Vimentin as a potential target for diverse nervous system diseases
Kang-Zhen Chen , Shu-Xian Liu , Yan-Wei Li , Tao He Jie Zhao Tao Wang , Xian-Xiu Qiu , Hong-Fu Wu
Abstract Vimentin is a major type III intermediate filament protein that plays important roles in several basic cellular functions including cell migration, proliferation, and division.Although vimentin is a cytoplasmic protein, it also exists in the extracellular matrix and at the cell surface.Previous studies have shown that vimentin may exert multiple physiological effects in different nervous system injuries and diseases.For example, the studies of vimentin in spinal cord injury and stroke mainly focus on the formation of reactive astrocytes.Reduced glial scar, increased axonal regeneration, and improved motor function have been noted after spinal cord injury in vimentin and glial fibrillary acidic protein knockout (GFAP–/–VIM–/–) mice.However, attenuated glial scar formation in post-stroke in GFAP–/–VIM–/– mice resulted in abnormal neuronal network restoration and worse neurological recovery.These opposite results have been attributed to the multiple roles of glial scar in different temporal and spatial conditions.In addition, extracellular vimentin may be a neurotrophic factor that promotes axonal extension by interaction with the insulin-like growth factor 1 receptor.In the pathogenesis of bacterial meningitis, cell surface vimentin is a meningitis facilitator, acting as a receptor of multiple pathogenic bacteria, including E.coli K1, Listeria monocytogenes, and group B streptococcus.Compared with wild type mice, VIM–/– mice are less susceptible to bacterial infection and exhibit a reduced inflammatory response, suggesting that vimentin is necessary to induce the pathogenesis of meningitis.Recently published literature showed that vimentin serves as a double-edged sword in the nervous system, regulating axonal regrowth, myelination, apoptosis, and neuroinflammation.This review aims to provide an overview of vimentin in spinal cord injury, stroke, bacterial meningitis,gliomas, and peripheral nerve injury and to discuss the potential therapeutic methods involving vimentin manipulation in improving axonal regeneration, alleviating infection, inhibiting brain tumor progression, and enhancing nerve myelination.
Key Words: astrocytes; axonal regeneration; bacterial meningitis; glial scar; gliomas; nervous system diseases; peripheral nervous system injury; spinal cord injury; stroke; vimentin
Introduction
Vimentin was the first member of the intermediate filament protein III coding genes to be cloned (Zehner and Paterson, 1983).Vimentin has been identified in the cells and tissues of many organs and has a molecular weight of approximately 57 kDa.Vimentin has mainly been described in a variety of cancers and immune system diseases in the past decades, and a large number of review articles have elaborated the role of vimentin in various cancers (Kidd et al., 2014; Chen et al., 2021).Other reviews have focused mainly on the role of vimentin in immunity (Kuna, 2012; Musaelyan et al., 2018) and on cellular functions (Ivaska et al., 2007; Patteson et al., 2020).Increasing findings have suggested that vimentin exerts diverse effects on nervous system injuries and diseases.The present review is aimed at elaborating on the biochemical cascade of vimentin and describing its implication in the pathological processes of diverse neurological diseases.
Vimentin knockout (VIM) mice are ideal models used by researchers to investigate the functions of vimentin (Ridge et al., 2022).Through comprehensive analysis of the phenotypes of VIMand wild-type (WT)mice, vimentin’s functional significance was easily explored.One of earliest and most profound studies on vimentin deficiency showed its ability to promote axonal regeneration after spinal cord injury (SCI).The lack of axonal regeneration in the injured central nervous system (CNS), especially in injured spinal cord, is closely related to the inhospitable environment formed by glial scar.Compared with WT mice, GFAPVIMmice exhibit significantly reduced glial reactivity and increased axonal sprouting of neurons as well as functional recovery (Menet et al., 2003).Lack of vimentin and GFAP could change the morphological features of reactive astrocytes by reducing glial scar formation, thereby creating an environment favoring axonal regeneration.In neural regeneration following SCI, vimentin might indirectly take part in determining axonal plasticity by influencing glial scar formation.However,other studies have found that the attenuated reactive astrocytes in GFAPVIMmice significantly reduced axonal regeneration and impaired normal neural plasticity.Increasing evidence suggests that reactive astrocytes limit neuroplasticity and neural regeneration in the CNS, and attenuated reactive astrocytes are also required for normal recovery of neuronal connection reconstruction in peri-infarct areas post-stroke (Liu et al., 2014; Aswendt et al., 2022).
Although vimentin is a principally intracellular cytoskeletal protein involved in regulating cell stiffness, cell motility, and cytoplasmic organization, it can also appear within the extracellular matrix via secretion and at the surface of various cells, often in association with axonal plasticity, inflammation,and bacterial infection.For instance, extracellular vimentin released from astrocytes is regarded as a novel axonal regeneration facilitator, and recombinant vimentin exerts neurotrophic effects by promoting axonal extension after SCI (Shigyo and Tohda, 2016).Additionally, extracellular vimentin interacts with von Willebrand factor (VWF) to induce spontaneous platelet adhesion in the vascular lumen, leading to microthrombus formation and stroke pathology (Fasipe et al., 2018).At the cell surface, vimentin functions as a receptor or co-receptor for multiple pathogenic bacteria,includingE.coli
K1,L.monocytogenes
, and group B streptococcus.Thus, cell surface vimentin is regarded as a central meningitis facilitator that mediates transport of multiple pathogenic bacteria across the blood-brain barrier(BBB), allowing them to colonize in the brain (Zou et al., 2006; Chi et al., 2012;Manzer et al., 2022).Recently, the novel functions of vimentin in neural stem cells (NSCs) havebeen found.These results showed that vimentin knockout NSCs had a reduced capacity to exit quiescence, and that quiescent NSCs recovered proteostasis through asymmetrical mitosis such that damaged proteins cosegregate with vimentin.Thus, vimentin is a critical regulator of quiescence exit and protein clearance in NSCs.In addition, vimentin knockout in NSCs reduced the ability of NSCs to recover from increased levels of misfolded or polyubiquitinated proteins.Interestingly, vimentin knockout NSCs exhibited increased autophagy flux, suggesting that vimentin knockout NSCs are more reliant on increased levels of autophagy to clear aggregated proteins (Morrow et al., 2020).The current understanding of vimentin’s function is limited, and more research is needed to continue to characterize the potent biological roles of vimentin.These new findings warrant a further understanding and elucidation of the roles of vimentin in neurological studies.
