Effects of exercise-based cardiac rehabilitation on inflammatory biomarkers in patients with cardiovascular disease:a systematic review with meta-analysis
Agustin Manresa-Rocamora,Susana Lopes,Ana Fernandez,Joana Martins,Vicente Climent-Paya,Jose Manuel Sarabia,Fernando Ribeiro
1Department of Sport Sciences, Sports Research Centre, Miguel Hernández University of Elche, Elche 03202, Spain. 2Institute for Health and Biomedical Research of Alicante(ISABIAL), Alicante 03010, Spain. 3Institute of Biomedicine-iBiMED and School of Health Sciences, University of Aveiro,Aveiro 3810-193,Portugal. 4Cardiology Department,Alicante General University Hospital(HGUA),Alicante 03010,Spain.
Abstract
Keywords:coronary artery disease; cytokines; cell adhesion molecules; exercise
Introduction
Cardiovascular disease (CVD) remains one of the most prevalent conditions worldwide,presenting a steady increase in incident cases of CVD from 1990 to 2015, in most of the European Society of Cardiology member countries [1]. CVD is the leading cause of death,being responsible for approximately 45% of all deaths in these countries (20% due to ischemic heart disease) [1]. Most of the CVD deaths share the same underlying pathology-i.e.,atherosclerosis-,as they result from the most common complications of coronary artery disease (CAD) (e.g., myocardial infarction), cerebrovascular disease,peripheral artery disease(PAD), as well as chronic heart failure(CHF)[2].
Atherosclerosis is a dynamic and gradual process of endothelial dysfunction and chronic, low-grade inflammation with an autoimmune component [3-9]. The recognition of the central role of chronic low-grade vascular wall inflammation in all steps of the atherosclerotic process, positioned the inflammatory pathways as promising therapeutic targets to improve clinical outcomes and overall prognosis. Therefore, the development of cost-effective secondary prevention strategies aiming to address the underlying causes of the acute cardiovascular events is paramount to reduce cardiovascular mortality and morbidity in people living with CVD. In this regard, exercise-based cardiac rehabilitation (CR) is an established intervention to reduce mortality, hospital admission, and improve the quality of life [10-12], receiving the highest class of recommendation and level of evidence [13-15].
The clinical benefits of exercise-based CR could be partially mediated by the reduction of the inflammatory profile of patients with CVD. Exercise-based CR programmes improve the circulating levels of inflammatory biomarkers such as C-reactive protein (CRP) [16],cytokines (e.g., interleukin-6 (IL-6) and tumor necrosis factor-alfha(TNF-α)) [17], and cell adhesion molecules (e.g., vascular cell adhesion molecule-1 (VCAM-1), intercellular adhesion molecule-1(ICAM-1), and E-Selectin]) [18]. However, previous systematic reviews and meta-analyses showed contrasting findings, reporting high heterogeneity in their pooled analyses [19, 20]. These contrasting results could be related to the study design (e.g.,randomised and non-randomised studies) [21], participants’characteristics (e.g., baseline inflammatory biomarker values and body composition) [19, 22], or training variables (e.g., type of exercise, intervention length, and training frequency) [20]. Previous reviews assessing the influence of training variables on the anti-inflammatory effect of exercise in patients with CVD show some limitations; for instance, continuous variables (e.g., intervention length) were categorised, and the analyses based on the type of exercise (i.e., aerobic exercise (AE), resistance exercise (RE), and combined aerobic and resistance exercise (CE)) were not carried out or accurately reported [23-25], limiting the clinical applicability of their findings [26].
Therefore, this systematic review with meta-analysis aims to investigate the effects of exercise-based CR on the circulating levels of inflammatory biomarkers (i.e., CRP, cytokines, and cell adhesion molecules)in patients with CVD based on the type of exercise(i.e.,AE,RE, and CE). Additionally, the influence of other training variables(e.g., intervention length, training frequency, and the total number of exercise sessions) will be investigated.
Methods
We conducted and reported a systematic review of the literature and a meta-analysis following the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines [27]. The systematic review with meta-analysis protocol was prospectively registered in the PROSPERO database (CRD42020199526).
Data search and sources
Potential studies were identified via a comprehensive strategy.Electronic searches were carried out in PubMed, Scopus, and Web of Science(all databases were included)from inception to October 2022.Free-text terms based on the PIO (participants, intervention, and outcomes) strategy were used. Language restrictions were not applied during this phase and the electronic search of individual databases was adapted as necessary (the full search strategy can be found in the Supplementary Material). Additionally, the reference lists of the previous systematic reviews and meta-analyses were manually checked to identify additional studies that met the eligibility criteria for the review. The authors of the included studies were contacted to identify unpublished or ongoing studies, and studies not identified by our search strategy.
Study selection
Eligibility criteria were established according to the PICOS(participants, intervention, comparisons, outcomes, and study design)guidelines. Study selection was performed using the following inclusion criteria: (participants)male and female adult patients(≥18 years) with CAD, CHF, or PAD; (intervention) supervised,home-based,or mixed supervised and home-based interventions based on AE, RE, or CE, either alone or in addition to psychosocial and/or educational interventions; (comparison) usual care and psychosocial and/or educational interventions but not a structured exercise training programme; (outcomes) acute phase proteins (i.e., CRP and serum amyloid A), cytokines/chemokines (i.e., interleukin-1ß [IL-1ß], IL-6,TNF-α, and RANTES) and/or cell adhesion molecules (i.e., selectins,VCAM-1, and ICAM-1); (study design) randomised and non-randomised, controlled, prospective trials. Studies written in English,Spanish or Portuguese were included.The selection of studies with more than one publication was limited to only one.
Two authors (AF and JM) assessed all identified titles/abstracts for possible inclusion. The same two authors reviewed the full texts against the inclusion criteria, and disagreements were settled by consensus. In cases where consensus was not achieved, a third author(AM) assessed the study to obtain agreement.
Data extraction, coding study characteristics, and potential moderator variables
Two authors (AM and SL) coded the characteristics of the included studies using a standardised data extraction form.In cases of doubts,a third author (FR) assessed the study to obtain agreement.
