Plastic surgery for giant metastatic endometrioid adenocarcinoma in the abdominal wall: A case report and review of literature
Jing-Yuan Wang, Zhi-Qi Wang, Si-Chen Liang, Guang-Xue Li, Jing-Li Shi, Jian-Liu Wang
Abstract
Key Words: Abdominal metastasis; Infection; Endometrial cancer; Reconstruction; Mesh; Case report
lNTRODUCTlON
Endometrial cancer (EC) is the most common gynecological malignancy in the United States and its mortality rate is on the rise[1]. The prognosis of patients is relatively good, with an overall five year survival rate of 90.88% for patients staged as IA according to the International Obstetrics and Gynecology (FIGO) 1988 surgical classification[2]. However, the prognosis of patients with advanced disease is extremely poor, with metastasis being the main cause of death[3]. EC commonly metastasizes to the lungs, bones, liver, and brain, but abdominal wall metastasis is extremely rare[4]. Traditionally, local and distant metastatic lesions have been identified using imaging modalities such as pelvic magnetic resonance imaging (MRI), computed tomography (CT), and positron emission tomographycomputed tomography (PET-CT). As patients with metastatic EC constitute a heterogeneous group, an individualized approach is required.
Herein, we report a case of endometrioid adenocarcinoma with extensive abdominal wall metastasis complicated by infection. In this case, we highlight the design of the abdominal wall reconstruction after resection.
CASE PRESENTATlON
Chief complaints
A 65-year-old woman, gravida 2, para 1, was referred to our institution for an identifiable abdominal mass.
History of present illness
Patient’s symptom started about one month ago.
History of past illness
Gynecological history revealed that she underwent a total abdominal hysterectomy and bilateral salpingo-oophorectomy 11 years ago, and the histopathology revealed a well-differentiated endometrioid adenocarcinoma, defined as FIGO stage II. Postoperatively, external beam radiation was prescribed with a total dose of 1000 Gy in 25 fractions. The patient had no evidence of recurrence during the eight-year regular check-ups after her initial therapy until she experienced abdominal pain in the right half of her abdomen in 2018. Subsequently, a right hemicolectomy was performed. This suggested that the colon metastasis was related to her initial gynecological cancer, and histopathology showed moderately differentiated endometrioid adenocarcinoma. Molecular subtyping showed a low copy number. The patient received no adjuvant therapy postoperatively.
Personal and family history
The patient had no family history.
Physical examination
A mass located on the lower abdomen was found on physical examination, extending down to the mons pubis, up to the laparotomy incision, and outward to the skin surface. The lesion was marked by ruptured vesicles that formed a white crust on the surface (Figure 1A).
Laboratory examinations
Considering that the patient’s body temperature was 37.6℃, further investigations were carried out. The white blood cell count was 11.90 × 109/L and the percentage of neutrophils was as high as 84.3%, but no pathogens were cultured. Taken together, the huge abdominal mass showed signs of infection, and ertapenem-based antibiotics were administered immediately until the hemogram dropped to the normal range.
Imaging examinations
A pelvic MRI was performed which showed an irregular 7.3 cm × 9.9 cm × 6.7 cm mass located on the middle of the anterior abdominal wall, invading the bladder and rectum and infiltrating the rectus abdominis muscle (Figure 2A). The patient then underwent a thoraco-abdomino-pelvic CT scan, which also revealed multiple lymph node shadows in the mediastinum, with the largest one approximately 0.8 cm in diameter (Figure 2B). Subsequent PET-CT examination showed increased fluorodeoxyglucose uptake in the mediastinum and hilum, lymph nodes, two nodules under the liver capsule, and a mass on the abdominal wall, with an SUVmaxof 3.5-6.0, 10.9, and 3.9, and 21.5 respectively (Figure 3).
