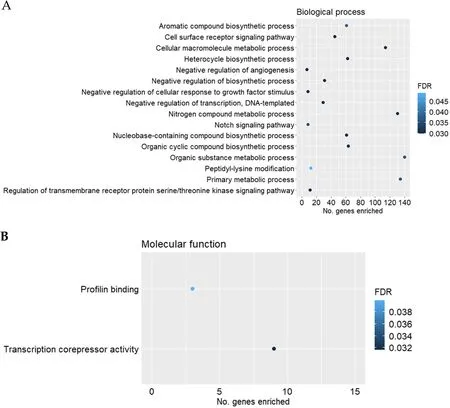
Fig.2 Scatterplots of gene ontology(GO)terms significantly enriched(FDR ≤0.05)by the upregulated genes for the comparison of heat stress(HS,n=17)vs.control(CON,n=10)in female fetal longissimus dorsi(LD) muscles at fetal d 60 age for the GO domains of A biological process,B molecular function
The comparison of HS vs.CON in females was chosen for GSEA as it was the primary focus herein.Gene set enrichment analyses identified that a total of nine pathways,including signalling by insulin receptor(FDR=0.049) and MAPK family signalling cascades(FDR=0.049),were significantly enriched in the REACTOME database for the comparison of HS vs.CON in females (Table 3).In addition,the GO term assembly of collagen fibrils and other multimeric structures showed a trend toward enrichment by the upregulated genes (FDR=0.08;Additional file 3:Table S3).DNA replication (FDR=0.018),adherens junction (FDR=0.013) and JAK-STAT signalling pathway (FDR=0.005) were among pathways significantly enriched (FDR<0.05) in the KEGG (Kyoto Encyclopedia of Genes and Genomes) database(Table 4).A total of 33 transcription factor binding motifs were significantly enriched for the comparison of HS vs.CON in females (Table 5).A full list of the GSEA results,including the associated genes,can be accessed in Additional file 3: Table S3.
Immunohistochemistry
Fetal LD muscles from pregnant gilts experiencing HS had decreased area density for positive CD31 staining compared to their counterparts from CON gilts (HS=0.52±0.03% vs.CON=0.68±0.03%,P=0.004,Fig.4).Female and male fetuses had a similar positive CD31 staining area density in LD muscles (Female=0.58±0.02% vs.Male=0.62±0.03%,P>0.1).The interaction between gestational temperature treatment and fetal sex for positive CD31 staining area density was not significant (P>0.1).
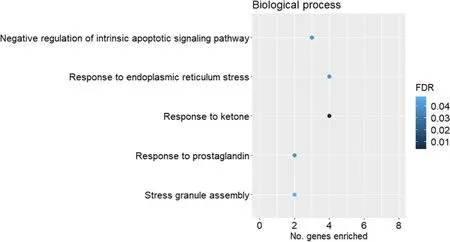
Fig.3 Scatterplots of gene ontology(GO:biological process)terms significantly enriched(FDR ≤0.05)by the downregulated genes for the comparison of heat stress(HS,n=17)vs.control(CON,n=10)in female fetal longissimus dorsi(LD) muscles at fetal d 60 age for the GO domain of biological process
Discussion
The principal findings of this study were that when pregnant gilts experienced HS during early to midgestation alterations in fetal skeletal muscle gene expression were exclusively in females.The molecular responses caused by gestational HS in the female fetus were associated with transcription repression,enhanced adipogenesis and fibrogenesis,while angiogenesis was downregulated.Moreover,muscle blood vessel density was reduced by gestational HS independent of fetal sexes.These data indicate that fetal LD muscles are susceptible to maternal HS during gestation and provide a novel explanation for the change in skeletal muscle phenotypes of the progeny born to heat-stressed sows.
