Maintaining the metabolic homeostasis of Helicobacter pylori through chronic hyperglycemia in diabetes mellitus: A hypothesis
Vasiliy Ivanovich Reshetnyak,Igor Veniaminovich Maev
Abstract
Helicobacter pylori (H. pylori) infection occurs in almost half of the world's population,most of whom are merely carriers of this microorganism. H. pylori is shown to be detected more frequently in patients with diabetes mellitus (DM)than in the general population,which is accompanied by a significantly increased risk of developing H. pylori-associated diseases. In addition,eradication therapy shows a low efficiency for H. pylori infection in patients with DM. There is a relationship between the level of chronic hyperglycemia and a higher detection rate of H. pylori as well as a lower efficiency of eradication therapy in patients with DM. The exact mechanisms of these phenomena are unknown. The authors make a hypothesis that explains the relationship between chronic hyperglycemia and the increased detection rate of H. pylori,as well as the mechanisms contributing to the improved survival of this bacterium in patients with DM during eradication therapy.
Key Words: Helicobacter pylori; Diabetes mellitus; Glycated hemoglobin A; H. pylori eradication; Amino acids and glucose as nutrients for H. pylori
INTRODUCTION
Forty years have passed since the description ofHelicobacter pylori(H. pylori) as a pathogen in the development of atrophic gastritis and peptic ulcer disease[1-3]. It has been shown thatH. pyloriinfection occurs in almost half of the population in the world,most of whom are merely carriers of this microorganism[4,5]. In addition,many researchers have indicated thatH. pyloriare detected more frequently in patients with diabetes mellitus (DM) than in the general population[6-11]. This is accompanied by a substantial increase in the risk of developingH. pylori-associated diseases[6,11,12]. At the same time,there are studies which report reverse results about the incidence of type 2 DM (T2DM) inH. pyloripositive patients[13-15]. However,the relationship betweenH. pyloriinfection and the risk of developing T2DM remains controversial and ambiguous. Hence,a prospective cohort study by Jeonet al[16]has shown thatH. pyloriinfection correlates with a high risk of T2DM. Similarly,a meta-analysis carried out by Mansoriet al[11] suggests thatH. pylorimay be one of the risk factors for T2DM. On the contrary,other studies report thatH. pyloriis not associated with either insulin resistance or the prevalence of T2DM[17-20]. Data from Tamuraet al[21] suggest that East Asian CagA-positiveH. pyloriinfection is not a risk factor for T2DM. The successfulH. pylorieradication rates in patients with type 1 and type 2 DM are 62% and 50%,respectively,which are much lower than those in people without these two forms of the disease[22-25]. The low efficiency of eradication therapy forH. pyloriinfection in diabetic patients is uniquely presented in many studies[26-29].
There is a clear correlation between the higher detection rate ofH. pyloriin diabetic patients and lower efficacy of eradication therapy,depending on the level of hyperglycemia[10,13,29]. Uncontrolled diabetes with the development of chronic hyperglycemia causes a number of metabolic changes[30].Chronic hyperglycemia in turn leads to increased susceptibility to infective agents in diabetic patients[9,10,30,31]. The exact mechanisms underlying the link of chronic hyperglycemia and the higher detection rate ofH. pylori,as well as the mechanisms that improve the survival of this bacterium in diabetic patients during eradication therapy remain unknown. An understanding of how chronic hyperglycemia is related to the maintenance of the metabolic homeostasis ofH. pylorifor its vital activity and reproduction in diabetic patients is of great scientific and practical importance.
It is hypothesized that chronic hyperglycemia is associated with: (1) The increased detection rate ofH.pylori; (2) possible metabolic changes in the bacterial cells ; and (3) the results of eradication therapy.
It is well known thatH. pyloricolonizes the gastric mucosa. To establish long-term colonization,the bacterium must sense and adapt to the nutritional conditions that exist in its habitat.Surprisingly,little attention has been paid to the preferred sources of nutrients and energy for the life,growth,and reproduction ofH. pylori,as well as changes in the source of food ingredients and energy forH. pyloriin diabetic patients. The available data suggest that for its life,growth,and reproduction,H. pyloriutilizes amino acids and carboxylic acids,which are produced in sufficient quantities in the stomach as a result of hydrolysis of food proteins[32-34].H. pyloricatabolize a large amount of amino acids with the most substantial being alanine,arginine,asparagine,aspartate,glutamate,glutamine,proline,and serine[32,35-37].H. pylorican also catabolize fumaric acid[38],malic acid[35],and lactic acid[39]. Thus,amino acids and carboxylic acids are sources of carbon,nitrogen,and energy.