FlNAL DlAGNOSlS
Pathology revealed a metastatic endometrioid adenocarcinoma with poor differentiation, and all surgical margins were tumor-free (Figure 4). Molecular subtyping showed copy number high with TP53 mutation.
TREATMENT
When the patient was admitted to our department, we focused on remedies. Chemotherapy was not recommended because of tumor volume, and radiotherapy was not possible because of the infection. Finally, we decided to perform a cytoreductive operation and consulted a plastic surgeon before the surgery to discuss the approach to abdominal wall reconstruction after resection. Exploration of the abdomen and pelvis revealed that the large abdominal mass extended to the top of the bladder and reached the pubic symphysis. Radical resection of the anterior abdominal wall tumor was performed (Figure 1B), and a part of the superior surface of the bladder was removed. No other metastatic lesions were observed. At the end of the operation, the abdominal wall defect appeared as a nearly round hole of 17.0 cm × 15.0 cm, which involved the total abdominal wall thickness, and a Bard composite mesh (20.3 cm × 15.2 cm in size) was used to reconstruct the abdominal wall (Figure 1C). A local flap, measuring 19.0 cm × 17.0 cm, was made to help close the resultant defect of the external covering of the abdomen, measuring 15.0 cm × 13.0 cm (Figure 1D). The wound healed well, and the sutures were removed two weeks after the operation.
OUTCOME AND FOLLOW-UP
The patient began chemotherapy four weeks after surgery with paclitaxel and carboplatin.
DlSCUSSlON
The abdominal wall is an unusual location for metastasis of internal malignancies and abdominal metastasis from gastrointestinal or genitourinary malignancies account for only 1%-3% of cases[5]. Therefore, metastatic spread to the abdominal wall is a relatively rare event in early-stage EC. In recent years, with the wide application of laparoscopic technology, increasing port site metastasis has been identified, with an estimated occurrence of 0.18%-0.33% in early-stage EC[6-10].
Although the pathophysiology is not fully understood, distant metastasis in any solid tumor is traditionally considered to be related to lymphatic and hematogenous dissemination. Therefore, the anterior abdominal wall, with an abundant arterial supply, anastomotic venous network, and lymphatic system that drains cranially and caudally to several lymphatic chains, including pelvic