This review presents the most recent studies and current understanding of vimentin functions in various nervous system injuries or diseases and discusses the possible therapeutic effects of vimentin manipulation in neurological function restoration and the clinical relevance of high vimentin expression in CNS disorders.
Search Strategy and Selection Criteria
For the present review, the literature was searched using the following terms:“vimentin” AND “nervous system”; “vimentin” AND “neural stem cells”OR “NSCs”; “vimentin” AND “spinal cord injury” OR “SCI”; “vimentin” AND“stroke”; “vimentin” AND “glial scar” OR “astrocytic scar”; “vimentin” AND“meningitis”; “vimentin” AND “brain tumors”; “vimentin” AND “gliomas”;“vimentin” AND “peripheral nerve”; “vimentin” AND “Alzheimer’s disease”OR “AD”; “vimentin” AND “multiple sclerosis” OR “MS”.A PubMed literature search of articles published during the period mainly from January 2000 to April 2022 was conducted.The results were further screened by title and abstract, and only those studies exploring the relationship between vimentin and neurosciences were included to investigate the effects of vimentin in nervous system diseases.
Vimentin in Spinal Cord Injury
SCI can result from a variety of causes, including direct trauma, inflammation,infection, tumor or neurodegeneration.By far the most common causes of damage to the spinal cord are traumatic injuries (Fernández et al., 2022).Regarding traumatic spinal cord injuries, the injury site determines the different degrees of motion and sensory dysfunction.Generally, cervical level injuries and extreme severe trauma frequently result in serious neurological problems that cause respiratory depression, motor-sensory system deficiency,and seriously threaten the lifespan of victims (Korovessis et al., 2021).Many SCI patients are young and make up the main part of the workforce, so their permanent disability imposes a heavy burden.Additionally, current treatments fail to meet the expectation of SCI patients around the world.Therefore, explorations of the SCI-induced microenvironment are imperative.Accumulating evidence indicates that astrocytic scar formation is widely perceived as a physical and chemical barrier to axonal regeneration after SCI(Orr and Gensel, 2018; Raspa et al., 2021; Zhang et al., 2021).The underlying mechanisms for this phenomenon may be associated with the inhospitable local environment formed by astrocytic scar and inhibitor molecules secreted by reactive gliosis.
Silence of vimentin and GFAP improves functional recovery after SCI
Vimentin, a major protein of the astrocyte cytoskeleton, and GFAP commonly contribute to glial scar formation.Vimentin is generally expressed in both the gray and the white matter of the spinal cord, and its levels are significantly increased after SCI, suggesting that vimentin may be involved in the pathogenesis of traumatic spinal cord.Early studies have found that silencing vimentin and GFAP alleviates over-proliferation of reactive astrocytes and promotes supraspinal axons regeneration, leading to neural circuit reconstruction and locomotor functional rehabilitation (Menet et al., 2003;Toyooka et al., 2011; Desclaux et al., 2015).Interestingly, only GFAP/vimentin double mutant mice exhibited remarkable functional restoration associated with reduced astrocyte reactivity after hemisection of the spinal cord.Single GFAP or vimentin mutant mice exhibited minimal alteration in astroglial response and acquired limited axonal sprouting (Menet et al., 2003).Glial scar acts as a firm wall that blocks spared axonal regrowth.Double vimentin and GFAP knockout reduced glial scar formation, which is beneficial for axonal regrowth (Zhang et al., 2021).Nevertheless, it is possible that vimentin and GFAP have some redundant functions in glial scar formation, and gene modification of either may not alter functional consequences.A separate study demonstrated that lentivirus-induced silencing of both GFAP and vimentin leads to an efficient reduction in astrogliosis, improves functional outcomes, and promotes spared axonal plasticity in a preclinical mouse model of SCI (Desclaux et al., 2015).Moreover, administration of atelocollagen combined with both GFAP and vimentin siRNA suppresses glial scar formation and improves acute urinary dysfunction caused by SCI.It can be inferred that RNAi against GFAP and vimentin might improve SCI-induced acute urinary dysfunction by downregulating intermediate filament proteins (Toyooka et al.,2011).Normal urination, a result of bladder detrusor contraction and urethral sphincter relaxation, is controlled by both spinal and supraspinal circuitry.The coordination between the bladder detrusor and the urethral sphincter is interrupted by thoracic SCI, mainly because of the loss of white matter in the lateral and ventral funiculi, which includes ascending and descending micturition reflex fiber tracts.It is plausible that inhibition of glial activity in white matter surrounding the micturition reflex pathways is beneficial for residual neurons to bridge the gap of the nerve defects.
