Meta-analysis of the efficacy and safety of Shenkang injection combined with Western medicine in the treatment of chronic glomerulonephritis
Yang Liu ,Yue Ji ,Zi-Xuan Zhao ,Lu-Xuan Guo ,Jin Lin ,Rong-Xin Zheng ,Guan-Ran Wang,Jin-Hua Si,Xin-Yuan Zhang,Na Hao*,Hong-Tao Yang*
1Nephrology Department,First Teaching Hospital of Tianjin University of Traditional Chinese Medicine,Tianjin 300381,China.2National Clinical Research Center for Chinese Medicine Acupuncture and Moxibustion,Tianjin 300381,China.3School of Integrative Medicine,Tianjin University of Traditional Chinese Medicine,Tianjin 301617,China.4Psychosomatic Department,First Teaching Hospital of Tianjin University of Traditional Chinese Medicine,Tianjin 300381,China.
Abstract Objective:The purpose of the research was to systematically evaluate the clinical efficacy and safety of Shenkang injection combined with Western medicine in treating chronic glomerulonephritis.Methods:Randomized controlled trials of Shenkang Injection combined with Western medicine in treating CGN were obtained from 8 Science databases including China National Knowledge Infrastructure (CNKI),China Biomedical Database(SinMed),Weipu Database (VIP),Wanfang Database (Wanfang),PubMed,The Cochrane Library,Embase,Web of Science from establishment to June 2022.Two researchers screened literature and extracted useful data according to inclusion and exclusion criteria,and used RevMan 5.3 and STATA SE 14.0 software to perform meta-analysis.Results:1,214 patients were included in 15 studies,with 607 in treatment group and 607 in control group.The results of meta-analysis showed that the treatment group could improve the clinical effective rate[RR=1.33,95%CI(1.25,1.40),P<0.00001],reduce serum creatinine[MD=-31.28,95% CI (-42.90,-19.66), P<0.00001],blood urea nitrogen [MD=-2.26,95%CI(-3.08,-1.44), P<0.00001],24-hour urine protein quantification [MD=-0.37,95% CI(-0.49,-0.25), P<0.00001],tumor necrosis factor -α [MD=-7.93,95% CI(-10.56,-5.30),P<0.00001],systolic blood pressure[MD=-10.81,95%CI(-13.66,-7.96),P<0.00001]and diastolic blood pressure [MD=-7.36,95% CI (-9.34,-5.38), P<0.00001].Conclusion:Shenkang injection combined with Western medicine can enhance the clinical effective rate of CGN and reduce the levels of serum creatinine,blood urea nitrogen,24-hour urine protein quantification,tumor necrosis factor-α,systolic blood and diastolic blood pressure.However,due to the low quality of literature,the conclusion still needs to be supported by more multi-center,large-sample studies with rigorous design and standardized implementation.
Keywords: Shenkang injection;chronic glomerulonephritis;curative effect;randomized controlled trials;meta-analysis
Introduction
Chronic glomerulonephritis (CGN),a representative chronic kidney disease (CKD),is characterized by haematuria,proteinuria,edema,and hypertension [1].In 2017,average prevalence of CKD was globally 9.1%,an increase of 29% compared with 1990 [2].CKD affects about 10% of adults worldwide,causing 1.2 million deaths per year [3].CKD,by 2040,is predicted to be the fifth leading cause of death worldwide [4].As renal function declines,patients will have different degrees of heart and lung function decline,malnutrition,psychological function and cognitive impairment,seriously affecting patients' quality of life [5,6].The studies have shown [7] that autoimmunity,infection and inflammation affect development of CGN,but specific pathogenesis and molecular mechanism of CGN remain unclear [8].Currently,antihypertensive,anticoagulant,hormonal and cytotoxic drugs are commonly used in treating CGN to alleviate severe symptoms and slow down the occurrence of renal failure.However,these treatment schemes also have disadvantages in efficacy,cost and side effects[9].More and more studies have verified the curative effect of Traditional Chinese medicine in the treatment of CGN [10,11].Shenkang injection (SKI) contains Dahuang (Rheum palmatum),Huangqi (Astragalus membranaceus),Danshen (Salvia miltiorrhiza) and Honghua (Carthamus tinctorius),which has the functions of relaxing bowel and lowering turbidity,it can invigorate qi and promote blood circulation,mainly applied in treating chronic renal failure [12-14].SKI can clear free radicals in the body,correct lipid metabolism disorder,delay the damage of renal function,inhibit protein decomposition,inhibit water and sodium retention,etc [15].SKI combined with Western medicine can significantly reduce the level of inflammatory factors such as interleukin-6 and tumor necrosis factor-α in patients,and improve the oxidative stress and inflammatory state of patients [16].Recent clinical observations have shown that SKI can significantly reduce the levels of serum creatinine,blood urea nitrogen and 24-hour urine protein quantification in CGN patients to improve clinical effective rate [17,18].At present,more and more clinical studies confirming positive effect of SKI on CGN patients have indicated clinical symptoms' improvement of CGN patients.However,there are differences in protocol design,evaluation criteria and observation indicators among different studies,and scientific evidence-based medicine methods are needed to evaluate its efficacy.Our study systematically gathered the randomized controlled trials (RCTs) of SKI combining Western medicine in treating CGN,so as to assist clinical practice in providing evidence.
