Evaluation of the chemical profile from four germplasms sources of Pruni Semen using UHPLC-LTQ-Orbitrap-MS and multivariate analyses
Zihn Zho,Yue Liu,Yushi Zhng,Zeyu Geng,Rin Su,Lipeng Zhou,Cho Hn,Zhnjun Wng,Shungcheng M,Weidong Li,*
aSchool of Chinese Materia Medica,Beijing University of Chinese Medicine,Beijing,102488,China
bInstitute of Forestry and Grassland Ecology,Ningxia Academy of Agricultural and Forestry Sciences,Yinchuan,750002,China
cInstitute for Control of Chinese Traditional Medicine and Ethnic Medicine,National Institutes for Food and Drug Control,Beijing,100050,China
ABSTRACT
Pruni Semen,the seed of several unique Prunus plants,is a traditional purgative herbal material.To determine the authentic sources of Pruni Semen,46 samples from four species were collected and analyzed.Ten compounds including multiflorin A(Mul A),a notable purative compound,were isolated and identified by chemical separation and nuclear magnetic resonance spectroscopy.Seventy-six communal components were identified by ultra-high performance liquid chromatography with linear ion trap-quadrupole Orbitrap mass spectrometry,and acetyl flavonoid glycosides were recognized as characteristic constituents.The flavonoids were distributed in the seed coat and cyanogenic glycosides in the kernel.Based on this,methods for identifying Pruni Semen from different sources were established using chemical fingerprinting,quantitative analysis of the eight principal compounds,hierarchical cluster analysis,principal component analysis,and orthogonal partial least squares discriminant analysis.The results showed that the samples were divided into two categories:one is the small seeds from Prunus humilis(Ph)and Prunus japonica(Pj),and the other is the big seeds from Prunus pedunculata(Pp)and Prunus triloba(Pt).The average content of Mul A was 3.02,6.93,0.40,and 0.29 mg/g,while the average content of amygdalin was 18.5,17.7,31.5,and 30.9 mg/g in Ph,Pj,Pp,and Pt,respectively.All the above information suggests that small seeds might be superior sources of Pruni Semen.This is the first comprehensive report on the identification of chemical components in Pruni Semen from different species.
Keywords:
Pruni semen
Acetyl flavonoids
Amygdalin
Multivariate analyses
Authentic sources
1.Introduction
Pruni Semen has had a medicinal history of more than 2000 years in East Asia(China,Japan,and Korea),and has been used for the treatment of edema,intestinal dryness,and constipation with remarkable curative effects.Pruni Semen is the dried ripe seed of Prunus humilis Bge.,Prunus japonica Thunb.,or Prunus pedunculata Maxim.[1],whose seeds are much smaller than apricot and peach seeds.
Counterfeits of Pruni Semen have been common for many years.The underlying reason for this confusion is that chemical profile characteristics of these seeds are yet to be elucidated.Amygdalin(Amy)is a quality control indicator of Pruni Semen according to the Chinese Pharmacopoeia[1].Given the wide distribution of Amy in a variety of Prunus plant seeds[2],counterfeits have flooded the market.These counterfeits are mostly seeds of Prunus triloba Lindl.,which have lower prices and better commodity traits than genuine Pruni Semen.In addition,the counterfeits are considered to have good quality because of their high Amy content.However,their efficacy and safety are unclear.Amy is also a quality control indicator for the traditional seed medicines Armeniacae Semen Amarum and Persicae Semen.However,the three medicinal materials demonstrate quite different functions[1].This suggests that other secondary metabolites besides Amy are important to the function of Pruni Semen.
Generally,the oil part of the seed is believed to be the major material basis for the intestinal purification effect of Pruni Semen,but this opinion may be narrow.Some investigations focusing on the seeds and seed coat of Prunus plants have revealed the presence of polyphenols[3].Multiflorin A(Mul A),an acetylated glycoside of kaempferol,has been isolated from the seeds of Prunus japonica(Pj)and exhibits specific purgative activity[4].The purgative mechanism of Mul A might be related to the activation of intestinal immunity and changes in intestinal barrier function.These effects are temporary and noncytotoxic[5,6].Mul A also demonstrated a potent suppressive effect on the postprandial elevation of blood glucose levels in glucose-loaded mice,which was directly related to the acetyl substituents[7].However,there is currently insufficient focus on the distribution of Mul A in Pruni Semen.
There is a limited number of studies on the seeds of Pruni Semen plant sources.Prunus humilis(Ph)and Pj belong to the dwarf cherry subgenus and have a close genetic relationship.Ph is a promising third-generation functional fruiter that bears a distinct aroma;the fruit is rich in polyphenols,which benignly regulate intestinal immunity[8-10].However,although Pruni Semen is a traditional herbal medicine,there are few reports on the chemical composition of their seeds.Prunus pedunculata(Pp)is a new type of sandy land oil tree that exhibits remarkable prevention against desertification.The Pp seed coat contains various polyphenols,which have outstanding antioxidant and antibacterial activities.It also inhibits the proliferation of HepG2cells and induces apoptosis[11].In summary,both Pruni Semen and its source plant have potential widespread applications.However,there is a lack of comprehensive horizontal analysis of these plant seeds.
Orbitrap mass spectrometry(MS)has become a powerful tool to characterize the chemical components in traditional Chinese medicines because of its high sensitivity,high mass resolution,and high mass accuracy[12,13].Owing to the different detection parameters,its reproducibility is often poor.Its reliability is unknown,especially for the samples that lack chemical composition reference and standard substances.In addition,quantitative analysis using liquid chromatography(LC)-MS is sensitive,but not stable.In terms of the samples with comparability,the markers obtained by metabolomics screening were mostly trace components.In contrast,the content differences of main components with definite bioactivity deserve more attention.
This study presents an unprecedented comprehensive analysis of the chemical components in Pruni Semen from four species(Ph,Pj,Pp,and Prunus triloba(Pt)),and their spatial distribution characteristics.The chemical composition was identified by means of chemical isolation,nuclear magnetic resonance(NMR)spectroscopy,and LC-MS analysis.The results of the LC-MS analysis are more reliable as they are based on the chemical separation in the early stage.The chemical profiles of the different species were described through chemical fingerprinting,quantitative analysis of eight principal compounds,and multivariate analyses(hierarchical cluster analysis(HCA),principal component analysis(PCA),and orthogonal partial least squares discriminant analysis(OPLS-DA)).This study provides a reference for the quality control of seed medicinal materials and evaluation of medicinal plant germplasm sources.