The following information was extracted from the included studies:(a) study characteristics (i.e., publication year, country, study design[randomised or non-randomised], and journal); (b) participant characteristics(i.e.,CVD(CHF,CAD,or PAD),sample size,percentage of men, age, left ventricular ejection fraction (LVEF), and cardiorespiratory fitness (e.g., peak oxygen uptake or metabolic equivalent)); (c) intervention characteristics (i.e., setting (supervised,home-based,or mixed intervention)and lifestyle programme(exercise only or multicomponent)); and (d) training variables (i.e., type of exercise (AE, RE, or CE), training frequency (sessions per week),intervention length (weeks), intensity (e.g., peak oxygen uptake percentage, percentage of heart rate reserve, or percentage of one-repetition maximum), and volume (i.e., session length, sets,number of repetitions, and interval length)).
Assessment of risk of bias
Two reviewers(AF and JM)independently assessed the systematic risk of bias of each included study using the Cochrane Collaboration’s core risk of bias items [28]. Disagreements between both reviewers were settled by consensus. This tool was used to assess the following domains: random sequence generation (selection bias), allocation concealment (selection bias), blinding of outcome assessment(detection bias), incomplete outcome data (attrition bias), and selective reporting(reporting bias).Studies were classified as having a high,unclear,or low risk of bias for each item based on the descriptive judgements proposed in the Cochrane Handbook [28].
Computation of effect size and statistical analyses
The standardised mean difference (SMD) was used as the effect size(ES)index.SMD was calculated by subtracting the mean change in the control group (CG) from the mean change in the exercise group (EG)divided by the pooled standard deviation (SD) at baseline and then corrected by a factor for small samples [29]. In studies reporting data as median and range (or interquartile range), the means ± SD were estimated by the formula previously proposed by Wan et al. [30]. In studies including more than one EG with the same type of exercise(e.g., AE), and a shared CG, the sample size of the CG was split by the number of EG to avoid overinflation of the sample size [31]. In this way, several analysis units were obtained from the same study.Subgroup meta-analyses based on the CVD (i.e., CHF, CAD, and PAD)were performed for each SMD index according to the type of exercise(i.e., AE, RE, and CE) and outcome measure (e.g., CRP).Random-models effects were applied for each meta-analysis in which the weighting factor was the inverse variance, defined as the sum of the within-study and between-studies variance [32]. We established a priori that at least three analysis units must have examined the anti-inflammatory effect of exercise on a CVD sublevel (i.e., CHF,CAD, or PAD) to include this condition in meta-analysis. Later, data were pooled when at least three analysis units reported the same outcome.
Analyses comprised the calculation of the mean ES with its 95%confidence interval (CI), a heterogeneity statistical test, chi-square,and theI2index to evaluate the degree of homogeneity of the ES around the average effect[33,34].The SMD magnitude was classified as trivial (< 0.2), small (0.2-0.6), moderate (0.61-1.2), large(1.21-2.0), or very large (> 2.0) [35]. The ES was considered statistically significant atP≤0.05. Heterogeneity was classified as low, moderate, or high at 25%, 50%, and 75%, respectively.I2index values greater than 50% were considered as indicative of substantial heterogeneity[36].When recommended(or necessary),heterogeneity analyses were carried out by analysing the relationship between the ES and the categorical and continuous moderator variables using subgroup analyses and simple meta-regressions, respectively. All analyses were performed using weighted least squares and assuming mixed-effect models [37]. Subgroup analyses were performed with a minimum of three analysis units for each moderator sublevel, while meta-regressions were carried out when at least 10 studies reported the specific continuous covariate. Finally, publication bias analyses were performed using funnel plots with the trim-and-fill method for imputing missed ES [38, 39]. Publication bias analyses were performed if 10 or more studies were included in the meta-analysis[31]. All analyses were performed using STATA software (version 16.0; Stata Corp LLC, College Station, TX, USA).
Results
Study selection
Figure 1 illustrates the systematic review process. In brief, the literature search revealed a total of 7,336 records after removing duplicates. Eighty studies were considered eligible for full-text analyses, of which, 51 were included in the qualitative synthesis.Twenty-nine studies were excluded from qualitative synthesis for several reasons: publications based on the same sample/participants(n = 9), did not have a usual care CG (n = 10), did not assess inflammatory parameters (n = 8), and were not exercise intervention studies (n = 2). Finally, 11 studies were excluded from quantitative synthesis due to insufficient data (n = 6) or the results were not pooled due to the low number of included studies (n = 5).
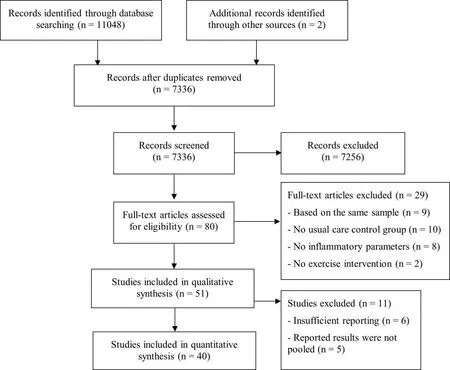
Figure 1 Flowchart of the systematic review process
Study and intervention characteristics
The characteristics of the included studies and exercise interventions are summarised in Tables 1 to 3. The studies were published from 2002 to 2021. Forty-three (84.3%) studies were randomised controlled trials and eight (15.7%) were non-randomised controlled trials.The 51 studies enrolled 3,843 patients with CVD(2107 received an exercise-based CR intervention,while 1,736 were allocated into the CG). Twenty (39.2%) studies enrolled patients with CHF (n = 1,846 patients), 28 (54.9%) were carried out with patients with CAD (n =1,822 patients), and three (5.9%) exclusively included patients with PAD (n = 175 patients). The total sample size ranged from 21 to 928 patients, with a mean ± SD age of 60.6 ± 5.1 years (min-max:49.9-75.5 years). Seven (13.7%) studies enrolled only men, 41(80.4%) included male and female patients, and three (5.9%) did not report this information.