An interesting finding of the present study was that fetal LD muscles showed a sex-specific response to gestational HS at the gene expression level,such that females,but not their male littermates,displayed the adaptive changes at mid-gestation when the gilts were exposed to HS.The reason for this sexually dimorphic molecular response to gestational HS is not clear.However,these findings can be potentially explained by temporal differences in the timing of muscle development and responses to prenatal stress between sexes.This is reflected by findings from the current study that males were heavier than females at the similar fetal age.Others reported that skeletal muscles of male fetuses develop greater than those of female fetuses in cattle [29],although this in utero sexual dimorphic effect remains to be verified in pigs.Postnatally though the semitendinosus muscles in female grower pigs are more susceptible to HS-induced mitochondrial damage than those in malepigs [30].Thus,the absence of gene expression changes in male muscles at mid-gestation might be due to their earlier adaptation to maternal HS,as a consequence of rapid muscle growth,hence the lack of differences at the time of sampling on d 60.Changes in fetal skeletal muscle characteristics are likely underpinned by placental insufficiency [31].We have previously demonstrated that placentae from the female fetuses exhibited a sexspecific reduction in placental efficiency (fetal/placental weight) in response to maternal HS at mid-gestation,and the placental insufficiency of those female placentae was associated with impaired nutrient transport capacity[15,17].The current data therefore reinforce and extend our previous findings by demonstrating that HS-induced placental insufficiency can be associated with altered skeletal muscle molecular responses in the fetus.
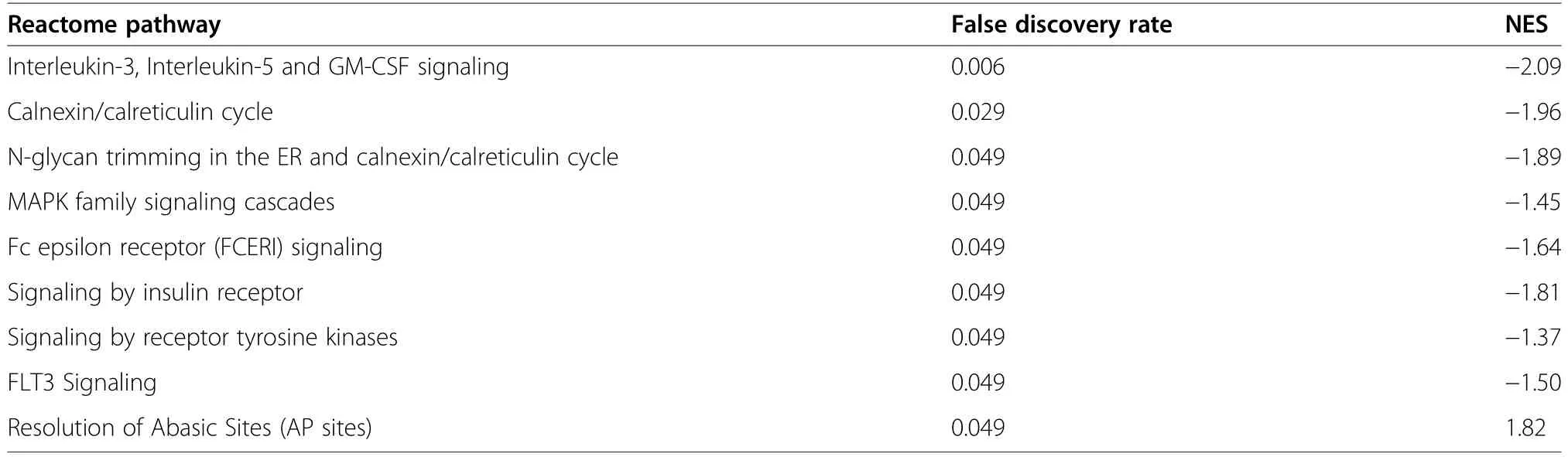
Table 3 Reactome pathways significantly enriched(false discovery rate (FDR)≤0.05)by the ranked gene list for the comparison of heat stress(HS)vs.control(CON)in female fetal longissimus dorsi(LD) muscles at fetal d 60 age
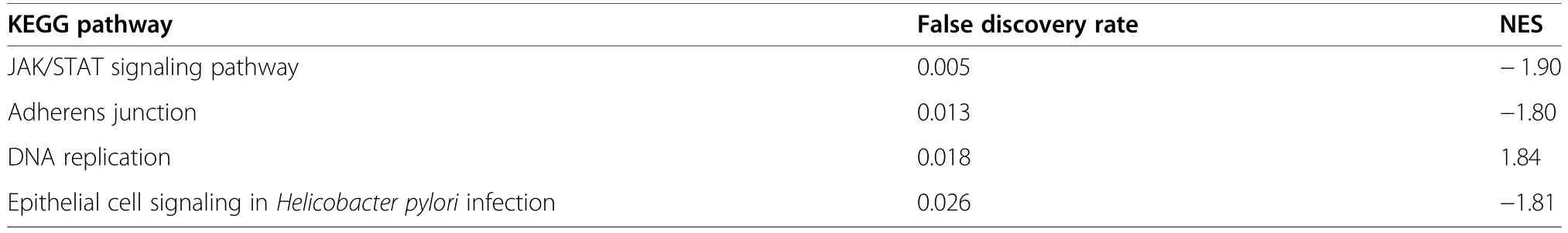
Table 4 KEGG pathways significantly enriched (false discovery rate (FDR)≤0.05)by the ranked gene list for the comparison of heat stress(HS)vs.control(CON)in female fetal longissimus dorsi (LD)muscles at fetal d 60 age
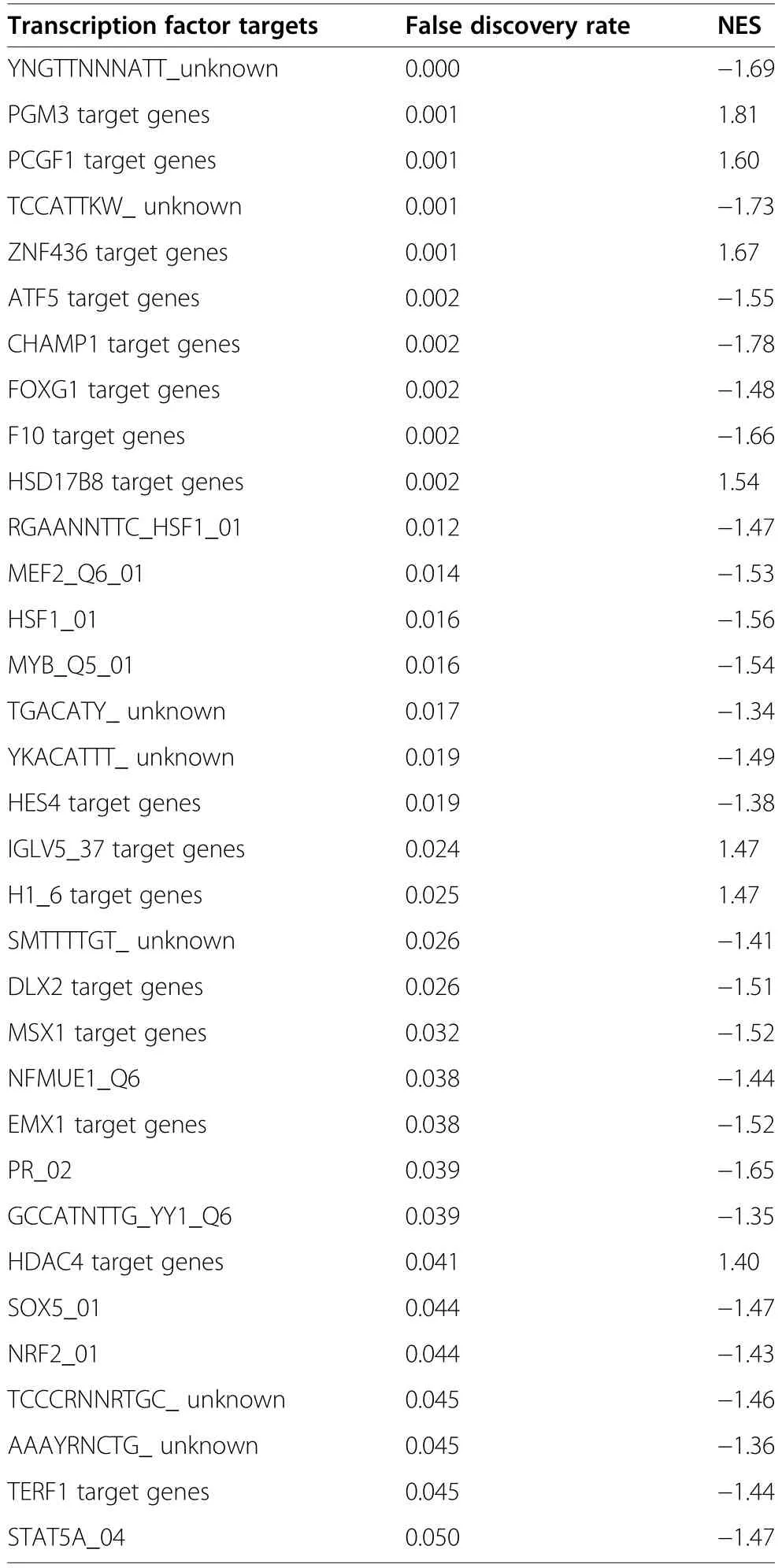
Table 5 Transcription factor prediction gene sets significantly enriched (false discovery rate (FDR)≤0.05) by the ranked gene list for the comparison of heat stress (HS)vs.control (CON)in female fetal longissimus dorsi (LD) muscles at fetal d 60 age
Notably,female LD muscles from heat-stressed pregnant gilts showed a lower capacity for gene transcription.This was evidenced by an upregulation of genes associated with transcription corepressor activity by gestational HS,suggesting transcription repression.Skeletal muscle development occurs via a balance between transcription activation and repression,and a defect in