2.Materials and methods
2.1.Chemicals and reagents
MS-and high performance liquid chromatography(HPLC)-grade acetonitrile,methanol,and formic acid were purchased from Fisher Scientific(Fair Lawn,NJ,USA).Analytical grade ethanol,methanol,dichloromethane,petroleum ether,sulfuric acid,aluminum chloride,and other reagents were purchased from Beijing Chemical Plant(Beijing,China).Ultrapure water was obtained from Watsons(Guangzhou,China).Column chromatography(CC)silica gel,thin layer silica gel G,and thin layer silica gel GF254were purchased from Qingdao Marine Chemical Plant(Qingdao,China).AB-8 macroporous adsorption resin and Sephadex LH-20 gel were obtained from Solarbio(Beijing,China).SiliaSphere C18was obtained from Silicycle(Quebec City,Canada).Dimethyl sulfoxide-d6and methanol-d4were purchased from Cambridge Isotopes Laboratories(Andover,MA,USA).Amygdalin standard compound(purity>98%)was obtained from Chengdu Pusi Biotechnology Co.,Ltd.(Chengdu,China).
2.2.Compound isolation and identification
The dried ripe seeds of Ph(1.4 kg)were crushed,and the oil was removed during two 4 h extraction periods,using petroleum ether and a Soxhlet apparatus.The remaining powder was reflux extracted twice for 1.5 h with 70% ethanol.The combined extracts were eluted through AB-8 macroporous resin(water,15%,45%,and 70% ethanol).The eluent of 45% ethanol was collected(17.7 g),and the compounds were separated by CC using a SiliaSphere C18column and gradient elution with methanol(MeOH)/H2O(25%,45%,65%,and 100%).The collected elutions were combined into 16 fractions(Fr.A-Fr.P)based on thin layer chromatography(TLC)and HPLC analysis.Fr.E(2.2 g)was chromatographed using a gradient elution with dichloromethane/methanol/water (65:25:10,65:30:10,and 65:35:10,V/V/V)using silica gel CC(2 cm×50 cm,200-300 mesh,35 g).Fourteen fractions(Fr.E1-Fr.E14)were obtained.Fr.E9 and Fr.E5 were further separated using Sephadex LH-20 CC,eluted with methanol,and purified by preparative HPLC(SiliaSphere C18,MeOH/H2O)to yield compounds 1(20 mg),2(52 mg),3(26 mg),and 4(25 mg).
Fr.K(2.7 g)was chromatographed using a gradient elution with dichloromethane/methanol/water(65:20:10,65:25:10,and 65:30:10,V/V/V)using silica gel CC(2 cm×70 cm,200-300 mesh,45 g).Ten fractions(Fr.K1-Fr.K10)were obtained.Fr.K6,Fr.K8,and Fr.K3 were further separated on a Sephadex LH-20 CC,eluted with MeOH,and purified by preparative HPLC(SiliaSphere C18,MeOH/H2O)to provide compounds 5(8 mg),6(270 mg),7(14 mg),and 8(29 mg).Fr.L(2.3 g)was chromatographed on a silica gel column to yield compound 9(160 mg).Fr.N(255 mg)was chromatographed using a gradient elution with dichloromethane/methanol/water(65:15:10 and 65:25:10,V/V/V)on silica gel CC,then further separated on a Sephadex LH-20 CC,eluted with MeOH,and purified by preparative HPLC(SiliaSphere C18,MeOH/H2O)to provide compound 10(7 mg).
The purity of the isolated compounds was confirmed using ultra performance liquid chromatography(UPLC)area normalization method(>95%).13C-NMR and1H-NMR data were obtained using a Bruker Avance Neo 600 NMR spectrometer(Bruker BioSpin GmbH,Karlsruhe,Germany).The compound structures were also determined using linear ion trap-quadrupole(LTQ)-Orbitrap-electrospray ionization(ESI)-MS(Thermo Fisher Scientific Inc.,San Jose,CA,USA).
2.3.Plant materials and sample preparation
A total of 46 Pruni Semen samples were collected,including the seeds of Ph,Pj,Pp,and Pt(Fig.1 and Table S1).Ripe fruits were collected,the flesh was removed,the core was broken,and the unbroken seeds were collected and dried to a constant weight at 25°C.All samples and original plants(Fig.1)were identified by Prof.Weidong Li of Beijing University of Chinese Medicine(Beijing,China).The seeds of Ph and Pj are often referred to as small Pruni Semen and Pp and Pt as big Pruni Semen,according to their morphological characteristics.
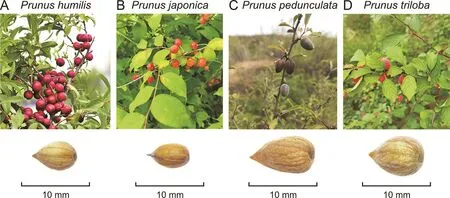
Fig.1.Plants and seeds of the four Prunus species.(A)Prunus humilis(Ph),(B)Prunus japonica(Pj),(C)Prunus pedunculata(Pp),and(D)Prunus triloba(Pt).
The seed samples were fully crushed,gently sieved(24 mesh),mixed well,and accurately weighed(200 mg).The oil was removed using petroleum ether and a Soxhlet apparatus for 4 h.Methanol(20 mL)ultrasonic extraction at 25°C for 30 min was performed to extract the vast majority of the secondary metabolites from Pruni Semen[1].The extract was centrifuged for 15 min at 16,000 g and 4°C,and passed through 0.22-μm filter membrane to obtain the sample solution for testing.All 46 samples were used for UPLC analysis,and four representative samples from four Prunus species(Ph-1,Pj-10,Pp-1,and Pt-8)were selected for LC-MS analysis based on their botanical character and the results of preliminary experiments.In view of the possible differences in chemical composition distribution,the kernel and seed coat were separated for the four representative samples.Solutions of the kernel and seed coat samples were prepared according to the above method.The concentration of the kernel and seed coat in the solution represented the natural concentration in plants.The seed coat accounted for approximately 9% of the total seed weight.These eight samples(four seed coats and four kernels)were also analyzed using LC-MS.
