Combined cell-based therapy strategies for the treatment of Parkinson’s disease: focus on mesenchymal stromal cells
Jannette Rodríguez-Pallares , María García-Garrote Juan A. Parga José Luis Labandeira-García
Abstract Parkinson’s disease is a neurodegenerative condition characterized by motor impairments caused by the selective loss of dopaminergic neurons in the substantia nigra. Levodopa is an effective and well-tolerated dopamine replacement agent. However, levodopa provides only symptomatic improvements, without affecting the underlying pathology, and is associated with side effects after long-term use. Cell-based replacement is a promising strategy that offers the possibility to replace lost neurons in Parkinson’s disease treatment. Clinical studies of transplantation of human fetal ventral mesencephalic tissue have provided evidence that the grafted dopaminergic neurons can reinnervate the striatum, release dopamine, integrate into the host neural circuits, and improve motor functions. One of the limiting factors for cell therapy in Parkinson’s disease is the low survival rate of grafted dopaminergic cells. Different factors could cause cell death of dopaminergic neurons after grafting such as mechanical trauma, growth factor deprivation, hypoxia, and neuroinflammation. Neurotrophic factors play an essential role in the survival of grafted cells. However, direct, timely, and controllable delivery of neurotrophic factors into the brain faces important limitations. Different types of cells secrete neurotrophic factors constitutively and co-transplantation of these cells with dopaminergic neurons represents a feasible strategy to increase neuronal survival. In this review, we provide a general overview of the pioneering studies on cell transplantation developed in patients and animal models of Parkinson’s disease, with a focus on neurotrophic factor-secreting cells, with a particular interest in mesenchymal stromal cells; that co-implanted with dopaminergic neurons would serve as a strategy to increase cell survival and improve graft outcomes.
Key Words: brain repair; cell replacement; co-grafts; dopaminergic neurons; fetal ventral mesencephalic tissue; mesenchymal stem cells; neural grafting; neural transplantation; neuroblasts; neurotrophic factors

Introduction 478 Search Strategy and Selection Criteria 478 Historical Overview 478 Co-Grafting Strategies: a Review 479 The Potential of Mesenchymal Stromal Cells for Cell Therapy 480 Mesenchymal Stromal Cells and Neural Repair 480 Mesenchymal Stromal Cell-Based Therapies for Parkinson’s Disease 481 Conclusions and Future Perspectives 481
Introduction
Parkinson’s disease (PD) is a neurodegenerative disorder characterized by the presence of motor manifestations such as tremor, rigidity, bradykinesia, and postural instability and a variety of non-motor symptoms that include sensory abnormalities, autonomic dysfunction, sleep disturbances, fatigue, apathy, and psychiatric disorders (Armstrong and Okun, 2020). PD pathology mainly lies in the degeneration of dopaminergic neurons in the substantia nigra (Poewe et al., 2017). Although there is a wide range of pharmacological treatments for PD, all treatments currently available provide only symptomatic relief. Levodopa is the most potent and effective treatment and remains as the gold standard. However, the