Methods
This research was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) and is registered in PROSPERO (CRD42022297770).
Search strategy
We gathered RCTs of SKI combining Western medicine in treating CGN from 8 Science databases including CNKI,VIP,Wanfang,SinoMed,PubMed,Embase,the Cochrane Library,and Web of Science from establishment to June 2022.Chinese search terms were as follows: 慢性肾炎,慢性肾小球肾炎,慢性原发性肾小球疾病,肾康注射液,肾康注射剂.English search terms were as follows: Shenkang injection,chronic nephritis,chronic glomerulonephritis,etc.Keywords combined with free words were used to search.Taking CNKI and PubMed as examples,their specific search strategies were as follows.The two researchers (Yang Liu and Yue Ji) extracted data from the included literature at the same time,including the authors,publication year,patient age,intervention measures,control measures,outcome indicators,course of disease and course of treatment.We used Excel spreadsheet tools to carefully record and check each other,and the third researcher (Na Hao) would help solve any differences.
Inclusion and exclusion criteria
Inclusion criteria.(1) Study types: RCTs of SKI combined with Western medicine in the treatment of CGN.(2) Study subjects:clinically diagnosed CGN patients presented with hematuria,proteinuria,edema,and hypertension.Regardless of race,sex,age and nationality,the intra-group basis linearity was good.Diagnosis basis:Guidelines for the diagnosis and treatment of chronic glomerulonephritis[19],Internal Medicine[20],Diagnosis,Syndrome differentiation and curative effect evaluation of Chronic glomerulonephritis (trial program)[21],Nephrology[22].(3) Interventions: The control group: either treatment of alprostadil injection,ACEI or ARB (no limits on drug dosage,daily use frequency,manufacturer,etc.).The treatment group was additionally given SKI (no limits on drug dose,daily drip frequency,manufacturer,etc.),and other treatments were the same as the control group.(4) Outcome indicators: Main outcome indicators:Clinical effective rate (referring toGuiding Principles for Clinical Research of New Chinese Medicine[23],Diagnosis,Syndrome differentiation and curative effect evaluation of Chronic glomerulonephritis(trial program)[24] andSummary of discussion on diagnosis and treatment of renal disease and efficacy criteria[25]),clinical efficacy rate=The number of significant and effective cases/total number of cases× 100%.Secondary outcome indicators: serum creatinine(Scr),blood urea nitrogen (BUN),24-hour urine protein quantification(24h-UP),tumor necrosis factor-α (TNF-α),systolic blood pressure(SBP)and diastolic blood pressure(DBP).Clinical studies must include one or more of these outcomes.
Exclusion criteria.(1) Studies did not meet the correct diagnostic criteria;(2) Studies using SKI combined with other traditional Chinese medicine therapies;(3) Studies that only have abstracts and the original data cannot be fully obtained;(4)Duplicate published studies;(5) review,cases of famous doctors,case reports,animal experiments and other non-clinical RCT studies.