Regarding the characteristics of the intervention,35(68.6%)studies assessed the effects of exercise training alone,while 16(31.4%)used a multicomponent CR programme including exercise training. The intervention length ranged from two to 48 weeks. Most of the interventions had a training frequency of three times per week(47.1%of the included studies), and the number of exercise sessions ranged from two to 12 sessions a week. The total number of exercise sessions performed throughout the intervention ranged from 18 to 192 sessions. Ten studies enrolled more than one EG, so a total of 63 analysis units were available for the systematic review. Fifty (79.4%)EG performed AE,three(4.8%)performed RE, and 10(15.9%)carried out CE. Forty-two (66.7%) EG performed supervised exercise sessions,seven (11.1%)carried out home-based sessions, 12(19.1%) combined supervised and home-based exercise sessions, and two (3.2%) did not report this information.
Inflammatory biomarkers
The studies that reported outcome data assessed the inflammatory profile of patients with CVD using various biomarkers. Thirty-four studies (40 analysis units) assessed CRP. Several cytokines were reported: four studies (four analysis units) determined IL-1ß; 15 studies (17 analysis units) measured TNF-α; 19 studies (22 analysis units) assessed IL-6; two studies (two analysis units) reported IL-18,four studies (five analysis units) evaluated IL-8; and seven studies(eight analysis units)assessed IL-10.The chemokine CCL5,also known as RANTES, was determined in two studies (two analysis units). Cell adhesion molecules were also assessed in many studies:12 studies(15 analysis units) evaluated VCAM-1; 12 studies (16 analysis units)reported ICAM-1; six studies (seven analysis units) assessed ESelectin;and four studies (five analysis units) assessed P-Selectin. Pooled analyses for IL-1ß, IL-18, and RANTES were not performed due to the low number of studies reporting the effect of exercise-based CR on these biomarkers.
Assessment of risk of bias
The assessment of the risk of bias of the studies included in the quantitative synthesis can be found in Table S1.Briefly,the strategy to generate the random sequence and how the allocation was performed was not explicitly reported in 20 (50%) and 30 (75%) studies,respectively. Therefore, the risk of selection bias was judged as unclear. The assessors were not blinded in 31 (78%) studies, and detection bias was judged as high risk. Furthermore, the number of patients who started and finished the experimental design, as well as the reasons for dropouts, were not explicitly disclosed in 24 (60%)studies, and attrition bias was judged as unclear risk. Finally,reporting bias was judged as low risk.
Meta-analyses Effect of the aerobic exercise-based CR on C-reactive protein.
There was a small, significant reduction in CRP levels after aerobic exercise-based CR compared with CG (k= 33 [CHF = 8; CAD = 22;PAD =3];P<0.01; SMD+= -0.33 [95% CI= -0.47, -0.20; Figure 2]. Nonetheless, moderate heterogeneity was found (P <0.01;I2=71.8%). Test for subgroup differences based on the type of CVD did not reach statistical significance (P= 0.47), and the influence of the potential moderator variables on the aerobic exercise-induced effect on CRP was analysed regardless of the CVD. Despite moderate inconsistency, there was no influence of any of the analysed moderator variables on the effect of aerobic exercise-based CR on CRP levels(P>0.05) (see Table S2).
There was no asymmetry, and no ES was imputed by the trim-and-fill method in the funnel plot for CRP (see Figure S1).Therefore, publication bias can be discarded as a threat against the validity of our findings.
Effect of the aerobic exercise-based CR on cytokines. There were non-significant differences between the two groups for TNF-α(k= 11[5; 6; 0];P= 0.15; SMD+= -0.15 [95% CI =-0.36, 0.05]) and IL-6(k= 14 [4; 10; 0];P= 0.09; SMD+= -0.13 [95% CI = -0.27,0.02]). Nonetheless, heterogeneity tests were significant with moderate inconsistency for TNF-α (P <0.01;I2= 59.5%) and low inconsistency for IL-6 (P= 0.09;I2= 37.7%). Moreover, the test for subgroup differences based on CVD was significant for TNF-α (P=0.01). The results showed a small, significant reduction in TNF-α levels after aerobic exercise-based CR compared with the CG in patients with CHF (k= 5, SMD+= -0.38 [95% CI = -0.59, -0.17]),while no differences were observed in patients with CAD (k= 6,SMD+= 0.04 [95% CI = -0.18, 0.26]). Heterogeneity tests were not significant,and no inconsistency was found in patients with CHF(P=0.50;I2= 0.0%), while low to moderate inconsistency was found in patients with CAD (P= 0.09;I2= 45.7%) (see Figure 3). Pooled analyses of the other cytokines were carried out exclusively with patients with CAD.There were non-significant changes in IL-8(k=5;P= 0.50; SMD+= -0.22 [95% CI = -0.86, 0.42]) and IL-10 (k= 7;P= 0.92; SMD+= -0.02 [95% CI = -0.40, 0.36]) after aerobic exercise-based CR compared with CG. Heterogeneity tests reached statistical significance with high inconsistency for IL-8(P <0.01;I2=89.0%) and IL-10(P <0.01;I2= 80.3%).
There was asymmetry in the funnel plot for TNF-α and IL-6. Two missed ESs were imputed for TNF-α (k= 13, SMD+= -0.07 [95% CI=-0.28, 0.14]), and one for IL-6(k=15, SMD+=-0.10[95% CI=-0.25, 0.04]) (see Figures S2 and S3). Nonetheless, the conclusions were the same as before imputing missed ESs.