beneficial effect of levodopa gets progressively shorter throughout the disease, and prolonged treatment is associated with drug-induced dyskinesia and other disabling side effects that limit its use (Armstrong and Okun, 2020; Lopez-Lopez et al., 2021). With a relatively localized neural degeneration, PD is considered a good target for cell therapy (Fan et al., 2020). Over nearly four decades, different restorative surgical therapies have emerged to replace lost dopaminergic neurons. First clinical trials based on intrastriatal transplantation of human fetal ventral mesencephalic (fVM) tissue showed that grafted cells survive and induce significant, long-lasting improvements in motor symptoms (Lindvall et al., 1990), although possible effects on non-motor symptoms were not explored. However, the low availability of human mesencephalic tissue limited its use. Currently, stem cells represent a promising alternative source for the generation of dopaminergic neurons (Jang et al., 2020; Rahimpour et al., 2022). In addition to important issues such as defining an optimal cell source, better criteria for PD patient selection, improved and standardized protocols, avoiding rejection, and promoting functional integration (Rahimpour et al., 2022), other factors that compromise the survival of grafted dopaminergic neurons, such as mechanical trauma, growth factor deprivation, hypoxia, oxidative stress, and neuroinflammation need to be addressed (Brundin et al., 2000; Moriarty et al., 2017). Low dopaminergic survival can be partially counteracted by neurotrophic factor addition. Although different strategies have been studied, direct, timely, and controllable delivery of neurotrophic factors into the brain faces important challenges (Gantner et al., 2020; Studer and Tabar, 2020). Different types of cells secrete neurotrophic factors constitutively and co-transplantation of these cells with dopaminergic neurons represents a feasible strategy to increase neuronal survival. In this review, we focus on the co-grafting of dopaminergic neurons and neurotrophic factorsecreting cells, with a particular interest in mesenchymal stromal cells (MSCs), as a possible strategy to reduce cell death after grafting, which represents one of the drawbacks faced by cell therapy to make the definitive leap to the clinic (Figure 1
).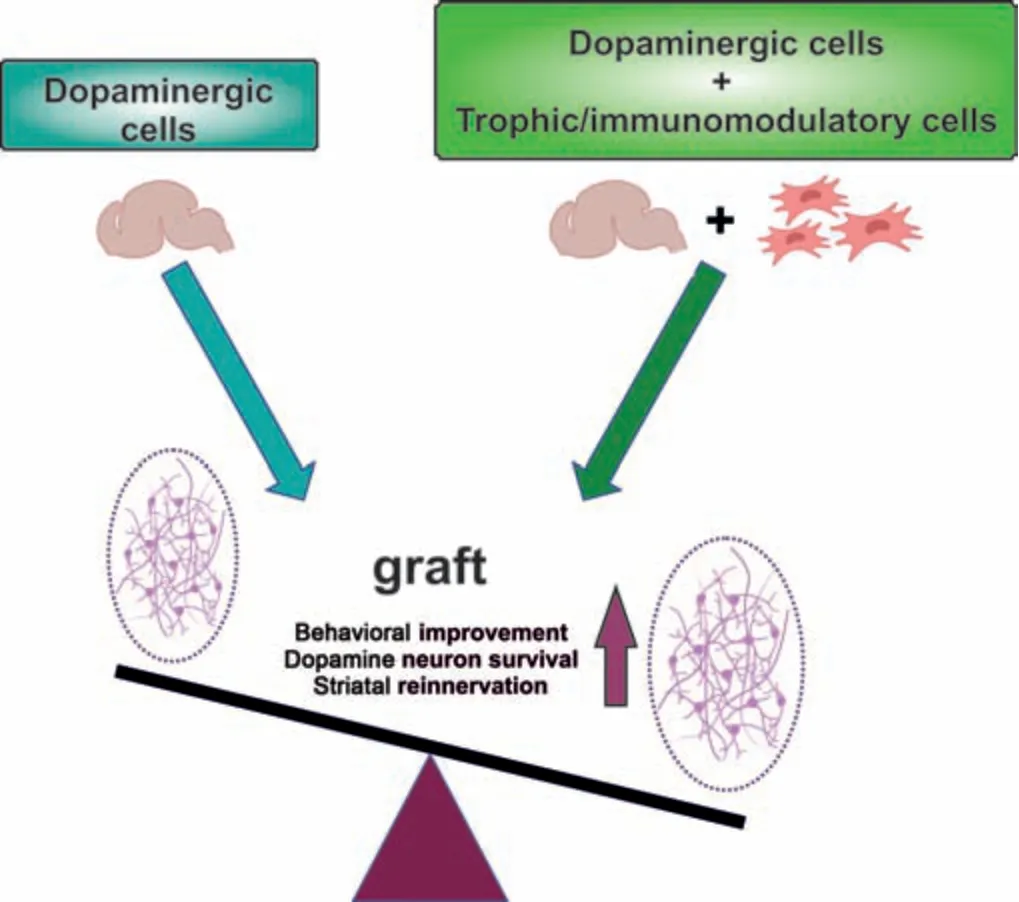
Figure 1 |Co-grafting of dopaminergic cells with trophic/immunomodulatory cells as a Parkinson’s disease cell therapy. Co-transplantation strategies are proposed as an approach to improve behavioral deficits, dopamine neuronal survival, and striatal reinnervation. Created with BioRender (https://biorender.com/).
Search Strategy and Selection Criteria
The search strategy and selection criteria were limited to articles published in peer-reviewed journals. A literature review of articles published in English from inception up to April 2022 was conducted by searching in the National Library of Medicine (PubMed) database (https://pubmed.ncbi.nlm.nih.gov/) using the following keywords: fetal ventral mesencephalic tissue, graft, Parkinson’s disease, co-grafts, mesenchymal stromal/stem cells, transplantation, neuroblasts, survival, embryonic stem cells, pluripotent induced stem cells and combination of the above terms. We also revised references of eligible articles.