Study selection and data extraction
Two evaluators (Yang Liu and Yue Ji) independently screened the literature according to the inclusion and exclusion criteria,excluded obviously irrelevant literature and cross-checked the results.In case of any disagreement,a third party (Hong-Tao Yang) participated in the discussion and decided.The software of NoteExpress was used to delete duplicate literature,and further exclude non RCTs studies such as reviews,case reports and animal experiments by reading the title and abstract of the literature.Finally,we read the full text of the literature,reasonably screen the literature according to the inclusion and exclusion criteria,and determine the final included literature.
Risk of bias analyses
Two evaluators(Yang Liu and Yue Ji)assessed the literature quality of the incorporated research according to the Cochrane manual 5.1[26],including the generation of random sequences,allocation concealment,blind measures,the integrity of outcome indicators,selective reports,and other biases.
Application of statistical methods
Revman 5.3 software was used for analysis.The relative risk(RR) and mean difference (MD) were used as efficacy indicators for counting data and continuity variables.If the numerical units were inconsistent,the standardized mean difference (SMD) was used for analysis.The Chi-square test was used to test the heterogeneity of results.IfP>0.10,I2<50%,it was considered that the heterogeneity among studies was small,and the fixed effect model was used.IfP≤0.10,I2≥50%,it was considered that there was great heterogeneity among studies,and the random effect model was used,and the source of heterogeneity should be further evaluated through subgroup analysis or sensitivity analysis.We Used the random effect model after excluding significant clinical and methodological heterogeneity [27].STATA SE 14.0 software was used for sensitivity analysis to explore whether a single study impacted on the total effect size of outcome indicators.The funnel plot was used to detect publication bias (n>10) for the major outcome indicators,and publication bias was quantitatively detected by Begg's test and Egger's Test[28,29].IfP<0.05,publication bias was indicated in the outcome indicators,and publication bias was corrected by trim and fill method[30,31].
Results
Study characteristics
According to the established retrieval formula,116 pieces of related literature were retrieved,55 duplicate literature were removed.Read the title and abstract further and exclude 10 articles that do not meet the inclusion exclusion criteria.Through reading the full text,36 studies that did not meet the inclusion and exclusion criteria were excluded.Finally,15 studies [17,18,32-44] were included,all of which were Chinese literature with publication years from 2017 to 2021 (Figure 1).
The key features included in the studies are shown in Table 1.15 RCTs were included,including 1,214 patients,607 in the treatment group and 607 in the control group.Sample sizes for individual studies ranged from 54 to 120 people,and the therapy course was 12 to 120 days.The treatment group was treated with Shenkang injection combined with Western medicine,and the control group was treated with Western medicine.(Tables 1 and 2).

Table 1 Included studies'characteristics.
Bias's risk analyses
We used the Cochrane Bias risk Assessment tool to assess bias's risk in the 15 RCTs.In terms of random sequence generation,9 literature[17,18,32,34,37,38,40,43,44] were grouped by random number table method and evaluated as "low risk".6 literature [33,35,36,39,41,42] neglected the randomization method and got an "unclear".In terms of allocation concealment and blinding,only one literature[35]proposed the "double-blind" scheme,which was evaluated as "low risk",and the rest neglected allocation concealment and blinding implementation,which were evaluated as "unclear".In terms of outcome data integrity,all the included studies had good integrity with no case loss.In terms of selective reporting,none of the included studies were published selectively.Other biases were not mentioned in any of the included literature (Figure 2).
Meta-analysis results
Clinical effective rate.14 studies [17,18,32-35,37-44] reported clinical effective rate,1,160 patients were enrolled,with 580 in treatment group and 580 in control group.The results showed that there was low heterogeneity among studies (P=0.10,I2=35%),so we used the fixed effect model for the meta-analysis.The results indicated significantly higher clinical effective rate of the treatment group than the control group with statistically significant difference[RR=1.33,95% CI(1.25,1.40),P<0.00001) (Figure 3).