Effect of the aerobic exercise-based CR on cell adhesion molecules. There was a small, significant reduction in ICAM-1 (k=13[5; 8; 0];P<0.01; SMD+= -0.38 [95% CI = -0.60, -0.16] after aerobic exercise-based CR compared with CG (Figure 4). There were non-significant changes in VCAM-1(k=12[5;7;0];P=0.07;SMD+= -0.30 [95% CI = -0.63, 0.03]). Nonetheless, heterogeneity tests reached statistical significance with moderate and high inconsistency for ICAM-1 (P <0.01;I2= 65.2%) and VCAM-1 (P <0.01;I2=83.2%), respectively. Test for subgroup differences based on the CVD type showed no differences between patients with CHF and CAD for ICAM-1 (P= 0.74) and VCAM-1 (P= 0.21). Therefore, the influence of potential moderator variables on the effect of aerobic exercise-based CR on cell adhesion molecules was analysed regardless of the CVD.
Heterogeneity analyses showed that there was no influence of any of the analysed variables on the effect of aerobic exercise-based CR on ICAM-1 (see Table S3). On the other hand, subgroup analyses for VCAM-1 showed significant between-group heterogeneity for the type of lifestyle programme (P= 0.05). Patients who exclusively received AE showed a higher effect (k= 8, SMD+= -0.48 [95% CI = -0.96,-0.01]) than those who received a multicomponent CR programme (k= 4, SMD+= 0.02 [95% CI = -0.13, 0.18]). In addition,meta-regressions showed that the reduction of VCAM-1 after AE programmes compared with CG was lower in studies which carried out longer interventions or higher number of exercise sessions (× 10)(k=12;P<0.01;B=0.053[95% CI=0.018, 0.088], andk=11;P<0.01;B= 0.270 [95% CI = 0.096, 0.445], respectively) (see Table S4).
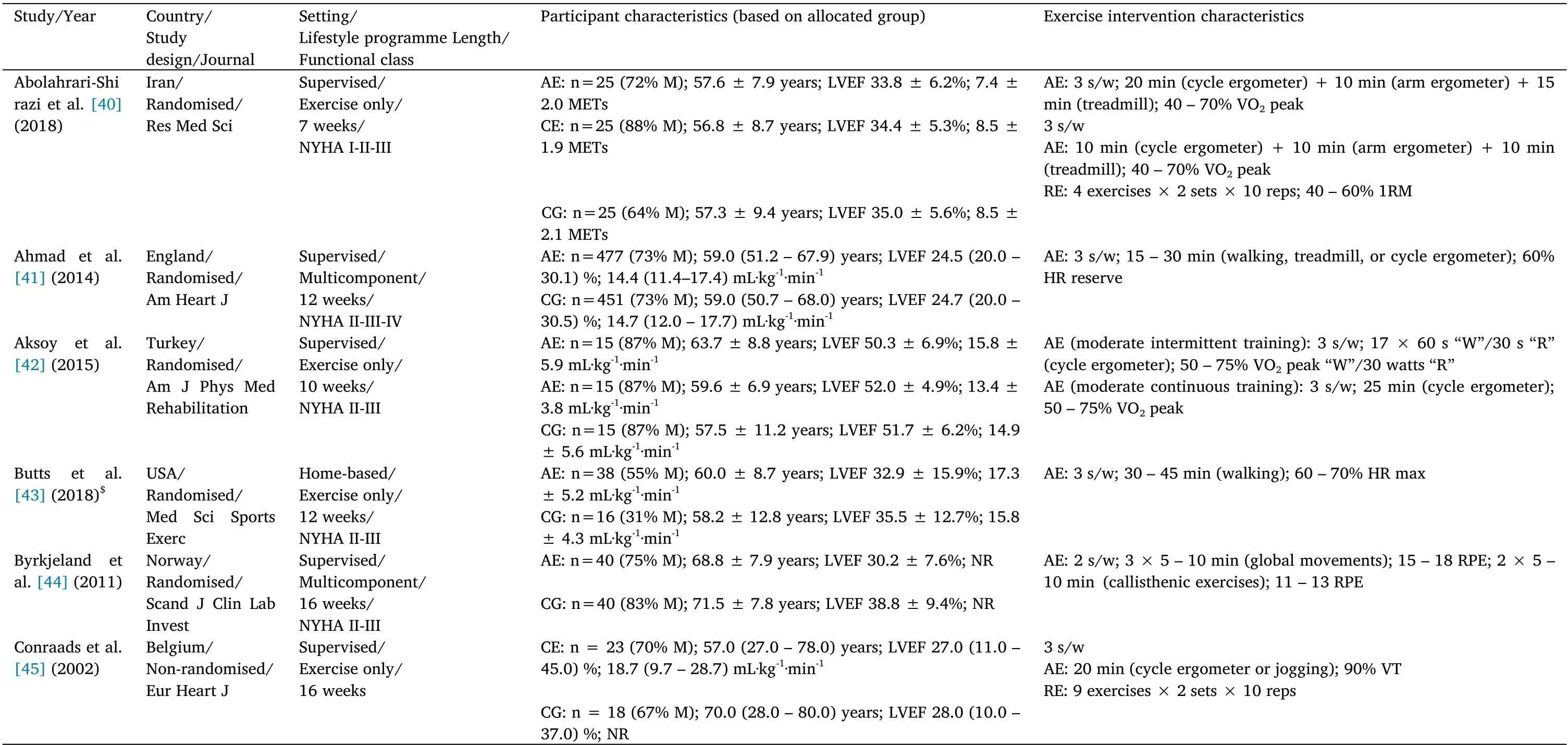
Table 1 Study, participant,and intervention characteristics in studies with chronic heart failure patients

Table 1 Study, participant,and intervention characteristics in studies with chronic heart failure patients (Continued)

Table 1 Study,participant, and intervention characteristics in studies with chronic heart failure patients (Continued)
