Ferroptosis and glaucoma: implications in retinal ganglion cell damage and optic nerve survival
Ming Yang, Kwok-Fai So, Wai-Ching Lam, Amy Cheuk Yin Lo
Glaucoma and visual pathway degeneration:
Glaucoma is the leading cause of irreversible blindness worldwide, which leads to a progressive loss of vision. Glaucoma can be classified into two types: primary open-angle glaucoma and primary closed-angle glaucoma. Primary openangle glaucoma can be caused by the blockage of the trabecular meshwork, and this results in elevation of the intraocular pressure (IOP), leading to retinal ganglion cell (RGC) death. However, many glaucoma patients have normal IOP; this is known as normal-tension glaucoma. Nevertheless, excitotoxic damage and oxidative stress can also lead to RGC damage in normal-tension glaucoma (Almasieh et al., 2012). Glaucomatous genes such as TIGR, OPTN, and CYP1B1 have been suggested to contribute to the pathogenesis of glaucoma. However, some glaucomatous patients may remain asymptomatic in the early, moderate, and late stages. Another type of glaucoma is primary closed-angle glaucoma. In this clinical condition, a relative pupillary block is contributed by the iris obstructing aqueous outflow. The patients may suffer from corneal swelling, headache, nausea, and blurred vision during the acute phase.Increasing evidence suggests that glaucoma damage is not limited to RGC in the retina, but also extends to the lateral geniculate nucleus, superior colliculus, and the visual cortex, interfering with information transmission in the visual pathway. Importantly, RGCs lack the ability to regenerate and reconnect with the visual pathway once injured. Therefore, the development of therapeutic strategies to prevent RGC degeneration is essential.
At present, clinical treatments of glaucoma mainly include drugs, lasers, and surgery. In general, lowering IOP is recommended as the first-line treatment for open-angle glaucoma. Selective laser trabeculoplasty treatment can also be considered when the angle of closure has not yet resulted in glaucoma. If the outflow channel is obstructed by large-area adhesion and the medical treatment is ineffective, surgery should be considered. However, pathological IOP elevation-induced RGC and optic nerve injuries remain the most challenging problems in glaucoma management. Proposed approaches to enhancing optic nerve survival include cell death prevention of RGCs, removal of factors limiting neural survival, gain-offunction of factors promoting neural survival, and axon projection to rebuild synaptic connections.
Ferroptosis, a distinct form of regulated cell death associated with neurodegenerative diseases:
Regulated cell death is a genetically programmed cell death associated with the maintenance of homeostasis and disease development. Apoptosis, pyroptosis, and necroptosis are classical forms of regulated cell death that play important roles in various diseases. Ferroptosis was a regulated cell death described by Dixon et al. (2012) who used elastin to treat cancer cells containing oncogene mutations. They found an iron-dependent and lipid peroxidation-triggered cell death pathway, which relies on iron-generated reactive oxygen species, and was independent of caspase, adenosine triphosphate exhaustion, mitochondria reactive oxygen species generation, the permeability of the mitochondria outer membrane, and increased concentration of intracellular calcium ion. This distinct form of cell death is significantly different from other forms of cell death morphologically, biochemically, genetically, and metabolically. Three mainstream pathways have been identified in ferroptosis: glutathione peroxidase 4-glutathione (GPx-4-GSH) pathway, ferroptosis suppressor protein 1-ubiquinone-reduced nicotinamide adenine dinucleotide phosphate [FSP1-CoQNAD(P)H] axis, and dihydroorotate dehydrogenase (DHODH) signaling (Figure 1
).GPx-4 was considered a mainstream ferroptosis suppressor. GPx-4 is one of the members of the GPx enzyme families that reduces hydrogen peroxide through the oxidation of GSH. Therefore, the activity of GPx-4 is directly related to glutathione metabolism, and the reduction of cysteine-glutathione metabolism can inhibit GPx-4 activity. GPX-4 has a broader substrate preference and is the only enzyme currently reported to directly reduce complex phospholipid hydroperoxides. Depletion of GPx-4 leads to cell death, therefore it is critical for cell survival. Phospholipid hydroperoxides initiates the Fenton reaction in the presence of free iron, leading to lipid peroxidation and cell death. Recently, FSP1 was identified as an independent anti-ferroptotic system. The FSP1-CoQ-NAD(P)H axis is shown to act synergistically with that of GPX-4 and GSH to inhibit lipid peroxidation and cell death in tumor cells (Bersuker et al., 2019).