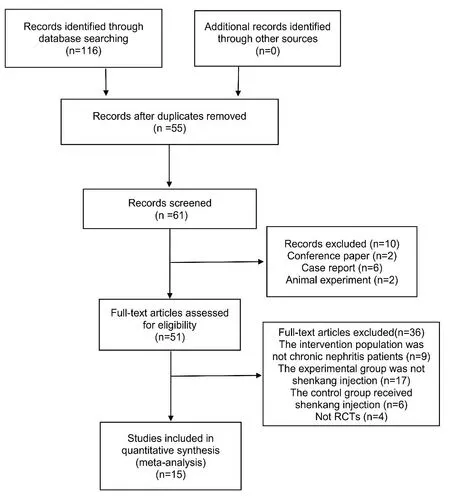
Figure 1 Literature retrieval and screening process
Serum creatinine.10 studies[17,18,32-35,37,40,43,44]reported levels of Scr before and after treatment,including a total of 834 patients,with 417 in the treatment group and 417 in the control group.The adopt random effect model was used due to the large heterogeneity among studies [P<0.00001,I2=97%].The results showed that the treatment group had more advantages in reducing the level of Scr [MD=-31.28,95% CI (-42.90,-19.66),P<0.00001].It was proved that SKI combining western medicine had more advantages in reducing the level of Scr than conventional western medicine alone (Figure 4).
We explored the source of heterogeneity through subgroup analysis.The results showed that the heterogeneity mainly came from the difference of treatment measures in the itreatment group.Conduct subgroup analysis basing different western drugs combined in the treatment group,which were divided into SKI+Alprostadil injection[18,33,35,40],SKI+Valsartan Tablets [17,34,44] and SKI +Benazepril Tablets [32,37,43].The results of heterogeneity test showed that the heterogeneity between SKI+Alprostadil injection was significantly reduced [P=0.06,I2=60%],no heterogeneity between SKI+Valsartan Tablets was seen [P=0.40,I2=0%],and the heterogeneity between SKI+Benazepril Tablets was still very high[P< 0.00001,I2=94%].The random effect model analysis indicated: SKI+Alprostadil injection [MD=-43.07,95% CI (-49.36,-36.78),P<0.00001],SKI+Valsartan Tablets[MD=-6.97,95%CI(-9.75,-4.18),P<0.00001],SKI+Benazepril Tablets[MD=-40.53,95% CI(-54.38,-26.68),P<0.00001].The subgroup analysis results confirmed statistically significant difference between treatment and control groups (P<0.05) (Figure 5).
Blood urea nitrogen.10 studies [17,18,32-35,37,40,43,44]reported changes in the level of BUN before and after treatment,involving a total of 834 patients,with 417 in treatment group and 417 in control group.We used the random effect model for analysis due to significant heterogeneity among studies [P<0.00001,I2=95%].The results of meta-analysis showed that combined SKI had more obvious advantages in reducing the level of BUN on thr basis of Western medicine alone [MD=-2.26,95% CI (-3.08,-1.44),P<0.00001] (Figure 6).
We analyzed the treatment group combined with different kinds of Western medicine to identify the source of heterogeneity.The heterogeneity test results indicated: there was no heterogeneity of SKI+Alprostadil injection [18,33,35,40] [P=0.45,I2=0%],the heterogeneity of SKI+Valsartan Tablets [17,34,44] was also significantly reduced [P=0.08,I2=60%],there was no heterogeneity among SKI+Benazepril Tablets [32,37,43] [P=0.94,I2=0%],the adopt random effect model for analysis:SKI+Alprostadil injection [MD=-2.11,95% CI (-2.38,-1.83),P<0.00001],SKI+Valsartan Tablets [MD=-0.66,95% CI (-1.09,-0.22),P=0.003],SKI+Benazepril Tablets [MD=-4.17,95% CI(-4.61,-3.72),P<0.00001].It shows that there were significant differences between the treatment group and the control group in reducing the level of BUN (P<0.05) (Figure 7).