Combination strategies targeting vimentin to reduce glial scar formation represent potential methods to promote axonal extension and functional recovery.In rat models of hemisected spinal cord, a combination of retroviruses carrying antisense vimentin cDNA and Chondroitinase ABC(ChABC) reduces glial scar and cystic cavity formation (Xia et al., 2008).Further study by this group found that vimentin inhibition combined with ChABC also plays an important role in stimulating axon regeneration and locomotor function recovery (Xia et al., 2015).Together with their previous research, the combined treatment targeting vimentin implied a beneficial role in SCI repair, emphasizing the role of vimentin inhibition and ChABC in reducing glial scar and degrading chondroitin sulfate proteoglycan (CSPG),which are inhibitory molecules for axonal sprouting.At present, spinal injury scar is generally recognized as an inhibitor of axonal regeneration and impairs functional recovery (Figure 1).However, there has been some recent debate about whether spinal cord scar is good or bad for spinal cord repair(Bradbury and Burnside, 2019).Two different viewpoints could be interpreted two different ways.Although widely regarded as causal in restricting axonal extension, astrocytic scars play a protective role in separating healthy tissue from injured spinal cord.Meanwhile, Anderson et al.(2016) found that contrary to the prevailing viewpoint, glial scar formation is not a main cause for failure of SCI-induced injured axons to regenerate across central spinal cord lesions.Further studies will clarify the function of vimentin in SCI repair.

Figure 1 | Astrocytic reactivity after SCI may play a detrimental role in neurological recovery.
Extracellular vimentin secreted by astrocytes is beneficial for axonal regeneration
In addition to the effect of vimentin on glial scar formation, the extracellular vimentin secreted by activated macrophages and reactive astrocytes has been investigated.Existing evidence has confirmed that extracellular vimentin is a novel neurotrophic factor that could promote the regrowth of axons and contribute to motor function rehabilitation following SCI (Shigyo and Tohda,2016).Denosomin, a novel compound, has been certified as a facilitator that increases the concentration of astrocyte-secreted vimentin, and this is closely linked to axonal regeneration in spinal cord-traumatic mice (Teshigawara et al., 2013).Another study from this group also showed that recombinant vimentin treatment enhances axonal growth and improves performance in motor function in SCI mice (Shigyo and Tohda, 2016).However, the signaling mechanisms of axonal regrowth arising from recombinant vimentin remain unclear.In order to elucidate these mechanisms, further studies found that recombinant vimentin, in a manner similar to insulin-like growth factor(IGF1), may function as a novel ligand that directly binds to insulin-like growth factor 1 receptor (IGF1R) to cause axonal regeneration in cultured cortical neurons (Shigyo et al., 2015).Many studies have shown that IGF1-IGF1R crosstalk contributes to neurite outgrowth.Additionally, the study above has shown that both IGF1 and extracellular vimentin significantly promote phosphorylation of IGF1R.In other words, the extracellular vimentin-IGF1R signaling pathway may exert a similar effect as the IGF1-IGF1R signaling pathway in enhancing axonal regeneration.Notably, inhibition of intermediate filament proteins, such as vimentin and GFAP, could promote axon extension by reducing the formation of glial scar after SCI.However, recombinant vimentin treatment is associated with neurite outgrowth.The reasons why intracellular and extracellular vimentin play different roles in axonal regrowth remain unclear.It is unlikely that intracellular vimentin is involved in glial scar formation but rather that extracellular vimentin functions as a ligand that interacts with IGF1R to promote axonal plasticity.Therefore, further studies are needed to explore the relationship between intracellular and extracellular vimentin, and intelligent regulation of vimentin may provide a potential axonal regrowth strategy (Shigyo et al., 2015).
Vimentin in Stroke
Stoke, which is also known as cerebrovascular accident, is attributed to interrupted brain blood supply, usually due to blood vessel hemorrhage or blockage.Disruptions in oxygen and nutrient supply due to reduced blood flow cause brain tissue damage.At present, stroke has become a serious issue worldwide and is associated with prominent morbidity and mortality.Reports of vimentin in connection with stroke mainly focus on its role in astrocytic reactivity.The effect of reactive astrocytes post-stroke remains controversial,despite several reports (Liu et al., 2014).Stroke-induced astrocyte activation may exert both detrimental and beneficial roles under certain temporal and spatial conditions (Huang et al., 2018; Daidone et al., 2021; Wen et al., 2021).For instance, the expression of inhibitory molecules on reactive astrocytes is inextricably linked to a reduction in axonal regeneration.However, astrocytic scar may also separate damaged sites from intact tissue, preventing healthytissue from uncontrolled cascade reactions (Faulkner et al., 2004).Moreover,reactive astrocytes may also alleviate neuronal damage due to ischemic stress due to its ability to secrete numerous neurotrophic factors (Jiao et al., 2020).
Absence of vimentin and GFAP impairs neurological recovery after ischemic stroke
Existing evidence indicates that astrocytic reactivity after stroke may play a protective role in neurological recovery (Liu et al., 2014; Liu and Chopp,2016; Aswendt et al., 2022).Seven days after ischemic stroke induced by middle cerebral artery occlusion, GFAPVIMmice exhibited enlarged infarct volume, 2.1–3.5 fold larger than that of WT mice, suggesting a protective role of reactive astrocytes (Li and Murphy, 2008).However, there was no significant difference in infarct size between GFAPVIMand WT mice after ischemic stroke induced by cortical photothrombosis.Liu et al.(2014) reported that similar cerebral infarct volume induced by the Rose Bengal technique was generated in WT and GFAPVIMmice.As expected,research demonstrated that attenuated reactive astrocytes in GFAPVIMmice delayed or impaired functional recovery by increasing CSPG expression,reducing corticospinal tract (CST) axonal length, and decreasing perilesion astrocyte density.Compared with WT mice, the expression of CSPG was significantly increased in the cortical area at the outer ischemic lesion boundary zone and in the contralesional cerebral hemisphere in GFAPVIMmice.CSPG is an inhibitory protein secreted by reactive astrocytes that prevents axonal regeneration (Liu et al., 2014).Thus, the reduced CST axonal regeneration in GFAPVIMmice may in part be attributed to increased CSPG expression (Figure 2).