2.4.LC-MS conditions and chemical composition analysis
A Waters AQUITY UPLC HSS T3 C18chromatography column(2.1 mm×100 mm,1.8μm;Waters Corporation,Milford,CT,USA)was used with mobile phases comprising 0.1% formic acid water(A)and acetonitrile(B).The gradient elution was as follows:0-3 min(8%-20% B),3-8 min(20%-25% B),8-12 min(25%-28% B),12-15 min(28%-60% B),and 15-18 min(60%-90% B).The flow rate was 0.25 mL/min,the column oven temperature was 35°C,and the injection volume was 3μL.High-resolution(HR)MS spectral analysis was performed using an LTQ-Orbitrap mass spectrometer(Thermo Fisher Scientific Inc.,Waltham,MA,USA)equipped with an ESI source.The optimized operating parameters in the negative ion mode were as follows:sheath gas flow rate of 30 arb,auxiliary gas flow rate of 10 arb,capillary voltage of-35 V,electrospray voltage of 3.0 kV,tube lens voltage of-110 V,and capillary temperature of 300°C.The constituents were detected using full-scan MS analysis from m/z 100 to 1500.Data-dependent ESI-MS/MS analysis was triggered by the three most abundant ions.
An Xcalibur 2.2 workstation was used for MS/MS data processing and analysis.Compound Discoverer 3.0 and Mzmine 2.53 software were used for background elimination,peak alignment,and common peak extraction of different quality spectrum data,and the common constituents of the four sources of Pruni Semen were tentatively determined.First,the data of known structural compounds were analyzed,and possible fracture mechanisms were predicted based on MS/MS fragments.The compounds were preliminarily identified by referring to the retention time and fragmentation pathway information provided by the Compound Discoverer 3.0 software,PubChem,and The Human Metabolome Database.The acceptable molecular mass error was less than 5 ppm.
2.5.UPLC fingerprint and quantification of major constituents
A Nexera LC-40 UPLC (Shimadzu,Kyoto,Japan)with ultraviolet(UV)detection was used for fingerprinting and constituent quantification.A UV wavelength of 264 nm was used to obtain chemical information.Eight major constituents were quantitatively analyzed.The reference substance Amy and the isolated compounds Mul A,Mul B,afzelin(Afz),kaempferol 3-O-β-D-xylopyranoside(Koxy),and juglalin(Jug)were used to prepare a mixed standard solution in methanol,which was then diluted to form a series of mixed standards of different concentrations.An external standard curve was used for quantification.Given the similar structure and UV absorption spectrum(Fig.S1),Mul A was used to quantify the two other acetyl flavonoid glycosides,multinoside A acetate(Ms A)and isomer Mul A(Is MA).Finally,the above UPLC quantitative methods were validated,regarding the repeatability,stability,precision,and recovery.The chromatographic fingerprint similarity evaluation system of traditional Chinese medicines was used to establish different chromatographic fingerprints and evaluate their similarities.
2.6.Statistical and multivariate analyses
All the 46 samples were quantified in triplicate(n=3),and the data were presented as the mean and standard deviation.The compound content data of each sample were imported into GraphPad Prism 8 software to create box diagrams,and analysis of variance was conducted.After normalized processing,the data were imported into Heml 1.0.3.7 software to visualize the data in a clustering heat map,and SIMCA 14.1 software for multivariate analyses.An unsupervised PCA model was constructed,and group clustering and outliers were observed using a score plot.OPLS-DA was used to find differential constituents between groups[14].
3.Results and discussion
3.1.Compound isolation and identification
Compounds of Pruni Semen were isolated selectively.To date,few studies have reported the chemical isolation of Ph.In this study,ten known compounds were isolated from Ph seeds for the first time,including four aromatic glycosides:(R)-mandelic acid-O-β-D-glucopyranoside(compound 1),(S)-mandelic acid-O-β-D-glucopyranoside(compound 2),piperwallioside B(compound 3),and piperwallioside C(compound 4);and six flavonoids:Koxy(compound 5),Mul B(compound 6),Jug(compound 7),Afz(compound 8),Mul A(compound 9),and kaempferol(compound 10)(Fig.2).
Compounds 1-4 were found to be the derivatives of mandelic acid.The negative-ion HR-ESI-MS results of compounds 1-4 reveal a deprotonated molecular ion[M-H]-at m/z 313.0933,313.0932,327.1086,and 327.1088,respectively.These data suggest that the compounds were two pairs of isomers.The molecular formula of compounds 1 and 2 were assigned as C14H18O8,and compounds 3 and 4 were assigned as C15H20O8.According to the mass and13C-NMR spectrum of compound 1(Table S2),a glucose group was connected to the mandelic acid group.The benzene ring monosubstitution was confirmed by six aromatic carbons atδC129.6(C-2 and C-6),129.4(C-3 and C-5),129.9(C-4),and 137.3(C-1)in the13C-NMR spectrum and the presence of five protons atδH7.39 and δH7.50 in the1H-NMR spectrum(Table S2).Compound 1 was established as(R)-mandelic acid-O-β-D-glucopyranoside,and compound 2 was identified as(S)-mandelic acid-O-β-D-glucopyranoside,based on the spectroscopic profiles and comparison to published data in the literature[15,16].The1H and13C NMR data of compounds 3 and 4 are similar to those of compounds 1 and 2,albeit with slight differences.There were an extra δC52.9 and three protons at δH3.72 in the spectrum(Table S2).These data suggest methylation of the carboxyl group.Therefore,compound 3 was identified as methyl(2R)-2-phenyl-2-(((3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl)oxy)acetate,commonly known as piperwallioside B,and compound 4 was identified as piperwallioside C,according to the literature[17].Compounds 1-4 were respectively observed in Prunus zippeliana leaves[15],Averrhoa carambola root[16],and Piper wallichii stem[17].