gene transcription implies developmental abnormalities [32].We have identified transcription corepressors,including metastasis associated 1 (MTA1),nuclear receptor corepressor (NCOR1),DNA methyltransferase 1 associated protein 1 (DMAP1),C-terminal binding protein 1(CTBP1) and E1A-like inhibitor of differentiation-1(EID1) among the upregulated gene lists.For example,overexpression ofNCOR1inhibits muscle cell myogenesis through directly inhibiting the activity of MyoD,the master regulator of muscle myogenesis [33].Similarly,overexpression of EID1 can inhibit skeletal muscle gene transcription by competitively binding with MyoD coactivators in skeletal muscles [34].A possible explanation for the transcription repression might be DNA methylation-induced silencing of gene expression.This is supported by the overexpression ofDMAP1,an activator of DNA methylation [35],in the HS group.The inhibition of transcription also suggests a lower accumulation of myonuclei in skeletal muscles,potentially affecting later muscle hypertrophy[36].These findings are also supported by our previous findings,showing myonuclei numbers were lower in fetal muscles from heat-stressed pregnant gilts [15].Collectively,these data show that gestational HS reduced gene expression potential in female fetuses,possibly programming cellular phenotypes.This may be associated with epigenetic modifications,and further studies are warranted to test the change in genome-wide DNA methylation in progeny skeletal muscles,due to maternal HS.