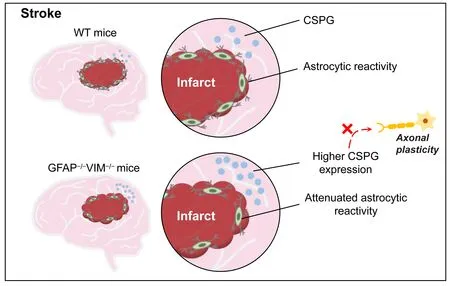
Figure 2 | Astrocytic reactivity after stroke may play a beneficial role in neurological recovery.
In a recent study of stroke-induced changes in GFAPVIMmice, decreased astrocytic reactivity impaired normal sensorimotor network recovery and induced new neural connections as well as altered neural regeneration responses around the infarct area.These findings suggest that stroke-induced astrocytic reactivity is necessary for axonal regeneration and optimal neuronal network reorganization (Aswendt et al., 2022).However, there are still many questions concerning reactive gliosis in stroke and SCI to be answered.First, glial scar formation in cerebral infarction leads to enhanced functional recovery after stroke; but glial scar formation is detrimental to functional restoration after SCI (Liu et al., 2020).When SCI-induced glial scar is formed,it may be a physical and chemical barrier for nerve regeneration that impedes regeneration of descending nerve fibers into the injured site, which leads to loss of connections with remaining neurons in the damaged site (Wilhelmsson et al., 2004).Thus, attenuated glial scar in injured spinal cord may have a beneficial role in facilitating neurite extension and functional recovery.Unlike SCI-induced glial scar, stroke-induced reactive gliosis around areas of infarct may not be a barrier for neurogenesis.Owing to the fact that there are no surviving neurons in the ischemic infarct tissue, it is unnecessary to promote nerve regeneration across the scar into the infarct area.By contrast,adjacent glial scar seems likely to play a beneficial role in improving functional recovery by protecting heathy tissue from ischemic infarcted tissue.Thus, it is not necessary to promote neurite extension across the glial scar and into the infarcted lesion.Collectively, reactive astrocytes play various roles with different effects on neuronal regrowth in SCI and stroke.Thus, instead of inhibition, adaptive modulation of reactive astrocytes within the correct time window may be a promising strategy for enhancing functional restoration after ischemic stroke.
Vimentin is associated with stroke pathology
Extracellular vimentin also participates in the pathogenesis of ischemic stroke.Endothelium surface vimentin binds to the A2 domain of newly released VWF strings in the vascular lumen (Da et al., 2014), which may lead to thrombogenesis and thereby cause stroke (Wieberdink et al., 2010).Briefly,vimentin/VWF interaction leads to the onset of ischemic stroke.Therefore,the targeted destruction of vimentin/VWF interactions can decrease injury due to ischemia reperfusion.To investigate the effects of vimentin in VWF string formation, cerebral arteries from WT and vimentin knockout mice were isolated to perform pressure treatment and histamine was used to activate endothelium and induce VWF string formation.Notably, less VWF string formation occurred in the pressurized cerebral artery of vimentin knockout mice compared with WT mice.In addition, VWF strings were significantly decreased in the presence of anti-vimentin antibodies and recombinant A2 protein in histamine-stimulated human umbilical vein endothelial cells (Fasipe et al., 2018).These findings demonstrate an important role of extracellular vimentin in VWF string formation via binding with the A2 domain and restricting vimentin/VWF interaction, which may improve reperfusion after ischemic stroke.
In addition, a recent study has found that high vimentin is strongly related to the incidence of ischemic stroke (Xiao et al., 2021).Xiao et al.(2021) carried out a prospective study to investigate the relationship between vimentin in serum and stroke incidence in the Malmö Diet and Cancer Cohort.The results showed that high plasma levels of vimentin and carotid plaque are both associated with a higher risk of ischemic stroke.One potential explanation of this relationship might be associated with the role of vimentin in atherosclerosis (Håversen et al., 2018; Gong et al., 2019).An earlier study suggested that elevated serum levels of secretory vimentin in patients are associated with coronary artery disease.In ApoE knockout mice, recombinant vimentin significantly promotes atherogenesis, which is one of the principal causes of stroke (Gong et al., 2019).Thus, vimentin may promote atherosclerosis and thereby induce stroke.Collectively, high vimentin is closely associated with stroke pathology, especially in individuals with carotid plaque.However, it is unclear whether high vimentin results in increased stroke incidence by promoting atherogenesis.
Vimentin in Bacterial Meningitis
Bacterial meningitis is an acute infectious CNS disease with rapid onset,epidemic potential and high case-fatality rate (Lorton et al., 2018).Unfortunately, 50% of bacterial meningitis survivors are left with some type of neurological or neuro-behavioral sequelae (Jumanne et al., 2018).The common mechanisms underlying the pathogenesis of bacterial meningitis include bacterial colonization and invasion of the mucosal barrier in the nasopharynx and survival and multiplication of bacteria in the bloodstream that penetrate the BBB and invade into the CNS (Travier et al., 2021; Tsang,2021; Wall et al., 2021).