and para-aortic lymph nodes, provides a favorable condition for metastases[11]. Other mechanisms have been postulated, including direct extension and spread through embryonic remnants[5,12]. Moreover, when the metastases are connected to the surgical incision, whether the surgical approach is laparotomy or laparoscopy, one of the potential explanations may be that tumor cells penetrate the uterine wall or the
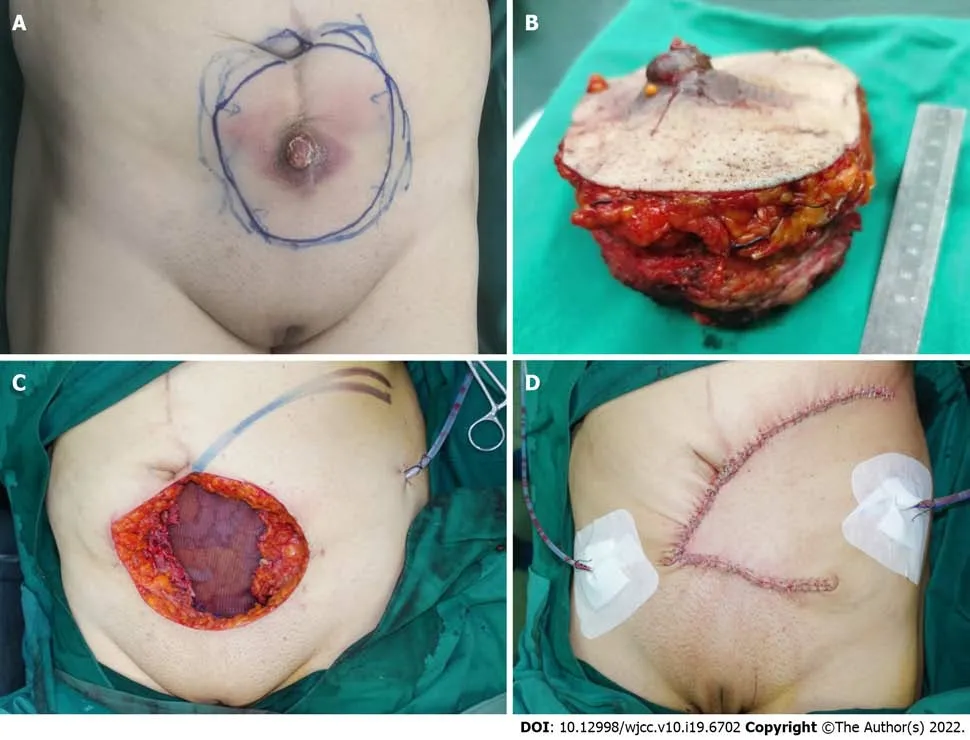
Figure 1 Radical resection of the anterior abdominal wall tumor was performed. A: A mass located on the lower abdomen with infection and the surface was covered with a white crust; B: The large defect after radical resection of the anterior abdominal wall tumor; C: The reconstructed abdominal wall with a Bard composite mesh and the local flap designed to help close the external covering of abdomen; D: Appearance of the abdominal reconstruction after surgery immediately.

Figure 2 Computed tomography images. A: A pelvic magnetic resonance imaging showed a huge mass located on the middle of the anterior abdominal wall; B: The computed tomography showed the mass on the anterior abdominal wall invading the bladder.
fallopian tube to disseminate intraperitoneally to the recent trauma, and hypothesis is that malignant cells spill through the cervical os during hysterectomy and implant directly in the incision[13]. It has been proposed that when a laparoscopic procedure is performed, high intra-abdominal pressure and implantation at the port site during specimen retrieval may contribute to the dissemination of tumor cells to the abdominal wall. All of these mechanisms may be related to the spread of neoplastic cells to the soft tissue of the abdominal wall; however, in our case, the abdominal wall metastasis of early stage endometrioid adenocarcinoma may be mostly related to local implantation of the free malignant cells shed during the surgery, considering that the patient underwent two laparotomies.
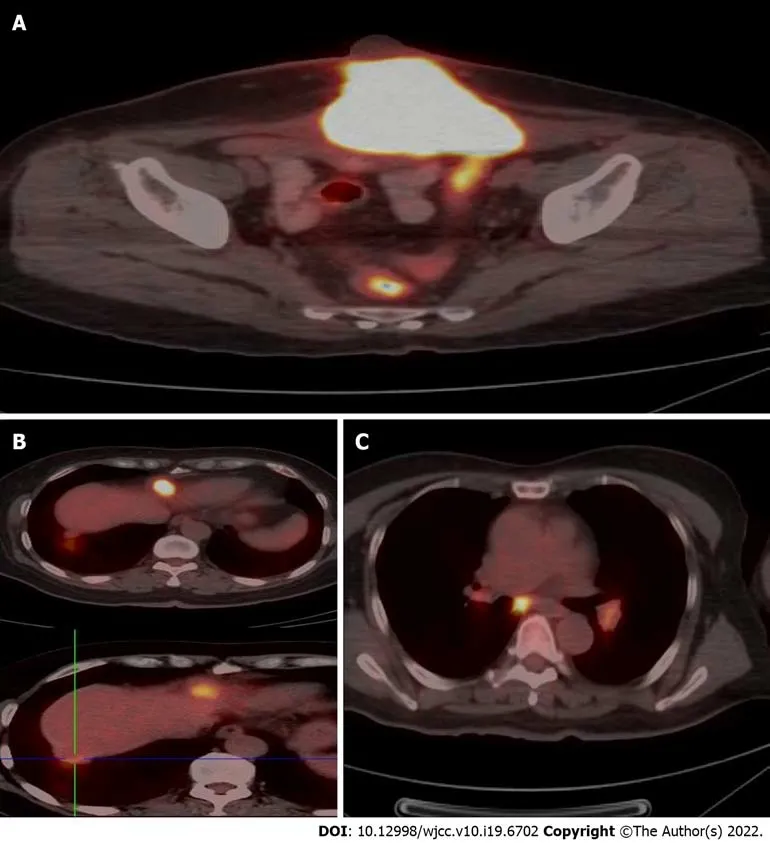
Figure 3 Positron emission tomography-computed tomography examination. A: Increased fluorodeoxyglucose (FDG) uptake in the mass on the abdominal wall, with an SUVmax of 21.5 on the fusion images; B: Increased FDG uptake in two nodules under the liver capsule, with an SUVmax of 10.9 and 3.9 respectively on the fusion images; C: Increased FDG uptake in mediastinum and hilum lymph nodes with an SUVmax of 3.5-6.0 on the fusion images.