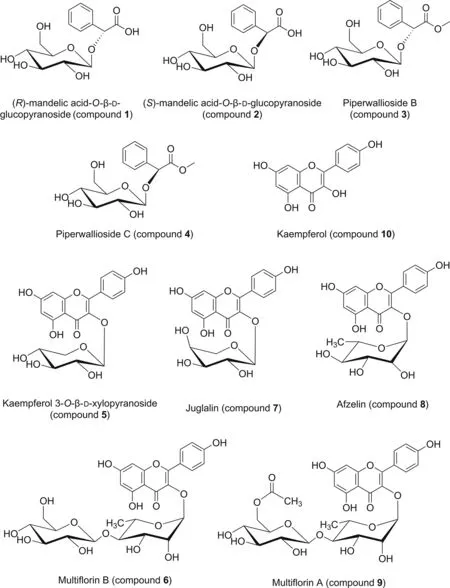
Fig.2.Structures of compounds 1-10.
Compounds 5-10 were obtained as yellow powder.TLC(AlCl3)appeared as a blue-green spot under 365 nm irradiation.These data suggest that the six compounds were flavonoids.The molecular formula of compound 10 was deduced to be C15H10O6,using negative HR-ESI-MS([M-H]-)m/z 285.0399.Fragment ions were observed at m/z 256.9937 ([C14H9O5]-),151.0083([C7H3O4]-)(Table S3 and Fig S2).The1H and13C NMR data reveal three phenolic hydroxyls C-5,C-7,and C-4'(Table S4).Therefore,compound 10 was determined to be kaempferol[18].Fragment ions of compounds 5-9 were all clearly observed at m/z 285,and the negative-ion HR-ESI-MS results revealed deprotonated molecular ions[M-H]-at m/z417.0834,593.1519,417.0822,431.0985,and 635.1627,respectively.These data suggest that thefive compounds were composed of a kaempferol with a sugar substituent.Compounds 5 and 7 were identified as a pair of isomers according to their exact molecular weights.The β-configuration of compound 5 was deduced because of the large J value of the anomeric proton atδH5.3(1H,d,J=7.2 Hz).Comparison with literature date[19,20],compound 5 was established as Koxy.Compound 7 was identified as kaempferol 3-O-α-L-arabinoside,commonly named Jug.Similarly,the structure of compound 8 was determined to be kaempferol 3-O-α-L-rhamnopyranoside,commonly named Afz[18].Compounds 5 and 7 were respectively observed in the flowers of Prunus spinosa[19]and leaves of Coriaria sinica[20].
According to the mass spectrum,compounds 6 and 9 were kaempferol disaccharide glycosides.The molecular formula of compound 6 was identified using negative HR-ESI-MS([M-H]-m/z 593.1503)as C27H30O15(Table S3).The1H and13C NMR data of compounds 6 and 9 revealed similarities.When compared with the date for compound 6,the signals δC170.2 and δC20.6 and the loss of C2H2O in mass spectrum suggest that compound 9 contained one acetyl group(Fig.S2 and Table S5).With reference to the literature[21],the structure of compound 9 was established as kaempferol 3-O-[(6′′′-O-acetyl-β-D-glucopyranosyl)-(1→4)-α-L-rhamnopyranoside],commonly named Mul A.Compound 6 was identified as Mul B.Compounds 6 and 9 have been observed in Prunus japonica seeds[4],Rosa multiflora fruits[21,22],and Neocheiropteris palmatopedata root[23].
3.2.LC-MS chemical composition analysis
Sample analysis was performed in the negative ion mode,in which most constituents displayed better sensitivity.The chemical compositions of Pruni Semen from the four Prunus species were similar.Nonetheless,the peak intensities of several of the major compounds were significantly different(Fig.3).After subtracting blank and removing peak adducts that were generated in source,the compounds were tentatively identified from their exact mass,isotope patterns,and mass fragmentation patterns.A total of 76 communal compounds were preliminarily identified by ultra-high performance liquid chromatography-LTQ-Orbitrap-MS(UHPLCLTQ-Orbitrap-MS),including 50 flavonoids,5 organic acids,3 cyanogenic glycosides,and 18 other compounds(Table S3).The structures of eight compounds were confirmed using standards and monomer compounds obtained by chemical separation:(S)-mandelic acid-O-β-D-glucopyranoside,amygdalin,Koxy,Mul B,Jug,Afz,Mul A,and kaempferol.The possible structures of the other compounds were tentatively identified based on the fragmentation pathways of the known structure compounds.
3.2.1.Generalized flavonoids
Kaempferol glycosides are representative flavonoids of Pruni Semen and display peaks with high relative intensity.Quercetin glycosides,isorhamnetin glycosides,procyanidins,and catechins were also identified.All identified flavonoid glycosides were single glycosides or disaccharide glycosides,of which Mul A(peak 58)exhibited the strongest relative peak intensity.Mul A has acetyl substituents on the glycosyl moiety,and exhibits specific purgative activity with an ED50of 30 mg/kg[21,22].This efficacy might be related to the activation of intestinal mast cells,thereby promoting intestinal immunity and enhancing intestinal smooth muscle contraction[5].Mul A is also involved in changes in intestinal barrier function and increased intestinal mucosal permeability,and this effect is temporary and noncytotoxic[6].Mul A can significantly inhibit intestinal glucose uptake in glucose-loaded mice,thereby significantly reducing the postprandial blood glucose peak and maintaining its stability in mice[7].This is associated with intestinal barrier function rather than changes in glucose metabolism[24].In addition,Mul A possesses aromatase inhibitory activity with an IC50of 15.5μM[25].