Cell surface vimentin interacts with multiple bacterial pathogens in the pathogenesis of meningitis
Escherichia coli
K1 (E.coli
K1), a common gram-negative bacteria, usually causes meningitis during the neonatal period (Huang et al., 2000).Research has indicated that the IbeA gene produced byE.coli
K1 is a virulence factor that binds with human brain microvascular endothelial cell (HBMEC) surface vimentin to form a IbeA-vimentin interaction.The main domain of HBMECs surface vimentin and middle region of IbeA (271–370 residues) act as binding sites for the IbeA-vimentin interaction (Zou et al., 2006).Hence, vimentin is involved in the pathological process of bacterial meningitis.In addition to vimentin acting as a primary receptor, studies published in 2012 showed that polypyrimidine tract-binding protein (PTB)-associated splicing factor (PSF) acts as a co-receptor that plays a cooperative role in IbeA-inducedE.coli
K1 invasion (Chi et al., 2012).The NF-κB signaling pathway, which was previously identified as a master regulator of innate immunity, is activated in cerebrospinal fluid cells in patients with meningitis and in epithelial cells withNeisseria meningitidis
invasion (Griffiths et al., 2007).Chi et al.(2012)found that two IbeA-binding proteins, the primary receptor vimentin and the co-receptor PSF, are required to activate the NF-κB signaling pathway in IbeAE.coli
K1 meningitis infection.Vimentin can form a complex with NF-κB during cell arrest, but the complex dissociates IbeA stimulation.Vimentin and PSF are required for IbeAE.coli
K1-induced activation and translocation of NF-κB, which subsequently upregulate the expression of vimentin and proinflammatory factors that result in bacterial infections (Chi et al., 2012).Moreover, the firstin vivo
study has verified that vimentin-dependent mechanisms underlie the triad of pathogenic features inE.coli
meningitis.These results suggest that vimentin deficiency is a favorable factor protecting neonatal mice fromE.coli
K1-induced bacterial meningitis and inhibiting the inflammatory response in CNS (Huang et al., 2016).The role of vimentin in inflammation in bacterial meningitis
In addition toE.coli
K1,L.monocytogenes
may also disrupt the integrity of the BBB and lead to threatening meningitis and encephalitis.Ghosh et al.(2018) found that theL.monocytogenes
surface protein, InlF, could interact with host cell surface vimentin to colonize the brain.However, vimentin absence contributes to severely compromisedL.monocytogenes
colonization in the mice brain.Notably, vimentin is a central meningitis factor that mediates the penetration and destruction of the BBB and colonization of the brain by multiple bacterial pathogens.Similarly, Deng et al.(2019) also found that host cell vimentin interacts with the group B streptococcal surface antigen I/II protein, BspC, to facilitate colonization in the brain endothelium and CNS inflammation during the pathogenesis of Group B Streptococcus(GBS) meningitis.In a mouse model of hematogenous meningitis, vimentin knockout mice were significantly less susceptible to GBS infection, resulting in a reduced inflammatory response.Recent data indicate that the BspC variable domain is the binding site of vimentin (Manzer et al., 2022).Conclusively,the interaction of BspC-vimentin is crucial in GBS adherence to the BBB and promotes the pathogenesis of GBS meningitis.However, the role of vimentin in inflammation is unclear.Thus, their lab further explored the roles of vimentin in mediating the inflammatory response during GBS meningitis.The results demonstrated that vimentin plays a direct role in mediating the expression of chemokines, and that vimentin regulates the activity of nucleotide-binding oligomerization domain containing protein 2 in HBMEC,which is a classical activator of the NF-κB signaling pathway.Additionally,localized disruption of vimentin by withaferin-A (WFA) ameliorates chemokine activation (Villarreal et al., 2021).Collectively, existing evidence shows that vimentin is mainly involved in the pathogenesis of meningitis at the level of the host cell surface receptors and mediates the crossing of pathogenic bacteria across the BBB and colonization in the brain by binding with surface ligands of a variety of pathogenic bacteria,causing meningitis.Blocking this receptor-ligand interaction might represent a promising strategy for preventing bacterial meningitis.
Vimentin in Gliomas
Gliomas are the most prevalent and malignant primary brain tumors of the CNS.They mainly emanate from neuroglial progenitor cells and develop into oligodendroglioma, oligoastrocytoma, astrocytoma, or ependymoma (Na et al., 2012; Ostrom et al., 2018).Glioblastoma multiforme (GBM) is the most common grade IV glioma, accounting for about 50% of glioma cases.GBM is highly invasive, well vascularized, and deadly, with a 5-year survival rate of less than 5%.The current gold standard therapy of GBM consists of fractionated radiotherapy and temozolomide (TMZ) and was implemented more than a decade ago.Unfortunately, this therapy represents only a palliative treatment for patients, and the average survival time after diagnosis is 14 months.Recently, vimentin, a pro-invasive tumor marker, was shown to be closely linked with malignant glial cell invasion (Van Meir et al., 2010).However, the definite mechanism of vimentin action in GBM is still unknown.
High vimentin is associated with the poorer outcomes in gliomas and TMZ resistance
Early studies have found that the expression of vimentin is relevant to the progression and outcome of glioblastoma.Compared with those patients with low vimentin expression, a significantly shorter overall survival and a poorer outcome has been observed in glioblastoma patients with high vimentin expression (Zhao et al., 2018).Several studies have demonstrated that vimentin is a canonical epithelial-mesenchymal transition marker, that is frequently expressed in various cancer types, including lung cancers (Al-Saad et al., 2008; Dauphin et al., 2013), oral squamous cell carcinoma (Islam et al., 2000), prostate cancer (Steinmetz et al., 2011), breast cancer (Chen et al., 2008), malignant melanoma (Li et al., 2010), and brain tumors (Satelli and Li, 2011).There is sufficient evidence to support the use of vimentin as a pro-invasion molecular marker, making it widely considered as a potential therapeutic target for GBM.
To further explore the effect of vimentin suppression in GBM cells, WFA, an inhibitor targeting vimentin protein, was used to treat human glioblastoma cell lines, U251 and U87.In both U251 and U87 cells, WFA exhibits a negative effect on proliferation at micromole doses that inhibit the ability of migration and invasion.Therefore, it is reasonable to presume that the anti-GBM effects were attributed to WFA-induced vimentin degradation (Zhao et al., 2018).Another study also indicated that high vimentin expression correlates with shorter survival time in high-grade glioma patients, which is in accordance with the previous data analysis.