In a healthy individual,H. pyloriare almost independent of sugars,such as glucose[32-34]. However,glucose is known to be one of the most important carbohydrates,which is used for life by many microorganisms,including inhabitants in the digestive system.Moreover,Wanget al[40] believe that glucose plays a key role in the outcome of bacterial infection in humans. A question is raised as to whetherH. pylorican utilize glucose as a plastic and energy material. Studies conducted in the 1990s and later indicate thatH. pylorihas enzyme systems capable of utilizing carbohydrates,D-glucose in particular[41-43]. These data suggest that in its evolutionary phylogenetic development and adaptation to life and reproduction in the stomach,H. pylorinot only acquire the ability to restructure its metabolism for the use of amino acids as a plastic and energy material,but most probably retain the ability to utilize carbohydrates for their life activity. There are experimental data showing that adding glucose to the nutrient medium when growingH. pylori,enhances its growth[29,44].
Chronic hyperglycemia in diabetic patients involves compensatory mechanisms aimed at normalizing the blood level of glucose[5]. To remove excess glucose in patients with DM and chronic hyperglycemia,it is most likely that the extradigestive (excretory) function of the gastric mucosa is switched on. This leads to the fact that in patients with DM and chronic hyperglycemia,H. pylorigain advantages for its growth,reproduction,and survival as it can use not only amino acids for its life,but also glucose available in excess in patients with DM. This hypothesis may explain the more frequent detection ofH.pyloriin patients with DM than in the general population.
Based on this hypothesis,it is possible to explain also the lower efficiency of eradication therapy in patients with DM.
H. pylorieradication regimens contain antibacterial drugs (clarithromycin,metronidazole,bismuths,etc.) and agents that reduce hydrochloric acid production. The use of antacids aimed at creating optimal conditions for acid-dependent antibacterial agents[45-48]. The data presented in recent studies suggest that it is extremely important to determine gastric pH forH. pylorieradication[45,46].In addition,the antacids have a double effect onH. pyloriwith an opposite effect. Increased gastric pH is a favorable factor for the vital activity ofH. pylori. But at the same time,the antacids depriveH. pyloriof nutrients.Exposure to hydrochloric acid in the stomach causes denaturation of food proteins and initiates their hydrolysis by the gastric juice enzymes pepsin and gastrixin. This gives rise to oligopeptides with different lengths and to a certain amount of amino acids,which are utilized byH. pylorifor its life activity. Taking antacids practically does not lead to denaturation of food proteins. Consequently,the rate of protein hydrolysis is considerably reduced. As a result,the stomach practically does not produce amino acids that are essential for maintaining the vital activity ofH. pylori.The lack of nutrients and the intake of antibacterial drugs result in the death of the microorganism or in its transition to a dormant form[49]. The latter is rare during powerful antibiotic therapy.
There is an opportunity forH. pylorito utilize glucose as an energy and plastic material in diabetic patients receiving eradication therapy against the underlying chronic hyperglycemia and amino acid deficiency. It is likely that this mechanism enables this microorganism to successfully survive the extreme conditions of eradication. But this can happen only in the presence of chronic hyperglycemia.That is to say,the survival ofH. pyloriunder extreme conditions of eradication should depend on the level of hyperglycemia. And the longer period of hyperglycemia is,the more likelyH. pylorisurvive the extreme conditions of eradication.