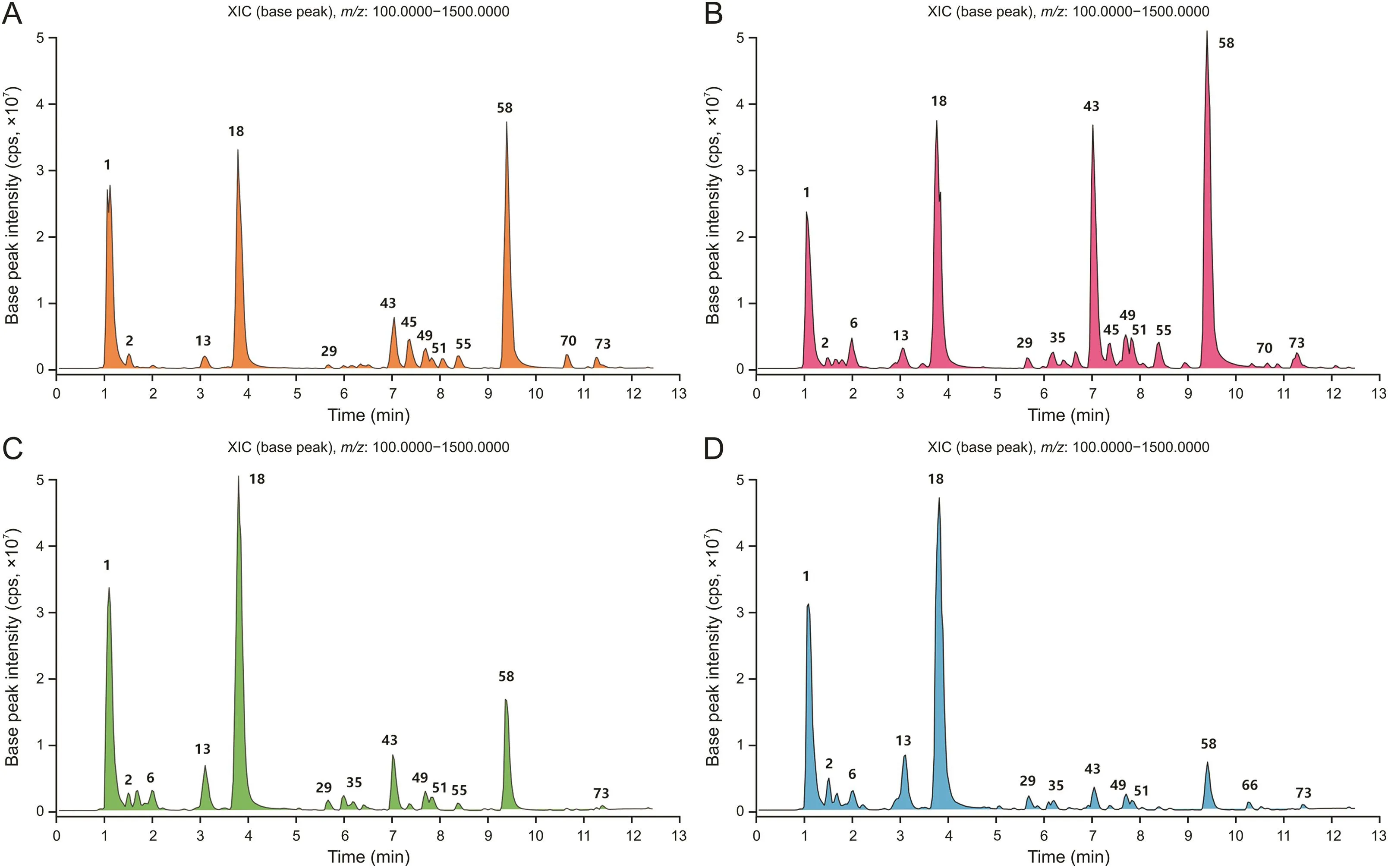
Fig.3.Liquid chromatography-mass spectrometry(LC-MS)base peak chromatograms of Pruni Semen from four sources in negative ion mode:(A)Prunus humilis seed,(B)Prunus japonica seed,(C)Prunus pedunculata seed,and(D)Prunus triloba seed.XIC:extracted ion chromatogram.
Mul B(peak 43),in which the purgative effects and reduction of postprandial blood glucose were significantly weakened,is the deacetylation product of Mul A[21,24].Therefore,the aforementioned activity of Mul A was directly related to the acetyl substituents.A total of 14 flavonoid glycosides with acetyl substituents were detected in Pruni Semen.Ms A(peak 51)also exhibited purgative activity.However,its activity was weaker than that of Mul A[21].Based on the above analysis,acetyl flavonoid glycosides were recognized as the characteristic constituents of Pruni Semen and were directly related to traditional efficacy,including purging of the bowels and alleviation of edema.Afz(peak 49)has been shown to exhibit anti-inflammatory and renoprotective effects[26].Moreover,it protects melanocytes and promotes melanin production[27];additionally,it protects the liver from injury caused by D-galactosamine/lipopolysaccharide through mitochondrial pathways[28].
The MS/MS fragmentation patterns of flavonoids were illustrated by the example of Mul A and kaempferol(Fig.S2),which mainly includes the loss of acetyl and other substituents,loss of glycosyl,and retro-Diels-Alder cleavage of aglycone.The secondorder fragment with the highest relative strength are typically flavonoid aglycones.The aglycone was determined first,and then the type and number of glycosyls were proposed according to the molecular weight of the lost fragments,and the connection position of the glycosyl could be inferred according to the relative abundance of fragments[29].
3.2.2.Cyanogenic glycosides and aromatic glycosides
Cyanogenic glycosides are amino acid-derived plant constituents that act as plant antibiotics against herbivores and pathogens[2].Three cyanogenic glycosides were identified in Pruni Semen.Amy(peak 18)had strong relative peak intensities in Pruni Semen from four species sources,especially in Pp and Pt(big seeds).Amy is widely available in the seeds of Rosaceae plants.It is commonly used in clinical settings as a cough expectorant and adjuvant anticancer drug.Amy also exhibits anticoagulant,antitumor,and analgesic activities[2].Furthermore,it often has synergistic effects with other small molecules[30].Benzyl b-sophoropyranoside(peak 13)is a representative example of aromatic glycosides in Pruni Semen,which lack a cyanide group compared with Amy.Amy is non-toxic but ultimately cleaves into hydrogen cyanide,which poses safety risks after oral administration.Fortunately,because almonds have long been consumed by humans,procedures for removing Amy are many and well developed in the food industry[31].The MS/MS fragmentation patterns of cyanogenic glycoside and aromatic glycoside constituents with similar structures mostly include the cleavage of glycosylation and loss of phenylethyl cyanide[32].