FSP1 transforms CoQ to dihydro ubiquinone (CoQH2) (a reduction reaction), which acts as an antioxidant to prevent cell membrane lipid peroxidation and inhibit ferroptosis (Bersuker et al., 2019). FSP1 inhibitors may decrease drug resistance in cancers. Although FSP1 serves as a ferroptosis protector on the cell membrane, studies addressing ferroptosis-defense systems that exist on the membranes of other organelles are essential.
Mitochondria is an organelle bound by the inner and outer membranes. It is responsible for aerobic respiration. However, a large amount of reactive oxygen species is generated during electron transfer in the inner membrane. In 2021, DHODH (Mao et al., 2021), a new GSH-independent anti-ferroptosis protein was discovered. This enzyme is located at the inner mitochondrial membrane, and is responsible for catalyzing the oxidation of dihydroorotic acid to orotic acid (the fourth step of the pyrimidine nucleotide synthesis pathway). At the same time, CoQ in the inner membrane is reduced to CoQH2, which is a free radical trapping antioxidant by receiving electrons. In addition, DHODH facilitates the production of CoQH2, preventing lipid peroxidation and ferroptosis (Mao et al., 2021). The association between DHODH and GPx-4 was further studied. Firstly, the expression level of DHODH was found to positively associate with the resistance of GPx-4 inhibitors in the Cancer Therapeutics Response Portal database. Secondly, inhibition of DHODH not only induced ferroptosis in cells and animals with low expression of GPx-4, but also triggered high sensitivity to ferroptotic death in high GPx-4-expressing cells and animals. Furtherin vitro
studies also confirmed that simultaneous inhibition of GPx-4 and DHODH, lead to ferroptosis. However, only mitochondrial DHODH was identified to exert an anti-ferroptotic effect.Overall, these three ferroptosis-defense systems are located in different subcellular locations: GPX-4 in the cytoplasm and mitochondria, FSP1 on the cell membrane, and DHODH in the inner mitochondrial membrane. In the mitochondria, DHODH and mitochondria-localized GPx-4 constitute the main ferroptosis defense system. Ferroptosis is involved in the progression of Parkinson’s disease, Alzheimer’s disease, and traumatic spinal cord injury (Ren et al., 2020). As neuronal damages are common in these diseases, this involvement suggests that ferroptosis contributes to the mechanisms of neuron and nerve damage. Therefore, the regulation of neuronal ferroptosis is promising for neural survival.
A potential involvement and role of ferroptosis in glaucoma:
There was increasing evidence supporting the association between ferroptosis and glaucoma in the past decade (Peng et al., 2020; Tang et al., 2021; Suo et al., 2022). A clinical study involving 17,476 participants showed that a high serum ferritin level was associated with increased risk and morbidity of glaucoma (Lin et al., 2014). Ferritin is a universal iron storage protein, which can store and release a large amount of free iron in humans. Upon facilitated release of ferric iron by increased ferritin, a redox reaction is triggered. This process is suggested to contribute to optic nerve degeneration in glaucoma. Indeed, silencing GPx-4 could markedly decrease cell viability while increasing the level of 4-hydroxy-2-nonenal in an immortalized rat retinal precursor cell line R28 (Sakai et al., 2015).N-methyl-D-aspartate (NMDA), an amino acid derivative that mimics the neurotransmitter glutamate, was shown in an early study to induce neurotoxicity through iron influx (Chen et al., 2013). In addition, anin vivo
study also discovered that intravitreal injection of NMDA not only induced RGC cell death but also increased the 4-hydroxy-2-nonenal level in GPx-4mice (Sakai et al., 2015). This condition was aggravated in GPx-4mice, suggesting the involvement of ferroptosis in RGC death. In the same year, another study demonstrated a significant elevation of malondialdehyde in sodium nitroprussideexposed porcine retinal homogenates, indicating the activation of retinal lipid peroxidation (Siu et al., 2015). However, when incubated with glutathione, sodium nitroprusside-treated porcine retinal homogenates showed a dosedependent increased malondialdehyde level, suggesting increased lipid peroxidation (Siu et al., 2015). These results may also indicate a possible activation of ferroptosis in sodium nitroprussideinduced porcine retinal homogenates. In another study, intravitreal injection of NMDA induced a prominent accumulation of Fein RGCs of Sprague-Dawley rat retina using SiRhoNox-1 fluorescent labeling after 24 hours, resulting in massive cell death after 7 days. However, administration of zinc-deferoxamine for 6 days markedly reduced Feaccumulation and 4-hydroxy-2-nonenal level with decreased number of TUNEL-positive cells. Proteomics analysis also showed the involvement of ferroptosis in NMDAinduced cell death in retinal and optic nerves in male Sprague-Dawley rats, uncovering a novel pathological cell death in this model (Suo et al., 2022). These results suggested that RGC ferroptosis may play an important role in NMDAinduced neurotoxicity in the retina. Recently, anin vivo