24-hour urine protein quantification.11 studies[17,18,32-34,36,37,41-44] reported the changes of the level of 24h-UP before and after SKI combining Western medicine in treating CGN,involving 878 patients,with 439 in treatment group and 439 in control group.The significant heterogeneity among the studies was still high after subgroup analysis based on different interventions and treatment courses in the treatment group.After eliminating individual studies one by one,the heterogeneity within the group has scarcely altered,and the confidence intervals are located on the left side of the invalid line of the forest map,demonstrating that the heterogeneity between studies has no impact on the results,so we adopted the random effect model,which showed that the treatment group was more advantageous in reducing the level of 24h-UP [MD=-0.37,95% CI(-0.49,-0.25),P<0.00001] (Figure 8).
Tumor necrosis factor-α.3 studies [38,40,44] reported inter-group the level of TNF-α,including 282 patients totally,with 141 in treatment group and 141 in control group.We used the random effect model due to significant heterogeneity between studies [P=0.08,I2=60%].The meta-analysis results showed that combined SKI had more obvious advantages in reducing the level of TNF-α on the basis of western medicine alone [MD=-7.93,95% CI (-10.56,-5.30),P<0.00001].We observed significant reduction of heterogeneity after excluding Rui Liu [40] [P=0.31,I2=3%].Considering that the heterogeneity might be caused by various interventions and treatment courses,we adopted the fixed effect model,which presented statistically significant reduction in TNF-α between treatment and control group [MD=-6.81,95% CI (-8.49,-5.12),P<0.00001](Figure 9).
Blood pressure.2 studies [32,43] reported the changes of SBP and DBP between groups,with 97 cases in the treatment group and 97 cases in the control group.The intra group heterogeneity of the two outcome indicators was low [P=1.00,I2=0%].The results of meta-analysis showed that the treatment group was significantly better than the control group in reducing the levels of SBP and DBP[MDSBP=-10.81,95%CI(-13.66,-7.96),P<0.00001],[MDDBP=-7.36,95% CI(-9.34,-5.38),P<0.00001] (Figures 10 and 11).
Adverse reactions rate.3 studies [18,32,34] reported the occurrence of adverse reactions,involving 266 patients totally,with 133 in treatment group and 133 in control group.The results showed that there was low heterogeneity among studies [P=0.46,I2=0%].We used the fixed effect model due to low heterogeneity between studies [RR=0.46,95% CI (0.18,1.18),P=0.11)],and significant difference was not seen in adverse reactions incidence between treatment group and control group (P>0.05) (Figure 12).
Sensitive analysis
We used STATA SE 14.0 software to conduct sensitivity analysis on clinical effective rate,Scr,BUN and 24h-UP respectively to test results'stability,and the results were shown below.The combined effect size was still within 95% CI of the total effect size after removing one by one,indicating that the results were stable and reliable(Supplementary Figure S1-S4).
Publication bias evaluation of clinical effective rate
Qualitative detection of funnel plot.The software STATA SE14.0 was used to draw funnel plot for publication bias analysis of clinical effective rate,with effect size(logrr) as the horizontal coordinate and standard error of effect size (selogrr) as the vertical coordinate.Results showed that most of the scattered points were evenly distributed within the 95% confidence interval,and only a few scattered points were distributed outside the interval.The funnel plot showed obvious asymmetry,informing possibility of publication bias risk(Figure 13).
Quantitative detection of Begg map and Egger's map.In order to clarify whether there was publication bias in clinical effective rate,Begg rank correlation test and Egger 's linear regression method were used for quantitative detection.Begg'test results showed that the Pr >|z|=0.001<0.05,Egger's test results showed that theP>|t|=0.000 <0.05,indicating significant publication bias in clinical effective rate results.Metatrim module in STATA SE 14.0 was used to identify and correct the publication bias of clinical effective rate,and the results were shown as: before using the trim and filling method,fixed effect model [RR=0.240,95% CI (0.186,0.294),P=0.000];random effect model [RR=0.254,95% CI (0.189,0.320),P=0.000].After 6 iterations of Linear method,6 studies were found to be missing.After including the missing studies,results were: after using the trim and filling method,fixed effect model [RR=0.184,95% CI(0.136,0.232),P=0.000];random effect model [RR=0.188,95%CI (0.133,0.263),P=0.000].The statistically significant 95% CI (P<0.05) of the fixed and the random effect model before and after using the trim and filling method indicated relatively robust clinical effective rate meta-analysis results,and the potential publication bias had little influence on it (Supplementary Figure S5-S7).