3.3.Composition distribution in the seed kernel and seed coat
The major chemical constituents in the seed kernel and seed coat were substantially different.Overall,the cyanogenic glycosides and aromatic glycosides were distributed in the kernel,whereas the flavonoids were observed in the seed coat(Fig.S3).The distribution characteristics of chemical components in kernel and coat of the four species of Pruni Semen were consistent.The distribution is proposed to be closely related to physiological function.The seed coat is formed by ovules that develop to varying degrees.Its primary function is to protect seeds from external mechanical damage and prevent pests and diseases from invading;its function is also closely related to seed germination[33].Considering the hard endocarp outside the seed,the structure of the seed coat of Prunus involves a simple membrane layer,and the mechanical protection effect is minimal.Flavonoids in the seed coat play a special evolutionary role.Flavonoids often possess outstanding antioxidant and antibacterial abilities,which not only maintain the physiological activity of nutrients in seeds,but also prevent seeds from being invaded by pathogenic microorganisms and pests.Some flavonoids,such as Mul A,possess specific purgative effect,prompting seeds to be discharged as soon as possible,which allows them to avoid possible damage in the digestive system of animals,and achieve species transmission for the plant.Afz positively regulates melanin production and antagonizes UV-induced cell damage,which protects the seeds from exposure to sunlight[34].
Several studies have reported the distribution and activity of flavonoid and polyphenol compounds in the seed coat of Prunus plants[3].The seed coat of Pp contains polyphenols,which have prominent antioxidant and antibacterial activities[11].Armeniacae Semen Amarum and Persicae Semen are generally peeled in traditional clinical applications,whereas Pruni Semen is not.This difference in processing could be related to the different distributions of the active ingredients in seeds.Our findings clarify the science behind this difference in processing.Based on the composition distribution,it would be more convenient to separate the different component groups to achieve different purposes in the functional food and herbal medicine industries.In addition,to ensure the accuracy of the experimental data,the broken seeds should not be used as qualified experimental materials,because the proportion of seed coat and kernel may not be typical.
3.4.UPLC fingerprinting and quantification of major components
UPLC chemical fingerprinting and quantitative methods for the major components were established.After methodological validation,the relative standard deviation of repeatability,stability,and precision was less than 5%.The recoveries ranged from 95.92% to 105.20%.The standard curve exhibited a good linear relationship,indicating that the method was stable and reliable(Table S6).There were differences in the UPLC fingerprints of the Pruni Semen samples from the four species.Pp and Pt seeds were similar(92.8%),as were Ph and Pj(95.9%).However,the similarity between the UPLC fingerprints of the four species samples was only 61.4%(Fig.4A).The difference was primarily reflected in the content of several major components.Therefore,using monomer compounds obtained by chemical separation and reference substances,the quantitative analysis of eight major constituents was carried out:Amy,Mul A,Mul B,Afz,Jug,Ms A,Is MA,and Koxy(Fig.4A).According to the peak area normalization method,the total peak area of the eight constituents accounted for 81.2%(Ph),84.6%(Pj),56.2%(Pp),and 64.1%(Pt)of the sample(Fig.4A),suggesting that these eight compounds reflected most of the chemical characteristics of the sample extract.The content of these components is listed in Fig.4B and Table S7.
Amy was the most abundant secondary metabolite in Pruni Semen.Its content in Pp(31.5 mg/g)and Pt(30.9 mg/g)was significantly higher than in Ph(18.5 mg/g)and Pj(17.7 mg/g)(Figs.4B and C and Table S7).Amy is ubiquitous in most Rosaceae plant seeds.In this study,it was found that the smaller the seeds,the lower the Amy content,which may be related to the difference in the biosynthetic function of different species[35].
The three acetyl flavonoid glycosides(Mul A,Is MA,and Ms A)and Mul B are all disaccharide glycosides of kaempferol,and acetyl flavonoid glycosides were characteristic constituents of Pruni Semen.Mul A had the highest content of the flavonoids;the average Mul A content in Pj was 6.93 mg/g,which was significantly higher than that in Ph(3.02 mg/g).Even so,the content in Pj and Ph was significantly higher than in Pp(0.4 mg/g)and Pt(0.29 mg/g)(Figs.4B and C and Table S7).The difference in Mul A content between different samples was higher than 60-fold,reflecting the physiological characteristics of stress resistance of plant secondary metabolites[36].The Is MA and Ms A content was much lower than the Mul A content.However,the distribution patterns among samples from different species were similar.These results support the conventional wisdom,which suggests that small Pruni Semen(Ph and Pj)are authentic Pruni Semen material.
Afz,Jug,and Koxy are kaempferol monoglycosides.Although the content of these three compounds in Ph was slightly higher than in Pj,the difference was not significant.Similarly,Ph and Pj had significantly higher content than Pp and Pt,and the distribution differences in Jug and Koxy content in the four species were notably striking than those of Mul A(Figs.4B and C and Table S7),which was consistent with the subsequent multivariate analyses results.At present,few studies have revealed the pharmacological activity of Jug and Koxy.Therefore,there were no data to demonstrate that these ingredients are associated with the known biological activity of Pruni Semen.It would be advantageous to use them as markers to distinguish the species of origin.
Notably,based on the literature and preliminary experiments,the flavonoid content of Armeniacae Semen Amarum was much lower than that of Pruni Semen[37].In particularly,Mul A had not been detected in Armeniacae Semen Amarum and Persicae Semen,to our knowledge.These results expand the understanding of the distribution of flavonoids in Prunus seeds,and indicate the advantage of small Pruni Semen among Prunus seeds.In this study,the total quantity of flavonoids in big Pruni Semen was generally lower than that in small ones.In terms of seed coat(approximately 9% of the intact seed weight),the total quantity of seven flavonoid compounds in small Pruni Semen was up to 100 mg/g,which is valuable for further study and development.
3.5.Multivariate analyses
Chemical profiling combined with multivariate analyses(PCA,OPLS-DA,and HCA)is an effective method for distinguishing samples from different sources and has been widely used in herbal medicine[14].Using the content of the above eight major compounds as variables,multivariate analyses were conducted to identify the plant species and screen out the marker compounds of Pruni Semen from the four species.