In addition, vimentin takes part in TMZ resistance, and high vimentin expression might counteract TMZ treatment efficacy (Lin et al., 2016).TMZ is a widely approved chemotherapy agent for GBM treatment (Messaoudi et al.,2015).Moreover, vimentin expression is strongly associated with the efficacy of TMZ chemotherapy.In low vimentin expression groups, patients treated with TMZ had better overall survival rates and progression-free survival than patients without TMZ treatment.However, in high vimentin expression groups, no significant survival benefit was observed in patients with or without TMZ treatment.
The 86C-vimentin complex can inhibit GBM progression
As mentioned above, vimentin plays a crucial role in the epithelial mesenchymal transition, proliferation and invasion.However, the exact roles of cell surface vimentin (CSV) are still unclear.Noh et al.(2016) described a novel monoclonal antibody (mAb), 86C, which can target CSV-activated rapid internalization of the 86C-vimentin complex.Once onto the GBM cells, the 86C-vimentin complex activates apoptosis signaling, leading to apoptosisin vitro
and inhibiting GBM progressionin vivo
.It was demonstrated that CSV-specific 86C mAb therapy may increase caspase-3 activity, making it a promising therapeutic target (Noh et al., 2016).Glioma stem cells(GSCs) are attractive candidate targets for anti-cancer therapy, owning to the fact that tumor initiation and recurrence are closely associated with GSCs.Destruction of GSCs is key to prolonging the survival of GBM patients(Singh et al., 2003).This group continues to characterize novel multifarious biological roles for CSV-targeted mAb 86C.The results found that 86C,acting as a TMZ chemosensitizer, could reverse GSCs chemoresistance.The combination of TMZ and 86C exerts better anti-tumor effects than TMZ alone in most GSCs lines.The synergistic anti-tumor effect of TMZ and 86C was further investigated in a GBM mouse model.The TMZ + 86C-treated mice survived longer than those treated with either drug alone (Noh et al.,2018).In addition to interacting with 86C, vimentin has been found to be a Nogo receptor (NgR) regulator.NgR maturation plays a role in inhibiting the migration and invasion of human glioma cells.The interaction between NgR and vimentin may suppress NgR maturation.Hence, vimentin knockdown may suppress GBM migration and invasion by promoting maturation of NgR (Kang et al., 2019).Collectively, vimentin is considered to be a promising glioma biomarker and target for GBM treatment.Vimentin in Peripheral Nervous System Injury
The peripheral nervous system (PNS) is indispensable for various physiological functions and behaviors that connect organs and the CNS.Common approaches for modulating peripheral nerve activities include surgery,pharmacology, and electrical stimulation (Chang, 2019).Although existing neuromodulation approaches promote peripheral nerve repair and are currently used in animal research and human therapies, its curative effect is poor because the PNS is highly intricate and heterogenous.Reports of vimentin in connection with peripheral nerve injury mainly focus on its role in negatively regulating myelination.
Vimentin negatively regulates myelination by interacting with TACE
Myelin thickness is essential for proper action potential propagation along axons and for structural integrity.Frequently, peripheral nerve injuries are characterized by impaired myelin thickness, which may lead to secondary damage in the axon.Triolo et al.(2012) found that vimentin acts as a negative regulator of myelination and neuregulin 1 (NRG1) type III.Compared with control fibers, the nerve fibers in vimentin-null mouse were thicker when assessed using electron micrographs.Additionally, hypermyelination in vimentin-null mice may be due to increased levels of NRG1 type III.In fact,tumor necrosis factor-α-converting enzyme (TACE) may convert the active form of NRG1 to the resting form, thus acting as a negative regulator of myelination (Hu et al., 2006; La Marca et al., 2011).In order to investigate whether vimentin exerts a synergistical role with TACE in inducing hypermyelination, double heterozygous VimTacemouse nerves were compared to control nerves.Of note, the protein level of myelin basic protein was significantly increased and hypermyelination was observed in double heterozygous mice (Triolo et al., 2012).It was demonstrated that vimentin interacts with TACE to regulate NRG1 type III and myelination.
MiR-138-5p negatively regulates vimentin and inhibits Schwann cell migration and proliferation
Early research found that upregulated vimentin expression enhances the ability of Schwann cells (SCs) to guide and promote axon regeneration after sciatic nerve injury (Perlson et al., 2005; Berg et al., 2013).SCs are the main glial cells of the PNS and play an important role in guiding peripheral nerve regeneration.Indeed, following injury, SCs and macrophages begin to clean debris at the injured site.In addition, SCs from distal sites proliferate and migrate to the injured site and form a framework to guide nerve regeneration.Eventually, the regenerated nerve fiber reinnervates its targets,and SCs remyelinate the regenerated axon (Abe and Cavalli, 2008).Sullivan et al.(2018) found that an increase in miR-138-5p decreases vimentin expression in SCs, and the 3′UTR of vimentin is a direct target of miR-138-5p.It is worth mentioning that low levels of miR-138-5p have been observed in the regenerated nerve, particularly at the time when SC migration peaks.Based on this, a potential mechanism of vimentin in regulating peripheral nerves became clear: the decreased expression of miR-138-5p facilitates the expression of vimentin, which is beneficial to SC migration (Sullivan et al.,2018).However, an early study found that vimentin is a negative regulator of myelination (Triolo et al., 2012).It is possible that SC migration inhibits myelination.