Figure 2 Bias's risk summary
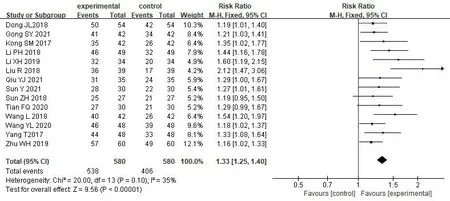
Figure 3 Forest map of clinical effective rate meta-analysis
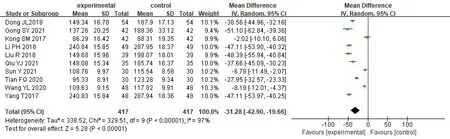
Figure 4 Forest map of Scr meta-analysis

Figure 5 Forest map of Scr subgroup meta-analysis of treatment group combining different Western drugs
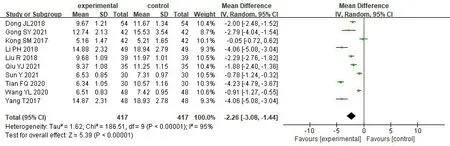
Figure 6 Forest map of BUN meta-analysis
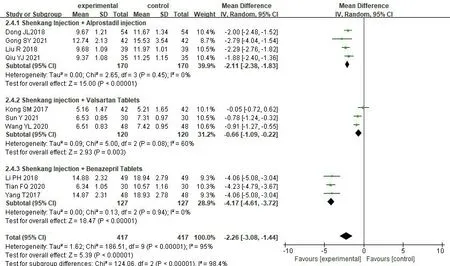
Figure 7 Forest map of BUN subgroup meta-analysis of treatment group combined with different Western drugs
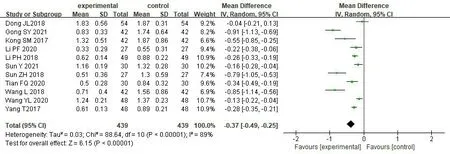
Figure 8 Forest map of 24h-UP meta-analysis

Figure 9 Forest map of TNF-α meta-analysis
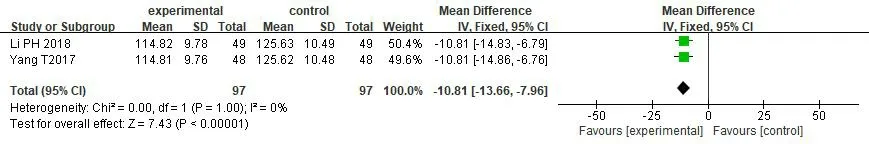
Figure 10 Forest map of SBP meta-analysis

Figure 11 Forest map of DBP meta-analysis
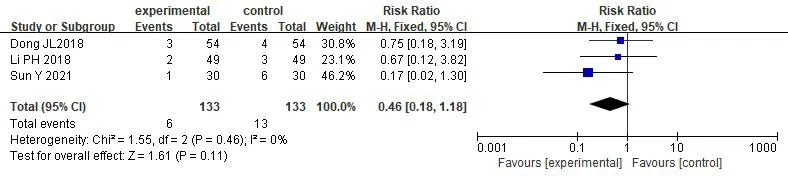
Figure 12 Meta-analysis of adverse reactions incidence

Figure 13 Funnel plot of clinical effective rate
Discussion
In Traditional Chinese Medicine,CGN belongs to the categories of"hematuria (血尿)","low back pain (腰痛)","edema (水肿)" and"fatigue (虚劳)" [45,46],and the pathogenesis is mainly attributed to spleen and kidney deficiency and the invasion of evil Qi,leading to symptoms of hematuria,edema and lumbago [47].Clinically,strengthening spleen and kidney,invigorating qi and promoting blood circulation are the main treatment methods to strengthen vital Qi and remove pathogens [48].Dahuang is the sovereign drug in SKI and has the effect of relaxing bowel and lowering turbidity,can promote blood circulation and remove dampness;Huangqi,as the minister drug,has the function of ascending the clear and descending the turbid,tonifying Qi and dispersing blood stasis;Danshen and Honghua,as the assistant drug,can help the sovereign drug promote blood circulation to remove blood stasis.The whole is skilled at invigorating Qi and activating blood circulation,relaxing bowel and lowering turbidity[49,50].Modern pharmacological studies have found that Dahuang contains anthraquinone derivatives,polysaccharides and other chemical components,which can reduce angiotensin converting enzyme production,improve the high metabolic status of residual kidney,increase glomerular filtration rate,reduce glomerular sclerosis and inhibit inflammation [51,52];Huangqi can reduce interleukin-6 and TNF-α [53,54] to regulate immune function and exert anti-inflammatory effect;Safflower yellow pigment,the main component of Honghua [55] can inhibit platelet aggregation and activation and prevent blood coagulation;Salvianolate [56],the main active component of Danshen can delay kidney injury by regulating transforming growth factor β1 (TGF-β1) and Monocyte Chemoattractant Protein 1 (MCP-1),and its water-soluble component[57]can inhibit or reduce lipid peroxidation by scavenging superoxide anions and hydroxyl radicals.In conclusion,SKI can be anti-inflammatory and anticoagulant,reduce platelet aggregation,promote the repair of kidney tissue,protect residual renal function,reduce the risk of transformation from CGN to chronic renal failure,and reduce the occurrence of various complications.