Through PCA,the first three principal components(PCs)extracted 87.3% of the total variance.PC1,PC2,and PC3 accounted for 60.9%,18.5%,and 7.9%,respectively.The loading of variables is shown in Table S8.PC1 was most closely related to the content of Mul A.The variable with the highest loading in PC2 was the content of Afz.PC3 was mostly correlated with the content of Ms A.As shown in the OPLS-DA score plot(Fig.5B)and clustering heat map(Fig.4C),the samples were first divided into two categories:group A included all samples from Ph and Pj,and group B included all samples from Pt and Pp.The 46 samples from the four species were completely separated in the PCA 3D score plot(Fig.5A),indicating that this method could accurately distinguish the four sources of Pruni Semen.The biplot reflected the information from score plot and loading plot simultaneously(Fig.5E).The biplot indicated the sample and variables(eight constituents)on the same coordinate axis,and the compounds distributed near the sample were the dominant constituents.Samples of group A were on the left side,towards seven flavonoids,while samples of group B were on the right side,towards Amy.Group A was further divided into two groups:Ph samples were nearer kaempferol monoglycosides(Afz,Koxy,and Jug),and distributed in the top left area,whereas Pj samples were distributed in the lower left part,nearer kaempferol disaccharide glycosides(Mul A,Mul B,Ms A,and Is MA).The spatial position also reflected the correlation between the compounds.Variable importance in projection(VIP)values could be used to further screen the marker compounds between different groups of samples.In this study,VIP>1 was used as a criterion to screen five compounds:Jug,Koxy,Mul A,Is MA,and Mul B.The contents of these five compounds in Ph and Pj were significantly higher than those of Pt and Pp,indicating their chemical uniqueness among the Prunus seeds.
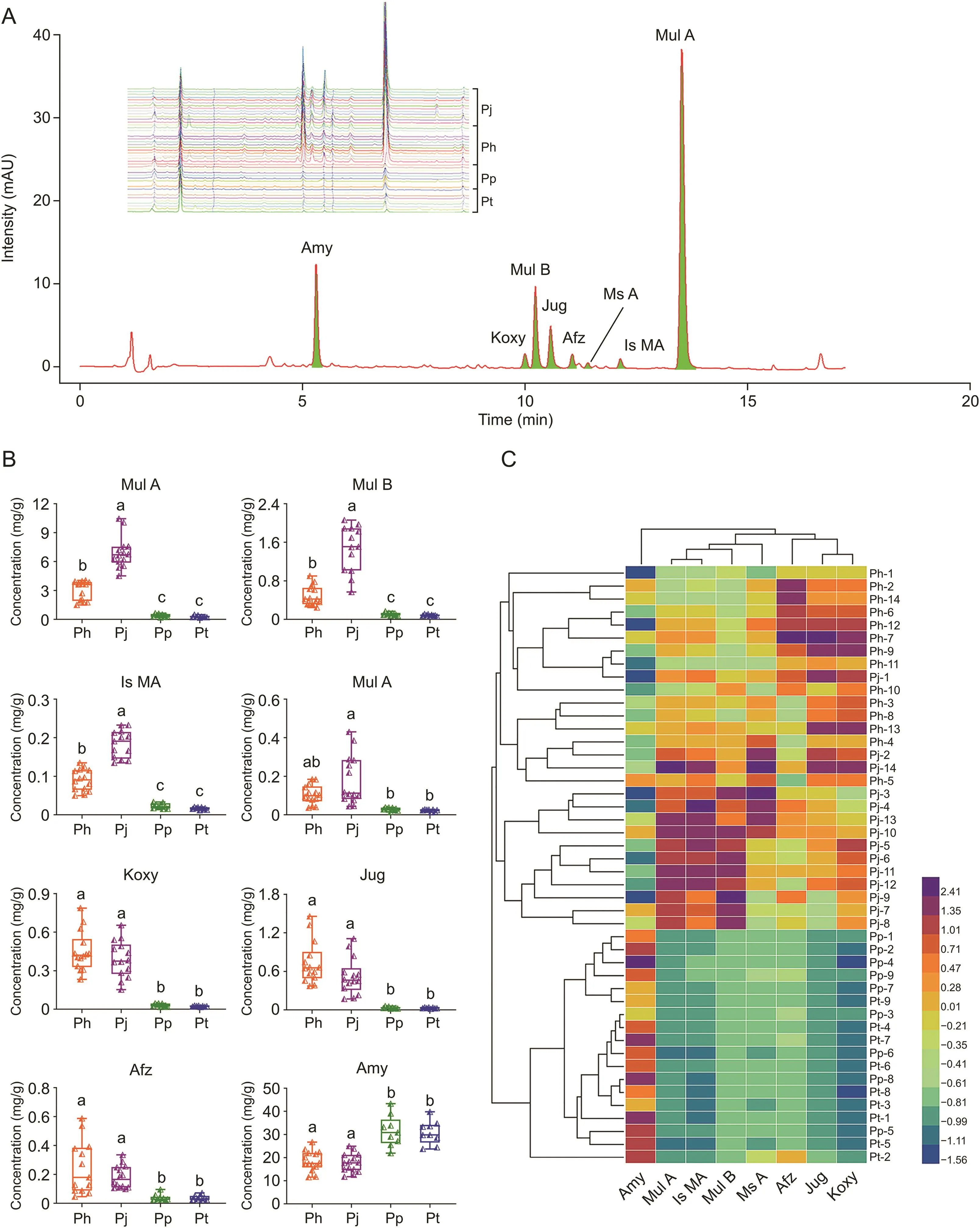
Fig.4.Ultra performance liquid chromatography(UPLC)fingerprint and quantitative analyses of Pruni Semen from four sources.(A)Eight quantitative constituents(sample Ph-13 as an example,quantitative constituents are green peaks)and UPLC fingerprint of Pruni Semen from four sources(inset:UPLC fingerprint of Pruni Semen from four sources).(B)Box diagram of the content of the eight constituents.There was significant difference between groups with different letters(P<0.05).The triangle indicates the single sample data.(C)Clustering heat map of the 46 samples.Ph and Pj:small seeds;Pp and Pt:big seeds.Amy:amygdalin;Mul A:multiflorin A;Mul B:multiflorin B;Afz:afzelin;Jug:juglalin;Koxy:kaempferol 3-O-β-D-xylopyranoside;Ms A:multinoside A acetate;Is MA:isomer multiflorin A;Ph:Prunus humilis;Pj:Prunus japonica;Pp:Prunus pedunculata;Pt:Prunus triloba.

