Concurrent alcoholic cirrhosis and malignant peritoneal mesothelioma in a patient: A case report
Liang Liu, Xiao-Yan Zhu, Wen-Jie Zong, Chuan-Lian Chu, Jing-Yu Zhu, Xing-Jie Shen
Abstract
Key Words: Malignant peritoneal mesothelioma; Abdominal distension; Ascites; Cirrhosis; Computed tomography; Case report
lNTRODUCTlON
Malignant peritoneal mesothelioma (MPM) is a rare malignancy originating from the peritoneal epithelium or mesothelium. The annual incidence of the tumor in the general population is 1-2 cases per million, and it was first reported in 1908[1]. In recent years, studies have shown that the incidence of the MPM in those with asbestos exposure is significantly higher than that in those without asbestos exposure[2]. Pathological and immunohistochemical examinations are the gold standards for its diagnosis. MPM has a hidden onset, and the clinical symptoms of patients are not typical; therefore, missed diagnosis and misdiagnosis may occur. Here, we here report a case where the clinical manifestations, such as abdominal distension and ascites and abdominal imaging findings, were consistent with alcoholic cirrhosis, due to which the diagnosis of MPM was missed.
CASE PRESENTATlON
Chief complaints
A 63-year-old man presented to our hospital with a history of abdominal distension for 20 d.
History of present illness
The patient's abdominal distension was persistent and worsened after meals. He vomited an average of 1-2 times per day, with no coffee ground vomitus. He was diagnosed with multiple hepatic cysts, alcoholic cirrhosis, and ascites by abdominal computed tomography (CT) in a community hospital. After treatment for alcoholic cirrhosis, the patient's symptoms did not improve significantly.
History of past illness
The patient had a history of schizophrenia for many years, and he denied a history of other diseases and surgery. The patient also had a history of alcohol consumption for nearly 30 years, with no history of special drug use or toxic exposure. He was diagnosed with hepatic cirrhosis by a liver biopsy 3 years ago.
Personal and family history
The patient did not report any personal and family history.
Physical examination
Physical examination on admission showed abdominal distension, full abdominal tenderness, and dullness in movement, but no splenomegaly was observed.
Laboratory examinations
Laboratory tests results were as follows: White blood cell count 7.59 × 109/L, platelet count 507 × 109/L, hemoglobin level 105 g/L, C-reactive protein 32.29 mg/L, erythrocyte sedimentation 51 mm/h, procalcitonin 0.82 ng/mL, albumin 33.9 g/L and D-dimer level of 2.15 mg/L. Exudate was detected in the examination of ascites, and the serum ascites albumin gradient level was 9.2 g/L. Results of other laboratory tests, including carcinoembryonic antigen, alpha fetoprotein, carbohydrate antigen 125, carbohydrate antigen 19-9, carbohydrate antigen 50, antin-uclear antibody, anti-mitochondrial antibody, anti dsDNA antibody, alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, gamma-glutamyl transpeptidase, coagulation function, hepatitis B surface antigen, and hepatitis C antibody were unremarkable. Malignant tumor cells were found in the exfoliated cells of ascites.
Imaging examinations
Contrast-enhanced CT scan confirmed the findings of the CT scan at the community hospital (Figure 1A). More importantly, a large amount of fluid was observed in the abdominal cavity, and the right peritoneum was irregularly thickened with nodular thickening of the greater omentum (Figure 1B).
FlNAL DlAGNOSlS
The patient then underwent exploratory laparotomy and a large amount of yellowish ascites was found in the abdominal cavity. There were extensive adhesions between the diaphragm, stomach, spleen and abdominal wall of the liver. Adhesions were severe in most of the small intestine and the mesentery was contracted. The greater omentum was pancake-shaped, multiple round masses were observed in the parietal and visceral peritoneum, with a diameter of 3-20 mm, and the right subphrenic peritoneum was thickened considerably, with an area of approximately 15 cm × 15 cm (Figure 2). Tumors over 3 mm in diameter, the partial right subphrenic peritoneum, and the greater omentum were resected. Pathologic examination showed an epithelioid MPM (Figure 3).
TREATMENT
In addition to general comprehensive treatments, the patient was administered pemetrexed in combination with cisplatin and intraperitoneal hyperthermic perfusion after surgery.
OUTCOME AND FOLLOW-UP
At present, the patient has been followed up for 11 mo, and he is in stable condition with no tumor recurrence.