LncRNA BC088259 directly binds with vimentin to promote SC migration
Previously, it was verified that lncRNA BC088259 affects neurite outgrowth(Yu et al., 2013).A recent study demonstrated that lncRNA BC088259 directly binds with vimentin to promote SC migration after peripheral nerve injury.The expression of lncRNA BC088259 was high 4 days following sciatic nerve injury,mainly within SCs.Bothin vivo
andin vitro
lncRNA BC088259 expression promote SC migration, and decreased expression of vimentin inhibits SC migration.To investigate the interaction between lncRNA BC088259 and vimentin, rescue experiments were performed.After the expression of vimentin was downregulated by siRNA technology, the migration ability of SCs decreased, followed by the overexpression of BC088259 and recovery of the migration ability of SCs.These data suggest that BC088259 may regulate themigration of SCs through vimentin (Yao et al., 2020).In summary, vimentin affects SC migration and myelin sheath formation in peripheral nerves and is also regulated by other factors, such as miRNAs and lncRNAs.These studies provide a new potential therapeutic target for peripheral nerve injury and contribute to understanding the molecular mechanisms of peripheral nerve injury.
Vimentin in Other Neurological Diseases
Vimentin participates in the neuronal damage-response mechanism of Alzheimer’s disease
Alzheimer’s disease (AD), a neurodegeneration disease, is the most common cause of dementia affecting elderly people worldwide (Quiroz et al., 2020).The role of vimentin in AD requires further elucidation.Previous study has found that human and mouse brains express vimentin in response to damage and/or disease.In addition, neuronal vimentin expression is positively correlated with amyloid deposition in AD brains.The role of vimentin in neuronal damage-response mechanisms can be roughly summarized as follows.In the initial pathological event, damage in neurons causes synaptic disruption and dendrite retraction.After several hours or several days, the neuron expresses and transports vimentin to the damaged dendrite where it participates in neuron repair (Levin et al., 2009).At present, there are still many questions to be answered regarding the increase in vimentin and GFAP around amyloid plaques in AD.The functions of upregulated vimentin in AD are still unclear (Kamphuis et al., 2015).
Vimentin is a 14-3-3 protein-interacting protein, which is expressed in reactive astrocytes in demyelinating lesions of multiple sclerosis
Multiple sclerosis (MS) is an autoimmune disease of CNS in which T lymphocytes cross the BBB, resulting in demyelination and axonal degeneration (Lassmann, 2018).Early studies found that the 14-3-3 protein acts as an adaptor to connect vimentin and GFAP in reactive astrocytes at the site of demyelinating lesions in MS.Vimentin is heavily expressed in reactive astrocytes found in the demyelinating lesions of MS, and vimentin is one of the 14-3-3 protein-interacting proteins found in cultured human astrocytes(Satoh et al., 2004).These observations suggest that the 14-3-3 protein may bundle vimentin and GFAP in the same assembled filaments.Compared with resting astrocytes, the proteins highly expressed in reactive astrocytes and the 14-3-3 protein require more effort to regulate the coordination of vimentin and GFAP.However, the functions of vimentin in AD and MS remain unclear and deserve further study.
Discussion
Vimentin is a multifunctional protein involved in multiple nervous system injuries and diseases
The aim of this review is to provide an overview of the current knowledge of vimentin in nervous system injuries and diseases, including SCI, stroke,bacterial meningitis, glioma, and PNS injury.From the above studies, it is evident that vimentin, a multifunctional protein, interacts with numerous factors involved in the pathogenesis of nervous system injuries or diseases(Table 1).However, the exact mechanism of vimentin deserves further investigation.
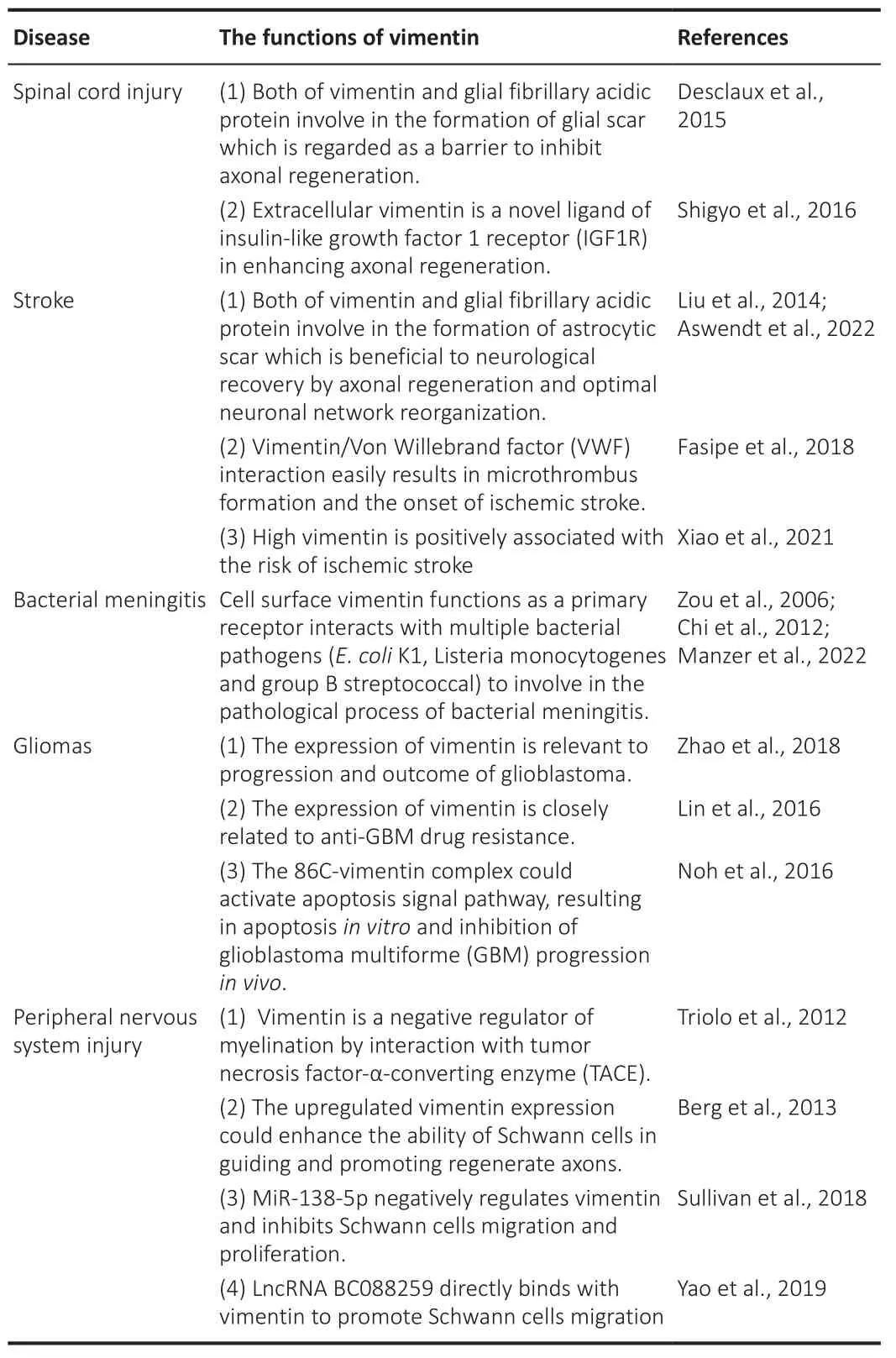
Table 1 |The roles of vimentin in diverse nervous system injuries and diseases
The brief summary of vimentin in nervous system injuries or diseases