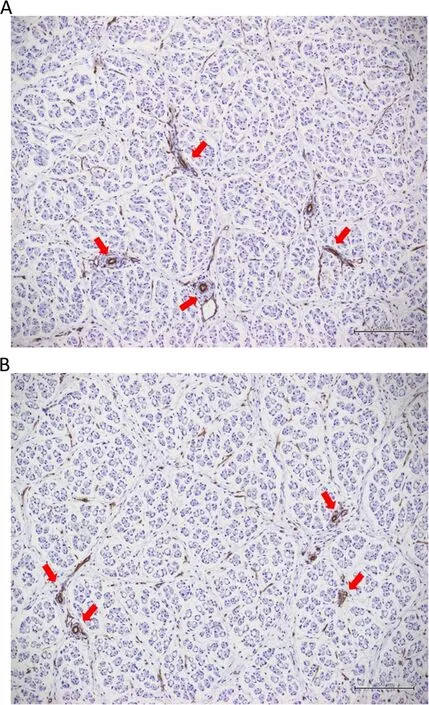
Fig.4 Photomicrographs of representative positive CD31 staining,the biomarker for blood vessel endothelium (examples indicated by red arrows),in fetal longissimus dorsi(LD)muscles of fetuses from the control(CON;A)or heat stress(HS;B)groups.CON:n=14 LD samples,HS: n=16 LD samples.Total magnification: 100×.Bars:200 μm
The present data showed that gestational HS inhibited the formation of new blood vessels in female LD muscles evidenced by a change in expression of genes that inhibited angiogenesis,while the endothelium abundance and vascularity were globally reduced in both sexes.The sexspecific change in gene expression might be related to timing of muscle adaptation between sexes as aforementioned.Furthermore,caution should be taken for reconciling the morphological and gene expression changes,because angiogenesis involves the formation of new capillaries continuing on from existing blood vessels,whereas endothelial cells line the interior of each individual blood vessel,hence are a proxy for the total area occupied by blood vessels.Therefore,these two measurements may represent different blood vessel characteristics and are not necessarily directly related or could be temporarily distinct events.For example,the initial response of LD muscles to gestational HS could be to decrease the density of blood vessels in both sexes,whilst changes in angiogenesis may be evident at d 60 only in female fetuses possibly due to greater placental insufficiency.This can be potentially explained by a lack of oestrogen (known to drive angiogenesis [37]) in female fetuses from the HS group,whilst testosterone production,which has a role in sustaining angiogenesis [38]similar to those of control fetuses,is elevated between d 40 to 60 of gestation in male fetal pigs [39].The current study design does not provide insights explaining the dichotomy between the female and male angiogenic gene expression relative to endothelial cell density in response to gestational HS on fetal LD muscles.Nevertheless,impaired angiogenesis and vascularity can further influence muscle nutrient uptake,oxidative function and muscle fibre development at later stages.
An important finding of this study was that gene expression of transcription factors involved in adipogenesis cascades was upregulated in female skeletal muscles from the HS group.Of note,fetal muscle expression of peroxisome proliferator-activated receptor (PPAR)gamma coactivator 1-beta (PPARGC1B/PGC-1β) was increased by gestational HS.ThePPARGC1Bregulates cell mitochondrial biogenesis,fatty acid oxidation and coordinates lipogenesis [40].In addition,the gene product ofPPARGC1Bcan bind and activate the function of PPARG,the essential transcription factor for adipogenesis [41].IncreasedPPARGC1B/PGC-1β expression can stimulate lipogenic gene expression and contribute to adipocyte differentiation [42,43].The PPARG mediates its function via heterodimerisation with the retinoid X receptor alpha (RXRα),as PPARs are ligand-activated transcription factors [44].In the present study,RXRα gene expression was also increased in female LD muscles from the HS group,reinforcing the upregulation of the PPARG/RXRα signalling.Furthermore,we also identified higher expression of the sterol regulatory elementbinding transcription factor 1 (SREBF1),also known as adipocyte determination and differentiation-dependent factor 1 (ADD1),in the female LD muscles from the HS group.TheSREBF1/ADD1is a potent activator of PPAR G/RXRα activity during adipocyte differentiation [45],possibly via the coactivation ofPPARGC1B[43].These data are consistent,in part,with another study showing increased gene expression of adipogenic transcription factors,includingPPARGandSREBP1C,in adipose tissues of neonatal piglets born to sows experiencing HS during late gestation and lactation [46].Taken together,these data demonstrated the change in expression of genes associated with enhanced adipogenesis cascades and altered energy metabolism in fetal LD muscles by gestational HS.Further studies need to investigate whether gene expression changes translate to protein expression in fetal skeletal muscles.Although fat deposition in fetal/newborn piglets is relatively low,it can be detected in fetal skeletal muscles at mid-gestation [47].Upregulation of adipogenic transcription factors at early developmental stages potentially programs fetal muscle growth and affects carcass traits of the offspring born to sows experiencing HS during early-mid gestation [10–12].