In this study,the meta-analysis was conducted on 15 RCTs,and the results indicated better clinical effective rate of SKI combing conventional Western medicine than Western medicine alone.Combining SKI with modern medicine can better reduce the levels of Scr,BUN,24h-UP,TNF-α and BP.Due to the large heterogeneity after the combination of outcome indicators,considering the risk of bias caused by simple combination,subgroup analyses were conducted based on various Western medicine and different treatment course between groups,and the results all confirmed the effectiveness of SKI.In terms of adverse reactions,one case of nausea occurred in the treatment group of Ying Sun[34];Ping-Hua Li[32]reported 1 case of rash and 1 pruritus;Jin-Li Dong [18] reported 2 vascular pain and 1 local redness,and the three control groups also suffered adverse reactions of varying degrees.No statistical significance of adverse reaction incidence was seen between the treatment and the control group (P>0.05),indicating that SKI may not reduce adverse reactions occurrence.According to the retrospective analysis of 19 patients with SKI-induced adverse reaction by De-Long Duo [58],63.16%of the patients had adverse reactions after 30 min intravenous infusions,among which the top three adverse reactions were nervous system damage,skin and accessory damage,and systemic damage.Those adverse reactions may be due to the inclusion of polysaccharides,proteins and other complex macromolecular antigens,easy to produce allergic reactions [59].Some of the reasons may be due to irregular infusion and individual differences,such as unreasonable dosage and duration,drug mixing,and age differences[60].As for the safety of SKI,more clinical evidence is still needed to confirm it since we only included few literature.
This study has some limitations: (1) the randomization of some studies was unclear,and few studies have used blinding and allocation hiding;(2) the included subjects were all from China,so there may be publication bias of specific population;(3) there were different diagnostic criteria in the diagnosis of CGN,and most of the literature did not explain the diagnostic criteria of CGN;(4) in the evaluation criteria of clinical effective rate,the accuracy of outcome indicators may be affected because the evaluation criteria have not been unified.
Conclusion
SKI combined with Modern medicine can improve the clinical effective rate of CGN and reduce the levels of Scr,BUN,24H-UP,TNF-α,SBP and DBP.However,due to the low quality of literature,the conclusion still needs to be supported by more multi-center,large-sample studies with rigorous design and standardized implementation.
 Clinical Research Communications2022年4期
Clinical Research Communications2022年4期
- Clinical Research Communications的其它文章
- Not just a sponge:novel functions of circRNAs in cholangiocarcinoma
- POEMS syndrome characterized by peripheral neuropathy as the first symptom: a rare case report
- Effectiveness of hip upslip correction method on ambulation and back pain in patients with lumbar discopathy:a case report
- Effect of Xingnao-Jianshen granules in treating AIS patients: study protocol for a non-randomized controlled intervention trial
- A study of ultrasonography for the treatment of Qingre Liangxue decoction on blood-heat psoriasis