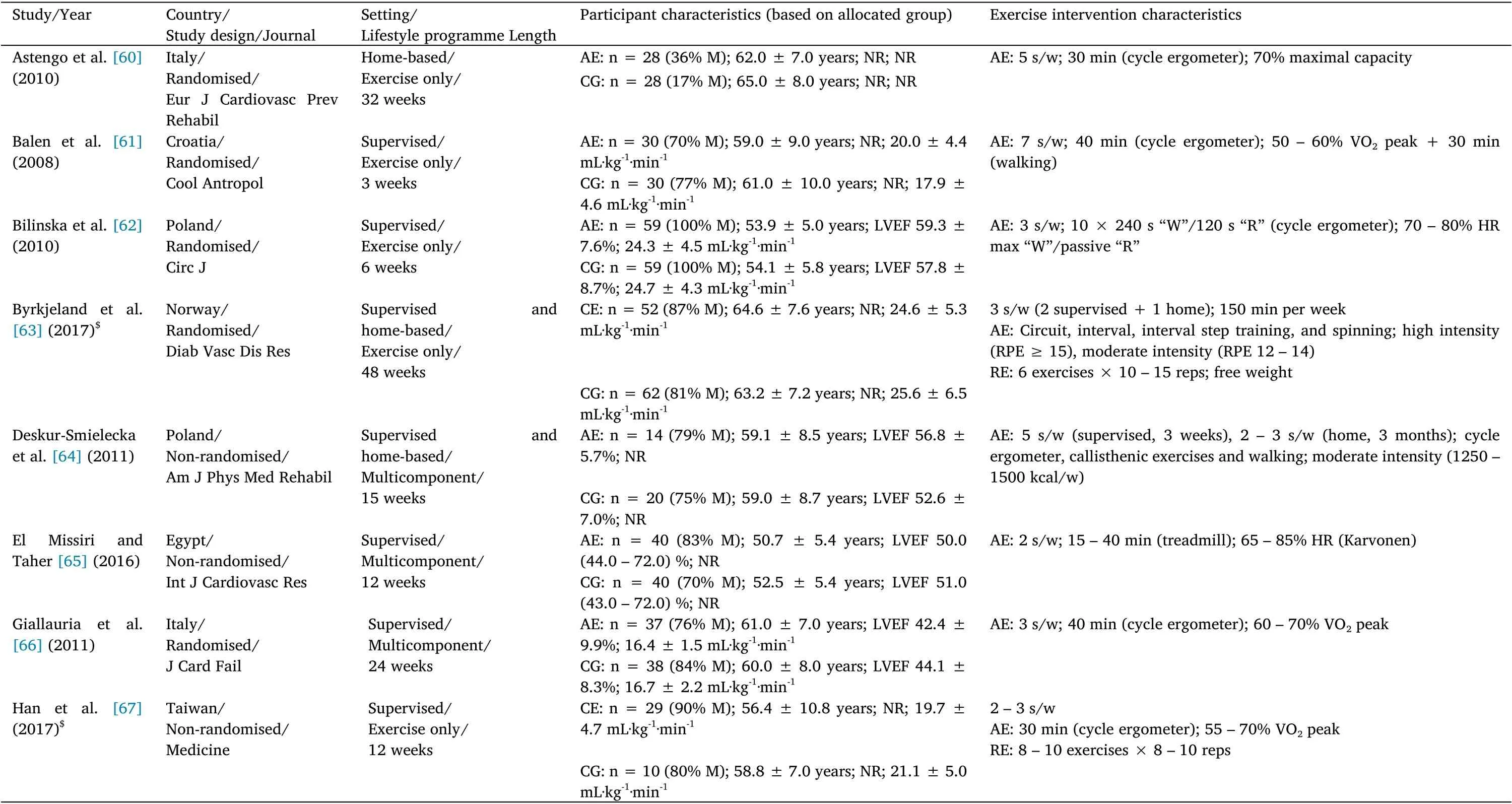
Table 2 Study,participant, and intervention characteristics in studies with coronary artery disease patients
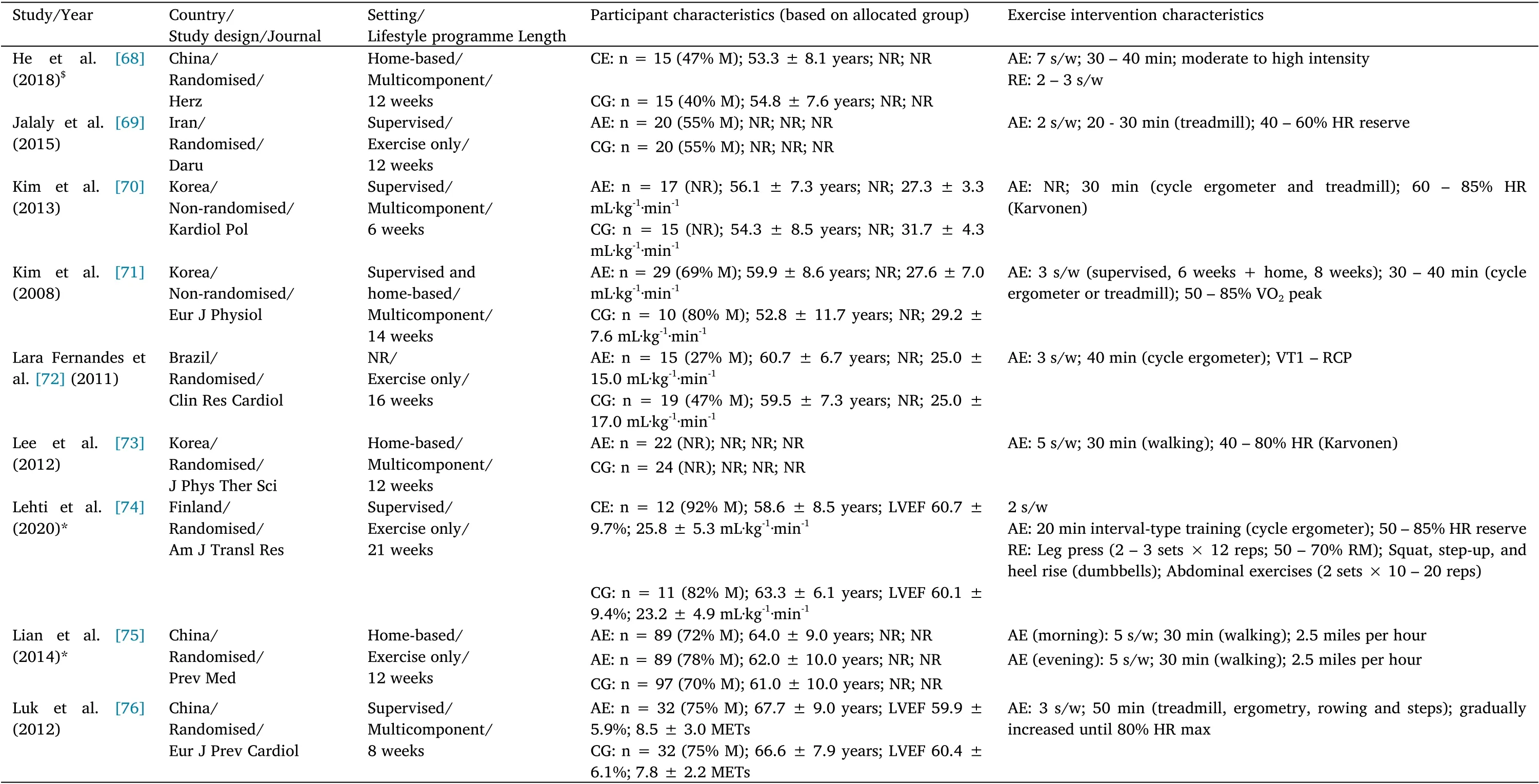
Table 2 Study,participant, and intervention characteristics in studies with coronary artery disease patients(Continued)
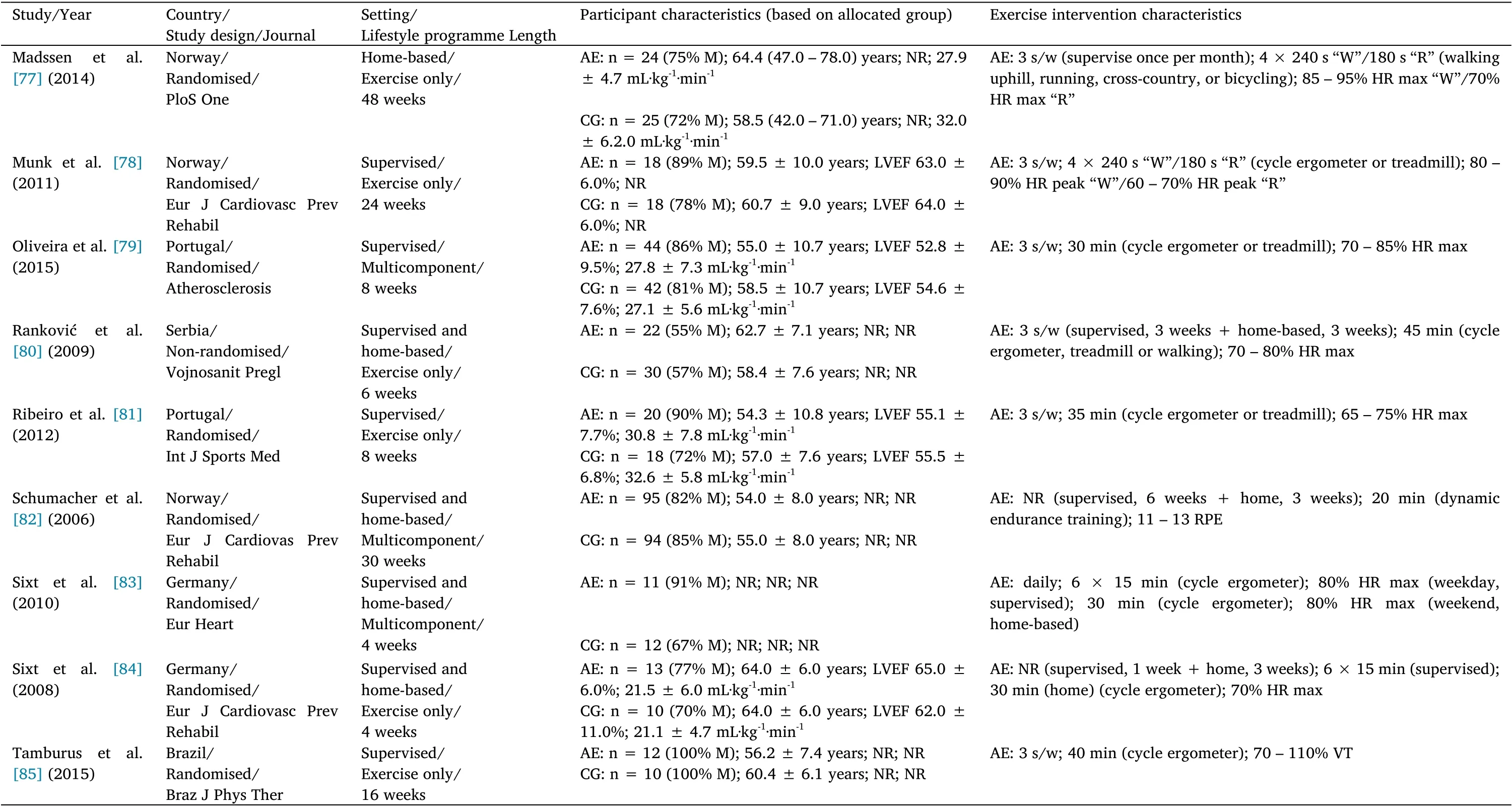
Table 2 Study,participant, and intervention characteristics in studies with coronary artery disease patients(Continued)

Table 2 Study,participant, and intervention characteristics in studies with coronary artery disease patients(Continued)

Table 3 Study,participant, and intervention characteristics in studies with peripheral artery disease patients
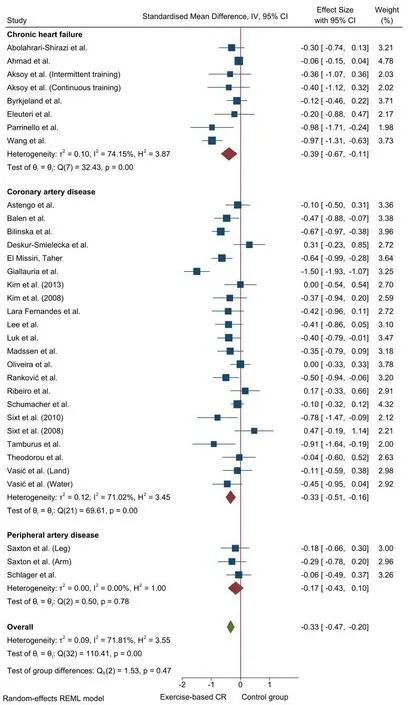
Figure 2 Forest plot for the effect of aerobic exercise-based cardiac rehabilitation on C-reactive protein (CRP)
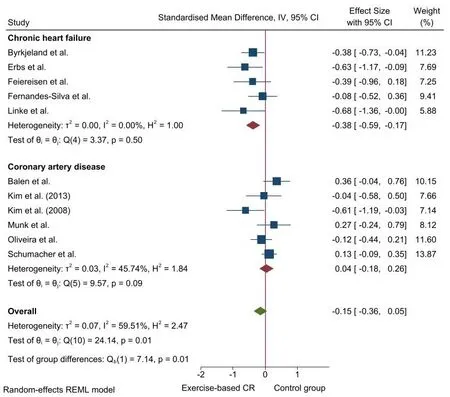
Figure 3 Forest plot for the effect of aerobic exercise-based cardiac rehabilitation on tumor necrosis factor-alpha (TNF-α)

Figure 4 Forest plot for the effect of aerobic exercise-based cardiac rehabilitation on intercellular adhesion molecule-1 (ICAM-1)
Despite a slight asymmetry in the funnel plot for ICAM-1,no ES was imputed for this outcome (see Figure S4). On the other hand, there was asymmetry with four missed ESs in the funnel plot for VCAM-1(k= 16, SMD+= -0.04 [95% CI = -0.40, 0.31]) (see Figure S5). The effect of AE compared with CG on VCAM-1 diminished from small to trivial magnitude.
Pooled analyses of E-Selectin and P-Selectin were carried out exclusively with studies which included patients with CAD. There was no difference between the two groups for E-Selectin (k=3;P=0.20;SMD+= -0.34 [95% CI = -0.86, 0.18]) and P-Selectin (k= 4;P=0.56; SMD+= 0.09 [95% CI = -0.22, 0.41]). The heterogeneity test reached statistical significance with high inconsistency for E-Selectin(P <0.01;I2= 78.5%), while there was moderate inconsistency for P-Selectin (P= 0.06;I2= 57.0%).