In addition to upregulation of adipogenic transcription factors,the current study identified increased expression of genes associated with collagen formation by gestational HS in the female fetuses,suggesting increased levels of fibrogenesis and connective tissue deposition.In particular,the genesCOL4A2andLAMA5encode subtypes of collagen IV and laminin,respectively,which are involved in the formation of basal lamina/endomysium surrounding individual muscle fibres [48].Moreover,the gene product ofPXDN,peroxidasin,mediates extracellular matrix formation and tissue fibrogenesis in myofibroblasts [49].These data imply an accumulation of endomysial connective tissues in LD muscles of female fetuses from the heat-stressed gilts.Although collagen and extracellular matrix are required to scaffold muscle fibres,interestingly,increased levels of connective tissues and fat were observed in skeletal muscles from smaller fetal pigs compared to their larger littermates [47],suggesting negative roles of increased intramuscular fat and collagen on fetal muscle development.As myocytes and adipocytes are derived from common mesenchymal stem cells [50],myogenic and adipogenic cell lineages are reported as negatively coordinated [51].Thus,the identified upregulation in gene expression of adipogenic and fibrogenic transcription factors indicates that myogenesis potential is less in those female fetal muscles by gestational HS.Future research is needed to investigate whether gestational HS shifts fetal skeletal muscles from myogenesis to fibrogenesis and adipogenesis.
The altered muscle metabolism in fetal skeletal muscles in females might be explained by fetal inflammation[52].In this study,we found that gestational HS induced low-grade pro-inflammatory responses in LD muscles,evidenced by increased gene expression of proinflammatory cytokines,including tumor necrosis factor(TNF) receptor associated factor 2 (TRAF2),interleukin-1 receptor activated kinase 1(IRAK1)and toll interacting protein (TOLLIP).For example,TRAF2is involved in TNF receptor signalling and NFkB activation,a major pathway involved in inflammatory signalling [53],whileIRAK1andTOLLIPplay a critical role in signalling transduction of interleukin-1 receptors (IL-1Rs) and tolllike receptors during inflammatory regulation [54,55].The increased inflammatory responses of the fetuses might be a consequence of an altered fetal immune system due to HS exposure in utero [56].
Conclusions
The present study provides evidence that gestational HS during early to mid-gestation alters gene expression profiles of LD muscles at mid-gestation,in female but not male fetal pigs.These data suggest female fetuses might be more adaptive,and/or there may be sexually dimorphic differences in the timing of molecular responses to gestational HS in fetal skeletal muscles.Nevertheless,gestational HS suppressed fetal LD muscle capacity for gene transcription and angiogenesis in female fetuses.Fetal muscle blood vessel density was reduced by gestational HS in both sexes.Gilt HS during gestation upregulated the expression of genes associated with fetal LD muscle adipogenesis cascades and connective tissue formation,suggesting a negative impact on skeletal muscle myogenesis.These changes might be associated with indirect evidence of low-grade inflammation.Collectively,this may have implications for later fetal and postnatal muscle growth trajectory.Future studies specifically investigating the relevant protein expression changes are necessary to provide further insights into the functional impacts of our findings.