study showed that 9 weeks’ treatment of deferiprone (an iron chelator) in the magnetic microbead-induced ocular hypertension model significantly increased the number of RGCs with decreased optic nerve loss, suggesting a potential protective effect of iron chelation in glaucoma (Cui et al., 2020). Importantly, deferiprone treatment did not significantly alter IOP in this model.Overall, these studies indirectly showed various associations between ferroptosis and glaucoma. Based on these data, we hypothesized that GPx-4-GSH-, FSP1-CoQ-NADH- and DHODH-mediated ferroptosis pathways may contribute to the axon degeneration mechanism in glaucoma. Targeting these pathways is of great interest in examining their roles in neuronal and axonal survival (Figure 2
). Therefore, more comprehensive studies illustrating the distinct role of ferroptosis in different glaucomatous models are essential in this field.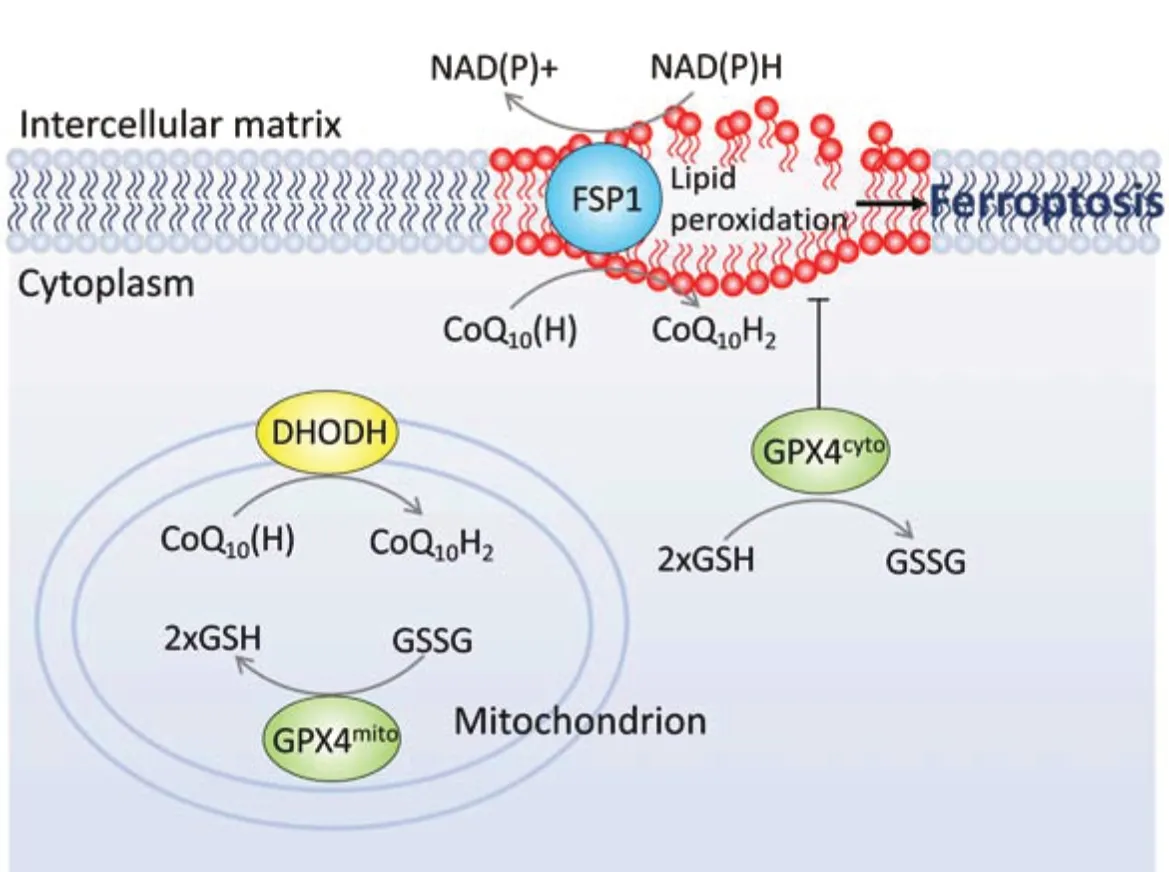
Figure 1 |We hypothesize three potential ferroptosis pathways in RGC.In the cell membrane, GPX4cyto and FSP1 work together to resist lipid peroxidation. The substrate of GPX4 is GSH, while FSP1 transforms NAD(P)H and CoQ10(H) to NAD(P)+ and CoQ10H2, respectively. In the mitochondrion, DHODH transforms CoQ10(H) to CoQ10H2, protecting the mitochondrial membrane from lipid peroxidation with GPX4mito. CoQ10(H): Ubiquinone; CoQ10H2: ubiquinol; DHODH: dihydroorotate dehydrogenase; GPX4cyto: cytoplasm GPX4; GPX4mito: mitochondrial GPX4; GSH: glutathione; GSSG: glutathione disulfide; NAD+: nicotinamide adenine dinucleotide; NADH: reduced nicotinamide adenine dinucleotide; NADP+: nicotinamide adenine dinucleotide phosphate; NADPH: reduced nicotinamide adenine dinucleotide phosphate; RGC: retinal ganglion cell.
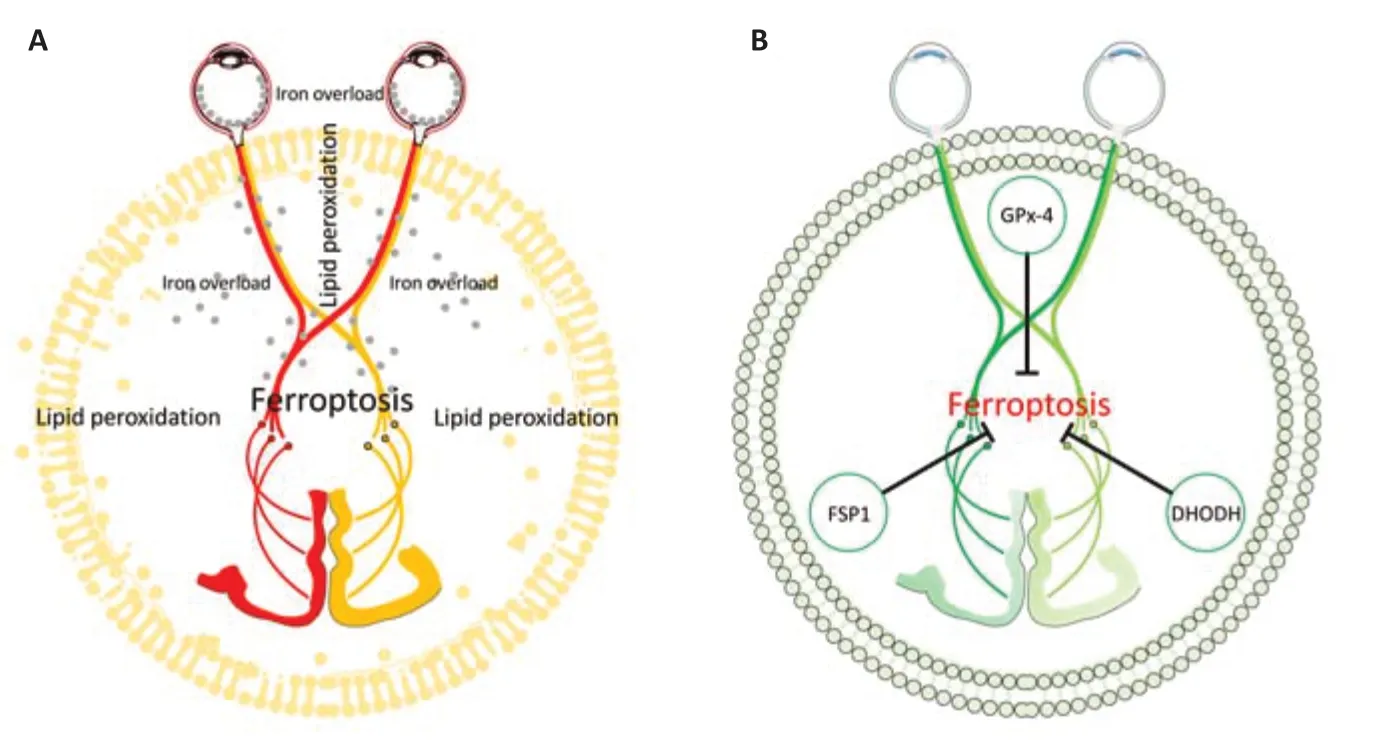
Figure 2 |A schematic diagram showing glaucoma caused axon degeneration and ferroptosis.(A) Ferroptosis is hypothesized to be involved in glaucoma and the visual pathway. RGC membrane lipid peroxidation and cytoplasm iron overload are two major features of ferroptosis. Gray dots: iron overload in axon. Yellow lipid bilayer: presence of lipid peroxidation. (B) Activation of GPx-4, FSP1, and DHODH may rescue axonal degeneration by suppressing ferroptosis. DHODH: Dihydroorotate dehydrogenase; FSP1: ferroptosis suppressor protein 1; RGC: retinal ganglion cell. Part of the elements in the figures were adapted from Servier Medical Art (http://smart.servier.com/ accessed on 10th December 2021), licensed under a Creative Commons Attribution 3.0 Unported License (https://creativecommons.org/licenses/by/3.0/).
Conclusion and future remarks:
In this perspective, we discussed the potential association between ferroptosis and RGC damage as well as optic nerve loss, and its impact on glaucoma. Ferroptosis may not only be involved in RGC and optic nerve degeneration in glaucoma models but also affect axonal survival. We propose that there are sufficient pieces of evidence to support the idea that ferroptosis may play a distinct role in glaucoma pathogenesis. Therefore, its contribution to RGC damage and optic nerve degeneration should not be overlooked.We thank Professor Christopher Kai Shun Leung (Department of Ophthalmology, The University of Hong Kong) for his kind advice and comments on the manuscript.
This work was supported by Albert Bing-Ching Young Professorship Endowment in Ophthalmology to WCL; Health and Medical Research Fund, the Food and Health Bureau, The Government of the Hong Kong Special Administrative Region (06171516) and General Research Fund, Research Grants Council, The Government of the Hong Kong Special Administrative Region (17112919) to ACYL.
Ming Yang, Kwok-Fai So, Wai-Ching Lam, Amy Cheuk Yin Lo
Department of Ophthalmology, School of Clinical Medicine, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong Special Administrative Region, China (Yang M, So KF, Lam WC, Lo ACY)
State Key Laboratory of Brain and Cognitive Sciences, The University of Hong Kong, Hong Kong Special Administrative Region, China (So KF)GHM Institute of CNS Regeneration, Jinan University, Guangzhou, Guangdong Province, China (So KF)
Correspondence to:
Ming Yang,hrmeym@connect.hku.hk; Kwok-Fai So, PhD, hrmaskf@hku.hk; Wai-Ching Lam, MD,
waichlam@hku.hk; Amy Cheuk Yin Lo, PhD, amylo@hku.hk.
https://orcid.org/0000-0001-8600-4325(Ming Yang)
https://orcid.org/0000-0003-4039-4246 (Kwok-Fai So)
https://orcid.org/0000-0003-2057-9374 (Wai-Ching Lam)
https://orcid.org/0000-0003-4239-6851 (Amy Cheuk Yin Lo)
Date of submission:
February 25, 2022Date of decision:
May 23, 2022Date of acceptance:
June 7, 2022Date of web publication:
August 2, 2022https://doi.org/10.4103/1673-5374.350196
How to cite this article:
Yang M, So KF, Lam WC, Lo ACY (2023) Ferroptosis and glaucoma: implications in retinal ganglion cell damage and optic nerve survival. Neural Regen Res 18(3):545-546.
Open access statement:
This is an open access journal, and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- Notice of Retraction
- Neuroprotective role of Noggin in spinal cord injury
- Combined cell-based therapy strategies for the treatment of Parkinson’s disease: focus on mesenchymal stromal cells
- Lights at night: does photobiomodulation improve sleep?
- β2-Microglobulin exacerbates neuroinflammation, brain damage, and cognitive impairment after stroke in rats
- The lymphatic drainage systems in the brain: a novel target for ischemic stroke?