Effect of the resistance exercise-based CR on inflammatory markers. Due to the low number of studies included based on the CVD (lower than three), pooled analyses to determine the effect of resistance exercise-based CR on inflammatory biomarkers were not performed.
Effect of combined aerobic and resistance exercise-based CR on cytokines. Pooled analyses for the effect of combined exercise-based CR on cytokines (i.e., TNF-α and IL-6) were carried out exclusively with studies including patients with CHF. There was a non-significant reduction in TNF-α and IL-6 levels after combined exercise-based CR compared with CG in patients with CHF (k= 4;P= 0.11; SMD+=-0.28 [95% CI = -0.63, 0.06], andk= 4;P= 0.31; SMD+= -0.20[95% CI = -0.57, 0.18]). Despite no statistical heterogeneity, there were moderate inconsistencies for TNF-α (P= 0.14;I2= 45.4%) and IL-6(P=0.09;I2= 53.9%). The summary of pooled findings and the results of individual analysis units excluded from meta-analysis can be found in Tables S5 and S6,respectively.
Discussion
This systematic review with meta-analysis aimed to i) determine the effects of CR programmes based on AE, RE, or CE on the circulating levels of inflammatory biomarkers (i.e., acute phase proteins,cytokines, and cell adhesion molecules) in patients with CVD (i.e.,CHF, CAD, or PAD), and ii) analyse the influence of potential moderator variables on the anti-inflammatory effect of exercise-based CR programmes. The main results are as follows: i) the great majority of studies included in the systematic review assessed the effect of aerobic exercise-based CR on inflammatory biomarkers in patients with CAD or CHF;ii)CRP,TNF-α,IL-6,VCAM-1,and ICAM-1 were the main reported biomarkers; iii) aerobic exercise-based CR diminished the levels of CRP in patients with CVD, as well as the levels of TNF-α in patients with CHF; and iv) the effect of aerobic exercise-based CR on VCAM-1 was greater in studies performing exclusively an exercise-based CR programme and in studies with a lower intervention length/number of exercise sessions.