DlSCUSSlON
MPM is a malignant tumor originating from mesothelial and subcutaneous cells of the abdominal cavity. Histologically, there are epithelioid, sarcomatoid, and biphasic types of MPM[3]. There is no significant difference in the degree of malignancy among the above types. Elderly men are at high risk for developing MPM. Recently, cases of MPM in young adults have also been reported[4]. MPM is a rare entity and has been linked to industrial pollutants and mineral exposure. There has been an increase in diffusion of chemicals and the incidence of cancer. The most common carcinogen associated with MPM is asbestos, with approximately 80% of cases being associated with asbestos exposure[5,6]. The pathogenesis of MPM is unknown. ABAP1mutation has been revealed in some patients with MPM by gene analyses, but it was not the only gene involving inherited predisposition to MPM[7]. MPM has insidious onset, and the most common initial symptoms of MPM are abdominal pain, abdominal distension, significant weight loss, ascites, anorexia, and night sweat[8]. Some patients have concurrent paraneoplastic syndromes associated with MPM, such as hypoglycemia, thrombocytosis, venous thrombosis, paraneoplastic liver disease, and wasting syndrome[8]. The diagnosis of MPM is difficult due to its nonspecific and vague symptoms and should be differentiated from alcoholic cirrhosis, as well as liver and pancreatic cancers. Serological tests and tumor markers are of little value, while, there is no specific imaging technique for the detection of MPM. Currently, CT, especially the contrast-enhanced CT, is widely used, with extensive and irregular thickening of the peritoneum, mesentery, and omentum accompanied by massive peritoneal effusion being the typical manifestations of MPM in a CT scan. Positron emission tomography (PET-CT) is valuable in early diagnosis, evaluation of curative effect, and judgment of distant metastasis. The prognosis of patients with MPM is poor. Tumor resection or palliative resection is preferred in the early stage. Pemetrexed combined with cisplatin is a widely accepted chemotherapy for inoperable patients. More clinical trials are needed to investigate the promising treatment of immunotherapy and targeted therapy[9].

Figure 1 Computed tomography images. A: Abdominal computed tomography revealed multiple hepatic cysts, liver cirrhosis and ascites; B: The right peritoneum was irregularly thickened.

Figure 2 The greater omentum was pancake-shaped, multiple round masses were observed in the parietal and visceral peritoneum, with a diameter of 3-20 mm and the right subphrenic peritoneum was thickened obviously, with an area of about 15 cm × 15 cm.
In the present case, the patient had a history of long-term alcohol consumption, and the CT scan showed cirrhosis and ascites, which resulted in a diagnosis of alcoholic cirrhosis in the community hospital. After the patient was transferred to our hospital due to poor treatment effect, we performed a contrast-enhanced CT examination and an ascites cytology test, which confirmed the MPM diagnosis. Therefore, a correct diagnosis of rare diseases, including MPM, is always necessary to adjust the treatments plan in a timely manner.
There are some limitations in this report. First, the relationship between alcoholic cirrhosis and MPM remains unknown. Further studies are needed to determine whether there is a correlation between the two diseases. Second, a long-term follow-up remains necessary.
CONCLUSlON
MPM is subjected to misdiagnosis and missed diagnosis because of its insidious onset. Clinicians should be aware of the disease and make a correct diagnosis to provide patients with timely and effective treatment. At the same time, further research on the pathogenesis of the MPM is urgently needed.
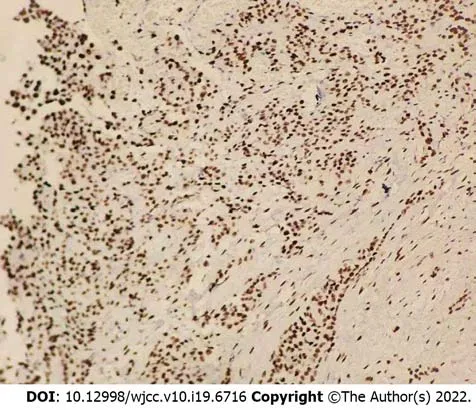
Figure 3 Pathological and immunohistochemical examinations showed an epithelioid malignant peritoneal mesothelioma.
FOOTNOTES
Author contributions:Liu L, Zhu XY, Zong WJ contributed equally to this work; Shen XJ, Zhu XY, Zong WJ, Chu CL and Liu L collected patient data; Liu L drafted the manuscript; Chu CL and Zhu JY revised the manuscript; all authors read and approved the final manuscript.
Supported byShandong Province Medical and Health Science and Technology Development Plan, No. 202003030878.
lnformed consent statement:Informed written consent was obtained from the patient for publication of this report and any accompanying images.
Conflict-of-interest statement:The authors declare that they have no conflict of interest.
CARE Checklist (2016) statement:The authors have read the CARE Checklist (2016), and the manuscript was prepared and revised according to the CARE Checklist (2016).
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:China
ORClD number:Liang Liu 0000-0002-7623-444X; Xiao-Yan Zhu 0000-0002-0026-6488; Wen-Jie Zong 0000-0001-7366-1502; Chuan-Lian Chu 0000-0001-9320-2311; Jing-Yu Zhu 0000-0002-1693-7370; Xing-Jie Shen 0000-0002-1340-7171.
S-Editor:Liu JH
L-Editor:A
P-Editor:Liu JH
 World Journal of Clinical Cases2022年19期
World Journal of Clinical Cases2022年19期
- World Journal of Clinical Cases的其它文章
- Current guidelines for Helicobacter pylori treatment in East Asia 2022: Differences among China, Japan, and South Korea
- Review of epidermal growth factor receptor-tyrosine kinase inhibitors administration to non-small-cell lung cancer patients undergoing hemodialysis
- Arteriovenous thrombotic events in a patient with advanced lung cancer following bevacizumab plus chemotherapy: A case report
- Endoscopic ultrasound radiofrequency ablation of pancreatic insulinoma in elderly patients: Three case reports
- Acute choroidal involvement in lupus nephritis: A case report and review of literature
- Choroidal thickening with serous retinal detachment in BRAF/MEK inhibitor-induced uveitis: A case report