As patients with metastatic EC constitute a heterogeneous group, an individualized approach is required. First, a comprehensive examination is necessary for patients with abdominal wall recurrence to rule out other metastases. An enlarged excision can be performed if there is no distant disease[11]. The surgical procedure consists of an enlarged excision of the tumor and reconstruction of the abdominal wall. Reconstruction poses a great challenge for multidisciplinary cooperation of gynecologists and plastic surgeons[14]. Advances in technology and the plethora of bioprosthetic or engineered tissues have provided more possibilities for doctors and patients[15-17]. A composite mesh is worthy of recommendation because they simplify the surgical treatment of large abdominal wall defects; otherwise, these defects would need further abdominal reconstruction by complex plastic surgery, which may increase morbidity[18]. The nature of the composite mesh makes it prone to providing lasting abdominal support through its safe placement directly onto the bowel, resistance to degradationin vitroand excellent biocompatibility[19,20]. Trauma injuries and tumor resection are the most common causes of large abdominal wall defects[16,21]. Complete resection of abdominal wall lesions is feasible, but preoperative assessment of the defect is the primary consideration for appropriate abdominal reconstruction[22]. Direct closure was performed if the defect was localized. However, it is impossible to advance or rotate the flaps obtained from the area around the defect for construction when the abdominal wall cannot be pulled back to its original position with excessive tension[23]. Therefore, it is highly recommended that a plastic surgeon be consulted before surgery to discuss how to reconstruct the abdominal wall after resection. In addition to covering the defect, the purpose of reconstruction is to prevent visceral eventration and acquire an acceptable cosmetic appearance[24,25]. In our case, the patient underwent extensive full-thickness resection of the abdominal wall tumor, hence, a reconstruction was required. A Bard composite mesh was placed into the abdominal defect area and sutured with 2/0 polypropylene sutures with a sufficient margin. Abdominal reconstruction was then performed by suturing the mesh to the muscular layers of the abdominal wall, and the flaps obtained from the area around the defect were advanced and rotated to achieve soft tissue coverage. Finally, one subcutaneous drain and one pelvic drain were placed.
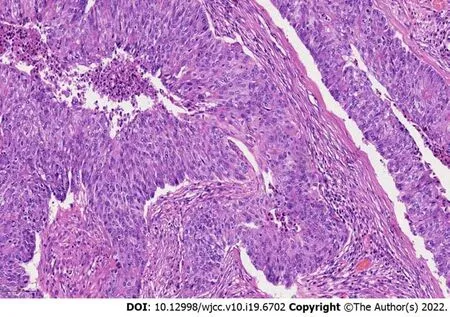
Figure 4 Hemotoxylin and eosin staining of endometrioid carcinoma, × 200 magnification.