Collectively, our results support that aerobic exercise-based CR is a non-pharmacological intervention able to decrease the circulating levels of CRP in patients with CVD, despite high heterogeneity. These results agree with previous evidence [20, 22-24]. This is of major importance since CRP changes seem to provide the highest evidence about the training-induced effect on inflammatory biomarkers [19,91]. Moreover, our findings also support previous evidence regarding a minimally favourable training-induced effect on the circulating levels of cytokines and cell adhesion molecules [24, 25].
The anti-inflammatory effect of exercise training may partially explain the reduction of the mortality risk in patients with CVD after an exercise-based CR programme [92-96]. Exercise training increases the tone and modulation of the parasympathetic nervous system branch of the autonomous nervous system (i.e., parasympathetic activity), which is normally assessed by heart rate recovery and heart rate variability,respectively[97,98].The anti-inflammatory response is under tonic parasympathetic control (i.e., cholinergic anti-inflammatory pathway), therefore, increased parasympathetic activity after exercise training enhances the anti-inflammatory response. This induces macrophage deactivation and decreased the concentrations of acute-phase proteins, cytokines, and cell adhesion molecules [99]. Several other mechanisms have been proposed to enlighten the decrease in acute-phase proteins (e.g., CRP) and cytokines levels induced by exercise training alone or as part of a CR programme. For instance, the exercise-induced reduction of central obesity with a consequent decrease in the adipocyte production of inflammatory cytokines; the reduction of inflammatory mediators releases the skeletal muscle and mononuclear cells; the improvement of endothelial function with a subsequent reduction of pro-inflammatory cytokines released by the endothelial cells; or the reduction of oxidative stress with a positive impact on LDL oxidation and monocyte activation [5].