Fig.5.Multivariate analyses of Pruni Semen from four sources.(A)Principal component analysis(PCA)score plot of the eight major compounds in Pruni Semen samples.(B)Orthogonal partial least squares discriminant analysis(OPLS-DA)score plot of groups A and B.Validation of the model by permutation tests for two categorical variables:(C)DA1 and(D)DA2.(E)Biplot of eight major compounds in Pruni Semen samples.Ph and Pj:small seeds;Pp and Pt:big seeds.Ph:Prunus humilis;Pj:Prunus japonica;Pp:Prunus pedunculata;Pt:Prunus triloba.DA:discriminant analysis.
OPLS-DA modelling cannot only distinguish between different groups but also predict the attribution of unknown samples.The model was verified seven times.R2represented the goodness of fit,and Q2represented the prediction ability of the model.The R2Y and Q2Y values of the model established in this study were 0.904 and 0.892,respectively,indicating that the model had good accuracy.To evaluate whether the model was overfit,200 permutation tests were conducted on the classified variables(Figs.5C and D).The abscissa indicated the correlation between the grouped variables after replacement and the original grouped variables.The intercept of the blue regression line on the longitudinal axis of Q2was less than zero,and the R2value of all variables after replacement was far lower than the original point of the top right corner(Figs.5C and D).The intercept of the regression line on the longitudinal axis was less than 0.05.Therefore,the model was effective and reliable(Figs.5C and D).
In summary,as a propagule,Pruni Semen is rich in nutrients,which are distributed in the kernels.The number of flavonoids in the seed coat was substantial.Small seeds(Ph and Pj)were found to be authentic sources of Pruni Semen in terms of chemical composition,which is consistent with records in ancient books on traditional medicine.Pp is a legitimate source according to the Chinese Pharmacopoeia.However,it is more similar to Pt in terms of both chemical composition and genetic relationships(subgenus Amygdalus).Whether Pt,currently considered as a common counterfeit of Pruni Semen,has traditional medical value remains unclear.The content of purgative compounds in the counterfeit Pruni Semen was much lower than that in the genuine Pruni Semen,although the inverse trend was observed for Amy content.Compensating for the deficiency in the content of purgative compounds in the counterfeit Pruni Semen by increasing the dosage may cause a safety risk.In contrast,the Amy content of small Pruni Semen was low,so it was considered to have better safety characteristics.
4.Conclusions
An unprecedented comparative study on the chemical constituents of Pruni Semen from three authorized Prunus species and one counterfeit Prunus species revealed their chemical composition and distribution characteristics.A total of ten compounds were isolated from Ph for the first time,including a notable purgative acetyl flavonoid glycoside,Mul A.Seventy-six communal constituents from the seeds of four Prunus species were identified using LC-MS analysis,including 50 flavonoids,5 organic acids,3 cyanogenic glycosides and 18 other compounds.The acetyl flavonoid glycosides were considered as the characteristic constituents.The flavonoids were distributed in the seed coat and cyanogenic glycosides were found in the seed kernel.
The UPLC fingerprint,a quantitative analysis method for major constituents,and a multivariate analyses identification method of Pruni Semen from different sources were established.The difference in the content of purgative constituents in different samples was extremely remarkable.Compared with the other three species,Mul A,Mul B,and Ms A were present in higher quantities in Pj;Afz,Jug,and Koxy were more abundant in Ph;and Amy was the dominant constituent of Pp and Pt.In terms of chemical composition,small Pruni Semen(Ph and Pj)was the preferred source of Pruni Semen.Pruni Semen has been in clinical use for over 2000 years and is a promising natural material that can be used to overcome the current challenges of diet disorders.
CRediT author statement
Zihan Zhao:Conceptualization,Methodology,Validation,Investigation,Data curation,Visualization,Writing-Original draft preparation;Yue Liu:Conceptualization,Methodology,Data curation,Visualization,Writing-Reviewing and Editing;Yushi Zhang and Zeyu Geng:Investigation,Formal analysis;Rina Su and Lipeng Zhou:Data curation,Resources;Chao Han:Writing-Reviewing and Editing;Zhanjun Wang:Funding acquisition;Shuangcheng Ma:Supervision,Validation;Weidong Li:Conceptualization,Funding acquisition,Supervision,Project administration.
Declaration of competing interest
The authors declare that there are no conflicts of interest.
Acknowledgments
This study was sponsored by the Key Research and Development Programs in the Ningxia Hui Autonomous Region,China(Grant No.:2020BBF02027)and Beijing Natural Science Foundation(Grant No.:5212014).We thank the Chinese Academy of Sciences Institute of Botany,Shenmu Ecological Association,Kunyu Mountain Farm,and Linyi University for their assistance in collecting the experimental materials.
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jpha.2022.06.007.
 Journal of Pharmaceutical Analysis2022年5期
Journal of Pharmaceutical Analysis2022年5期
- Journal of Pharmaceutical Analysis的其它文章
- Potential therapeutic effects and applications of Eucommiae Folium in secondary hypertension
- Screening of immune cell activators from Astragali Radix using a comprehensive two-dimensional NK-92MI cell membrane chromatography/C18column/time-of-flight mass spectrometry system
- Fluorescent intracellular imaging of reactive oxygen species and pH levels moderated by a hydrogenase mimic in living cells
- Sensitive detection of microRNAs using polyadenine-mediated fluorescent spherical nucleic acids and a microfluidic electrokinetic signal amplification chip
- Visualizing the spatial distribution and alteration of metabolites in continuously cropped Salvia miltiorrhiza Bge using MALDI-MSI
- The dynamic metabolic profile of Qi-Yu-San-Long decoction in rat urine using UPLC-QTOF-MSEcoupled with a post-targeted screening strategy