Surgical resection followed by adjuvant therapy may improve patient survival. However, palliative treatment alone is feasible in some circumstances. Previous studies have shown that radiotherapy for isolated abdominal metastases is associated with high local control rates in EC[26]. Chemotherapy, with paclitaxel combined with carboplatin or cisplatin, may also be frequently used for recurrent EC, especially in cases with unresectable lesions or distant metastases. Hormonal therapy can also be used. A phase II study evaluated the efficacy of everolimus, a mammalian target of rapamycin inhibitor, in combination with letrozole in patients with recurrent EC. The results were encouraging, with a clinical benefit rate of 40% and a confirmed objective response rate of 32%[27]. Data from a recent phase II study evaluating the same combination with metformin showed satisfactory results in patients with recurrent endometrioid endometrial cancer, with a clinical benefit rate of 50% and a confirmed objective response rate of 28%[28]. Considering the large size of the abdominal wall metastasis in our case, surgical resection may have been preferred, followed by chemotherapy.
Additionally, patients with recurrent EC should consider molecular subtyping to guide additional systemic therapy options. It is also recommended for recurrent EC to detect NTRK gene fusions, which are oncogenic drivers of various adult and pediatric tumor types[29]. It has been reported that the application of a first-generation TRK inhibitor, such as larotrectinib or entrectinib, in patients with NTRK fusion-positive cancers achieved satisfactory results with high response rates (> 75%), regardless of tumor histology[30]. Distant metastasis of the abdominal wall in our case was staged as FIGO II; therefore, there may be high-risk factors for molecular subtyping. The results showed copy number high with TP53 mutation and negative NTRK fusion, which may imply a poor prognosis.
CONCLUSlON
When patients with EC develop large abdominal wall metastasis with infection, an individualized approach is proposed. A plastic surgeon should be included in the planning before contemplating resection of large tumors.
FOOTNOTES
Author contributions:Wang JY participated in the management of the patient and wrote the manuscript; Wang ZQ participated in the medical management of the patient; Liang SC and Wang JL were involved in the surgical management of the patient; Li GX carried out plastic surgery; Shi JL performed immunohistochemistry and contributed to pathological characterization.
Supported byThis work was supported by the National Key Technology R&D Program of China, No. 2019YFC1005200, and No. 2019YFC1005201; the Natural Science Foundation of Beijing, No. 7202213; and the National Natural Science Foundation of China; No. 82072861, 81672571, and 81874108.
lnformed consent statement:All study participants, or their legal guardian, provided informed written consent prior to study enrollment.
Conflict-of-interest statement:The authors declare that they have no conflict of interest.
CARE Checklist (2016) statement:The authors have read the CARE Checklist (2016), and the manuscript was prepared and revised according to the CARE Checklist (2016).
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:China
ORClD number:Jing-Yuan Wang 0000-0001-6285-400X; Zhi-Qi Wang 0000-0002-5656-1072; Si-Chen Liang 0000-0002-0108-2322; Guang-Xue Li 0000-0002-2084-1152; Jing-Li Shi 0000-0002-4813-6527; Jian-Liu Wang 0000-0001-8932-317X.
S-Editor:Liu JH
L-Editor:A
P-Editor:Liu JH
 World Journal of Clinical Cases2022年19期
World Journal of Clinical Cases2022年19期
- World Journal of Clinical Cases的其它文章
- Current guidelines for Helicobacter pylori treatment in East Asia 2022: Differences among China, Japan, and South Korea
- Review of epidermal growth factor receptor-tyrosine kinase inhibitors administration to non-small-cell lung cancer patients undergoing hemodialysis
- Arteriovenous thrombotic events in a patient with advanced lung cancer following bevacizumab plus chemotherapy: A case report
- Endoscopic ultrasound radiofrequency ablation of pancreatic insulinoma in elderly patients: Three case reports
- Acute choroidal involvement in lupus nephritis: A case report and review of literature
- Choroidal thickening with serous retinal detachment in BRAF/MEK inhibitor-induced uveitis: A case report