As pointed out previously, the inconsistent results of previous meta-analyses (i.e., high heterogeneity) [20, 22-24] could be related to the influence of potential moderator variables (e.g., type of exercise) on the anti-inflammatory effect of exercise-based CR. We hypothesized that this inconsistency could be due to the type of exercise.Yet,in our study,the type of exercise did not seem to explain the high heterogeneity. When pooling AE studies alone or CE studies the heterogeneity remained high (see Table S5). Moreover, a low number of studies analysed the effect of RE alone on inflammatory biomarkers, preventing us to carry out pooled analyses.
Although there was the moderate heterogeneity,no influence of any of the analysed moderator variables on the effect of AE on CRP was found. Swardfager et al. [19] in their meta-analysis of patients with CAD, found that studies with higher baseline CRP values or worse baseline lipid profile showed a greater reduction in CRP. Similarly,Fedewa et al. [22] reported higher CRP reductions in studies with lower body mass index and low-fat percentage participants.Nonetheless, it should be noted that these meta-analyses, as well as the current study, were carried out based on the aggregated information. There is evidence that spurious relationships can be found between participant characteristics and the training-induced effect when meta-regressions are performed at study level (i.e.,aggregated data) [100]. Therefore, we chose not to analyse the influence of patient characteristics on the anti-inflammatory effect of exercise. Moreover, participant characteristics (e.g., baseline CRP values) were dichotomised before performing heterogeneity analyses in these previous studies, which is not considered a good practice[26]. Likewise our findings, Swardfager et al. [19] and Hammonds et al. [23] did not find any influence of training variables (i.e., training frequency, session duration, and intervention length) on the effect of exercise-based CR on the circulating levels of CRP. Also, Fedewa et al.[22] carried out heterogeneity analyses using meta-regressions and reported no influence of the training frequency, session duration, and intervention length on the effects of exercise training on the levels of CRP.
We observed a significant reduction in the levels of TNF-α in patients with CHF, but not in patients with CAD, after aerobic exercise-based CR. These results are similar to those reported by Pearson et al. [25]. Interestingly, heterogeneity analyses showed that the effect of aerobic exercise-based CR on the levels of VCAM-1 was higher in studies with shorter intervention length or with a lower number of exercise sessions. A previous study [20]also suggested that the effect of exercise-based CR on the levels of CRP is higher in studies lasting 3 weeks or less. A possible explanation for these findings may be related to the progression of the exercise dose during the intervention. There is mounting evidence showing that proper management of the training variables is essential to reach long-term adaptations [101]. Nonetheless, it seems that training principles (e.g.,progression) have not been properly applied in several studies which analysed the effect of exercise-based CR [102]. Therefore, we hypothesise that the stagnation or mitigation of the anti-inflammatory effect of long-term exercise interventions could be related to unoptimised management of the training variables throughout exercise-based CR programmes.
On the other hand, combined exercise-based CR presented no effect on TNF-α and IL-6 in patients with CHF. However, this result should
be read with caution due to the small number of studies (n = 4) and the moderate to high heterogeneity. It should be noted that in the present study,aerobic exercise-based CR decreased the levels of TNF-α in patients with CHF. Abolahrari-Shirazi et al. [40] found that AE enhances the levels of CRP to a greater extent than CE in patients with CHF, while Feiereisen et al. [49] indicated that the reduction in cytokines is independent of the type of exercise (i.e., AE, RE, and CE)in patients with CHF. Likewise, Theodorou et al. [86] found no influence of the type of exercise on the effect of exercise-based CR programmes on CRP levels in patients with CAD.
Strength and limitations
This is the first systematic review with meta-analysis evaluating the anti-inflammatory effect of CR based on the type of exercise (i.e., AE,RE, and CE).Moreover, heterogeneity analyses were carried out based on the original scale of the moderator variable. Nonetheless, some limitations deserve attention. Randomised and non-randomised controlled studies were included, which may have increased the heterogeneity of our findings. Moreover, even though patient characteristics seem to influence the anti-inflammatory effect of exercise-based CR,we did not include the sample characteristics in the heterogeneity analyses because aggregated information at study level was used to carry out analyses.
Conclusion
Current research seems to support the anti-inflammatory effects of exercise in patients with CVD. Aerobic exercise-based CR programmes improve the circulating levels of CRP in patients with CVD, as well as the levels of TNF-α in patients with CHF. Most of the included studies assessed the effect of aerobic exercise programmes on inflammatory biomarkers in patients with CAD or CHF, which limits findings of combined and resistance exercise alone. Also, the most common systemic biomarkers used to determine the anti-inflammatory effect of exercise training in CVD patients are CRP and circulating cytokines(e.g., IL-6). The effect of aerobic exercise-based CR on VCAM-1 was greater in studies testing an exercise training programme alone compared to a multicomponent CR programme and in studies with lower intervention length or a lower number of exercise sessions. It is important to note that the anti-inflammatory effect of exercise is observed on top of the usual care. Despite the documented benefits of exercise-based CR in patients with CVD, including improvement of endothelial dysfunction and low-grade inflammation, the exercise recommendations (e.g., type of exercise, intensity, frequency, and duration) are generic (i.e., specific exercise recommendations targeting low-grade inflammation are lacking). Further studies aiming to provide insight into the most effective exercise prescription parameters to tackle inflammation in the context of cardiac rehabilitation are clearly needed.
- TMR Non-Drug Therapy的其它文章
- Impact of resistance exercise training on physical performance of patients undergoing hemodialysis
- Active sham cupping therapy technique:identification of limitations and suggesting enhancements
- Implementation of alternative methods in the health system through evidence-based medicine
- The role of pain neuroscience education in the management of chronic musculoskeletal pain:a physiotherapeutic approach