Chronic hyperglycemia can be assessed by the blood level of glycated hemoglobin A (HbA1c)(Figure 1). The HbA1c level is the result of nonenzymatic glycosylation of hemoglobin,with the formation of a bond between glucose and the free N-terminal proline amino group in the hemoglobin βchain[50]. The indicator plays an important role in monitoring the time course of changes in blood glucose levels in diabetic patients and for evaluation of the efficacy of hypoglycemic drugs[51]. In 2011,the World Health Organization officially recommended an HbA1c level of ≥ 6.5% as a diagnostic cut-off value for DM[52]. This indicator reflects the integrated blood glucose level for the last 3-4 mo[53-55].The association betweenH. pyloriinfection and HbA1c in diabetic patients has been confirmed in many studies[51,56,57]. Glycated hemoglobin A levels were significantly higher in patients with DM andH.pyloriinfection than in those with DM and withoutH. pyloriinfection (WMD = 0.50,95%CI: 0.28-0.72,P< 0.001)[51]. Subgroup analysis by the subtype of DM has revealed a correlation betweenH. pyloriinfection and an elevated glycated hemoglobin A level in type 1 DM (I2= 74%,P< 0.001,WMD = 0.46,95%CI: 0.12-0.80) and in T2DM (I2= 90%,P< 0.001,WMD = 0.59,95%CI: 0.28-0.90,P< 0.001)[51].
Bektemirovaet al[58] used the HbA1c level to evaluate the efficacy of hypoglycemic drugs taken by 83 patients with T2DM andH. pylori-associated diseases during eradication therapy. Glycated hemoglobin A was shown to reach a target level of < 6.5% in 62 of the 83 examinees,while it remained elevated (> 7.0%) in 21 patients. This means that despite the use of hypoglycemic drugs,the level of hyperglycemia persisted in these patients for at least 2-3 mo. And it was in these patients who did not reach the target HbA1c level had a significantly (P< 0.017) lower efficiency of eradication therapy than those who achieved the target level of HbA1c < 6.5%. The data obtained by Bektemirovaet al[58]indirectly suggest thatH. pylorimost likely take advantage of chronic hyperglycemia to survive under the extreme conditions of eradication.
According to Tseng,the use of insulin to normalize blood glucose levels in patients with T2DM substantially increases the rate ofH. pylorieradication compared to those with DM without insulin administration[25].The higher efficiency ofH. pylorieradication in T2DM patients taking insulin suggests that these patients are more likely to normalize their blood glucose levels during insulin therapy. And this is most likely to cause an increase in the efficiency ofH. pylorieradication.
CONCLUSION
The data available in the literature advance the following hypothesis that in diabetic patients,H. pyloriare most likely to utilize both amino acids and glucose for its vital activity. The hypothesis makes it possible to explain the high detection rate ofH. pyloriin diabetic patients,as well as their lower eradication therapy efficiency. Undoubtedly,this hypothesis requires further conformations by biochemical,microbiological,molecular genetics,and other studies. Further multicenter studies are needed to confirm this hypothesis. But if this hypothesis is correct,then beforeH. pyloriare eradicated in DM patients,there is a need for mandatory monitoring and targeted correction of blood glucose and HbA1c levels according to the algorithm given in Figure 1. The algorithm can be used for the management of patients with DM and concomitantH. pylori-associated diseases,which is of great practical importance for their successful eradication therapy.
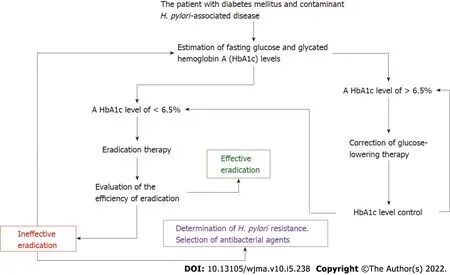
Figure 1 Algorithm for monitoring and targeted correction of glycated hemoglobin A levels in patients with diabetes mellitus and Helicobacter pylori-associated diseases. H. pylori: Helicobacter pylori.
ACKNOWLEDGEMENTS
The authors express their gratitude to Alexandr Igorevich Burmistrov for technical assistance in preparing this article.
FOOTNOTES
Author contributions:All the authors have equally contributed to the study conception and design,literature review and analysis,manuscript drafting,critical revision and editing,and approval of the final version.
Conflict-of-interest statement:All authors declare that they have no conflict of interest.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license,which permits others to distribute,remix,adapt,build upon this work non-commercially,and license their derivative works on different terms,provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:Russia
ORCID number:Vasiliy Ivanovich Reshetnyak 0000-0003-3614-5052; Igor Veniaminovich Maev 0000-0001-6114-564X.
S-Editor:Liu JH
L-Editor:Ma JY-MedE
P-Editor:Liu JH