Abbreviations
LD: Longissimus dorsi;HS: Heat stress;CON: Control;RNA-seq: RNA sequencing;FDR: False discovery rate;RIN:RNA integrity number;GO: Gene ontology;MSigDB: Molecular Signatures Database;GSEA: Gene set enrichment analyses;KEGG: Encyclopedia of Genes and Genomes;IHC: Immunohistochemistry;DEG: Differentially expressed genes;CD31: Platelet endothelial cell adhesion molecule;MTA1: Metastasis associated 1;NCOR1: Nuclear receptor corepressor 1;DMAP1: DNA methyltransferase 1 associated protein 1;CTBP1: C-terminal binding protein 1;EID1: E1A-like inhibitor of differentiation-1;PPARGC1B:Peroxisome proliferator-activated receptor(PPAR)gamma coactivator 1-beta;RXRα: Retinoid X receptor alpha;SREBF1: Sterol regulatory element-binding transcription factor 1;COL4A2: Collagen type IV alpha 2 chain;LAMA5: Laminin subunit alpha 5;PXDN: Peroxidasin;TRAF2:Tumor necrosis factor(TNF) receptor associated factor 2;IRAK1: Interleukin-1 receptor activated kinase 1;TOLLIP: Toll interacting protein;IL-1Rs: Interleukin-1 receptors.
Supplementary Information
The online version contains supplementary material available at https://doi.org/10.1186/s40104-022-00730-2.
Additional file 1: Table S1.Full list of the DEG for each pair of comparison.
Additional file 2: Table S2.Full list of significantly enriched gene ontology terms.
Additional file 3: Table S3.Full list of the GSEA results.
Acknowledgments
The authors acknowledge the support provided by Melbourne Bioinformatics,The University of Melbourne.The authors would like to thank Laura Leone from Melbourne Histology Platform,The University of Melbourne,for providing technical advice.
Authors’contributions
Conceptualization,WZ,MPG,FL,AWB,BJL,FRD and JJC;Funding acquisition,FL,BJL,FRD and JJC;Methodology,WZ,MPG,CDM,FL,GSL,AWB,BJL,FRD and JJC;Data collection,WZ,FL,and HHL;Formal analysis and bioinformatics,WZ and CDM;data curation,WZ and JJC;Writing—original draft preparation,WZ;Writing—review and editing,WZ,MPG,CDM,FL,GSL,AWB,BJL,FRD and JJC.The author(s)read and approved the final manuscript.
Funding
This study was partially funded by Australian Pork Limited (APL;2017/2216).Weicheng Zhao was a recipient of the Postgraduate Research Scholarship and the Melbourne Research Scholarship from APL and The University of Melbourne,respectively.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The animal procedures were reviewed and approved by the Animal Ethics Committee (AEC) of the University of Melbourne,Australia (Ethics Id:1714365.2).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Author details
1School of Agriculture and Food,Faculty of Veterinary and Agricultural Sciences,The University of Melbourne,Parkville,VIC 3010,Australia.2School of BioSciences,Faculty of Science,The University of Melbourne,Parkville,VIC 3010,Australia.3Melbourne Veterinary School,Faculty of Veterinary and Agricultural Sciences,The University of Melbourne,Werribee,VIC 3030,Australia.4Rivalea Australia Pty Ltd,Corowa,NSW 2646,Australia.5Centre for Muscle Research,Department of Anatomy and Physiology,The University of Melbourne,Parkville 3010,Australia.6Department of Animal Science,Cornell University,Ithaca,NY 14853-4801,USA.7Faculty of Biological Sciences,The University of Leeds,Leeds LS2 9JT,UK.
Received:11 January 2022Accepted:8 May 2022
 Journal of Animal Science and Biotechnology2022年6期
Journal of Animal Science and Biotechnology2022年6期
- Journal of Animal Science and Biotechnology的其它文章
- Toxicological effects of nanoselenium in animals
- The health benefits of selenium in food animals:a review
- The role of extracellular vesicles in animal reproduction and diseases
- The m6A methylation regulates gonadal sex differentiation in chicken embryo
- Integrative analysis of miRNA and mRNA profiles reveals that gga-miR-106-5p inhibits adipogenesis by targeting the KLF15 gene in chickens
- Genome-wide identification of functional enhancers and their potential roles in pig breeding