After spinal cord injury and stroke, vimentin is overexpressed, resulting in glial scar formation.Several studies have shown that glial scar formation severely affects neurogenesis (Menet et al., 2003; Liu et al., 2014; Aswendt et al.,2022).Reactive astrocytes not only impair axonal regrowth following SCI but also have beneficial roles in improving normal neural plasticity and neuronal connection reconstruction after stroke (Aswendt et al., 2022).Studies continue to elucidate the similar and different roles of SCI-induced and strokeinduced glial scar in future.In addition, vimentin has been proposed to serve as a potential biomarker for the progression of stroke and brain tumors and as a possible therapeutic molecular target in multiple nervous system disorders.Until now, the exact mechanisms of vimentin involvement in the onset of disorders and neural repair after injury are not entirely clear, but it is evident that vimentin is a multifunctional protein involved in scar formation, axonal regeneration, the inflammatory response, and apoptosis activation.
In the majority of tumors, vimentin is overexpressed.For instance, in brain tumors, high vimentin expression is regarded as an important marker of poor prognosis (Zhao et al., 2018).Interestingly, brain tumor-related and meningitis-related studies have mostly addressed the functions of cell surface vimentin, while the functions of intracellular or extracellularassociated vimentin remain unclear.Additionally, vimentin not only promotes the migration of SCs but also inhibits myelination.There is a possibility that SC migration might inhibit myelination (Triolo et al., 2012).Therefore,understanding the mechanism of vimentin regulation may contribute to a better understanding of various nervous system injuries and diseases.
Limitations of review
There are two major limitations in this review.First, this review only included studies on the roles of vimentin in some common neurological diseases and the functions of vimentin in other nervous system diseases deserves to be further summarized.Second, the exact role of vimentin in nervous system injuries and diseases is still unknown.Because vimentin is a cytoskeletal protein involved in several basic cellular functions, its potential roles in pathologic processes of neurological diseases are easily neglected.
Conclusions and perspectives
While current knowledge on the functions of vimentin has recently increased,this knowledge is just the tip of the iceberg and further studies are needed to better understand the novel functions of vimentin in neurological diseases.Vimentin is a predominantly cytoskeletal protein that plays important roles in basic cellular processes, such as cell migration, proliferation, and division.Therefore, vimentin has complicated implications in the pathophysiology of various neurological diseases.In view of the available data, implications that vimentin plays an important role in nervous system diseases provides us with a novel biomarker and therapeutic target.How to accurately assess disease progression and ensure the stability and specificity of vimentin as a serum marker of disease remains an objective of research efforts.In addition,because vimentin may serve as a therapeutic target of multiple meningitis pathologies and gliomas, interfering with the vimentin cascade may delay or inhibit the processes of brain tumors and meningitis.Most importantly,developing multi-targeted therapies that target vimentin may enhance treatment outcomes; however, current studies using these strategies are at the experimental stage.Hence, how to fully utilize vimentin, how to further improve the therapeutic effects of therapies targeting vimentin, and how to achieve effective treatment in nervous system diseases remain topics of further studies.
Author contributions:
Literature search and manuscript writing: KZC, SXL and YWL; figure preparation: KZC, TH and JZ; manuscript revision: HFW and XXQ; review supervision: TW.All authors approved the final version of the manuscript.
Conflicts of interest:
The authors declare no conflicts of interest.
Open access statement:
This is an open access journal, and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- The effects and potential of microglial polarization and crosstalk with other cells of the central nervous system in the treatment of Alzheimer’s disease
- Blocking postsynaptic density-93 binding to C-X3-C motif chemokine ligand 1 promotes microglial phenotypic transformation during acute ischemic stroke
- The critical role of the endolysosomal system in cerebral ischemia
- Clemastine in remyelination and protection of neurons and skeletal muscle after spinal cord injury
- Artificial nerve graft constructed by coculture of activated Schwann cells and human hair keratin for repair of peripheral nerve defects
- Novel therapeutic strategies targeting mitochondria as a gateway in neurodegeneration