Historical Overview
In 1975, Seiger and Olson grafted fetal brain tissue into the anterior eye chamber, laying the groundwork for future transplants in the nervous system. Cells derived from fVM tissue, enriched in dopaminergic cells, were the first type of cells transplanted in an animal model of PD. These initial preclinical studies confirmed that dopaminergic grafts were able to survive, extend projections into the host striatum and ameliorate behavioral deficits in dopamine-depleted animals (Bjorklund et al., 1980). Such was the impact of these results that in the early 1980, the first PD patients in the world received intracerebral grafts. However, these patients were grafted with autologous chromaffin cells obtained from the adrenal medulla, which had also been used in animal models of PD, to avoid the practical and ethical issues associated with human fetal tissue (Freed et al., 1981). Although it was initially assumed that adrenal medulla and fVM cells had similar mechanisms of action based on dopamine production, further studies showed that adrenal medulla graft benefits were due to neurotrophic actions that promote the sprouting of striatal dopaminergic fibers and not to dopamine production (Freed et al., 1990). However, the transient and highly variable improvements observed both in animal models and in PD patients, the frequent surgical complications, and the low cell survival observed in post-mortem studies led to the abandonment of the use of adrenal medullary tissue for transplantation (Date et al., 1990). A breakthrough in the field was the use of dissociated cell suspensions derived from fVM tissue, which permitted the targeting of deep structures as well as multiple graft placements (Bjorklund et al., 1983). A crucial step forward in clinical translation was the demonstration that dopaminergic neurons isolated from human embryonic/fetal tissue survive, provide extensive graft-derived striatal innervation, and ameliorate motor deficits after transplantation in preclinical models of PD (Brundin et al., 1986). Based on almost 10 years of transplantation studies in animal models, the first patients receiving grafts of human fVM tissue were implanted in 1987 (Lindvall et al., 1990). This pioneering study demonstrated for the first time that fVM grafted cells can survive in a human brain affected by neurodegenerative disease and could restore dopamine levels, monitored in the striatum using [C]-raclopride positron emission tomography, for as long as 10 years after surgery (Piccini et al., 1999). Interestingly, post-mortem studies carried out 24 years after grafting showed dense graft-derived reinnervation in the putamen (Hallett et al., 2014). The outcomes of a series of open-label trials showed promising results and generated high expectations in the field, leading the way to two National Institutes of Health-funded, double-blind, randomized, placebo-controlled trials. However, the results were disappointing, with some grafted patients showing normalized dopamine signaling while other participants showed modestly or no recovery compared to patients subjected to sham surgery (Freed et al., 2001). Nevertheless, even more discouraging was the fact that a subset of grafted patients developed graft-induced dyskinesias years later (Hagell et al., 2002), possibly due to the composition of the transplanted cell suspension. Experimental studies using different proportions of dopaminergic and serotonergic neurons in the cell suspension showed that higher proportions of serotonergic neurons, which are usually present in the graft, led to the dyskinesias, since serotonergic terminals could release dopamine as a false transmitter in an unregulated fashion (Politis et al., 2010). Additional evidence suggests that endogenous serotonergic neurons and serotonergic and dopaminergic interactions would contribute to the development of graft-induced dyskinesias (Muñoz et al., 2020). With the knowledge acquired in subsequent research, it is now considered that these studies were carried out prematurely and some of the side effects could have been avoided through careful selection of patients, improving the method of implantation and the composition of the grafted cell suspension, and optimizing the post-transplant immunosuppressive regimes (Bjorklund and Lindvall, 2017). The cumulative reanalysis of previous trials led a European research consortium called TRANSEURO to design a step-by-step optimized open-label trial, which is currently ongoing, with the main objective of developing an efficacious and safe methodology for fVM cell-based therapy for PD (ClinicalTrials.gov identifier NCT01898390) (Barker and TRANSEURO consortium, 2019).
Due to the abovementioned shortage of human fVM tissue for cell-based therapy and advances in stem cell knowledge, many efforts have focused on generating dopaminergic neurons for transplantation from stem cells, which potentially offer an unlimited number of cells. Different stem cell types have been considered as a possible source of neurons for the treatment of PD. However, the best positioned seem to be embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs) (Jang et al., 2020). Notable advances in differentiation protocols have led to the achievement of highly pure dopaminergic neurons suitable for clinical trials that showed satisfactory therapeutic effectiveness in animal models of PD and did not cause tumors in these animals (Xiong et al., 2021). The first transplantations using clinically graded hiPSC-derived neurons have already been carried out (Schweitzer et al., 2020). Piao et al. (2021) have recently reported the generation of the first ESC-derived dopaminergic cell product for the treatment of PD, which was approved for a clinical trial in the United States (Takahashi, 2021;Figure 2
). There is no doubt that stem cell technologies have the potential to be at the forefront of such PD treatments (Parmar et al., 2020). The results of ongoing and upcoming trials would be highly anticipated since the success of these trials would open up new possibilities for using cell therapy for the treatment of PD (Fan et al., 2020). However, it should be noted that, beyond the motor symptoms, the disease exhibits non-motor symptoms that can be also disabling for the patient, which currently are not targeted by cell therapy and should also be considered in future therapies (Pantcheva et al., 2015).
Figure 2 |Timeline of breakthroughs in cell therapy for Parkinson’s disease. Relevant contributions in the field of cell therapy are shown in black. Key scientific advances are shown in blue. DA: Dopamine; fVM: fetal ventral mesencephalic; hESCs: human embryonic stem cells; hiPSCs: human induced pluripotent stem cells; MSC: mesenchymal stromal cell; PD: Parkinson’s disease.
Despite the encouraging results obtained, cell-based therapies face important limitations and challenges. In addition to issues concerning safety and reproducibility, a critical aspect is the survival rate of the grafted dopaminergic neurons. Different evidence suggests that only 3‒20% of grafted dopaminergic cells survive after the procedure (Brundin et al., 2000; Kim et al., 2020; Li and Li, 2021; Hiller et al., 2022). Triggers that may initiate dopaminergic cell loss in grafts include mechanical trauma, neuroinflammation, poor vascularization, and growth factor deprivation in the host brain (Barker et al., 1996; Brundin et al., 2000; Moriarty et al., 2017). The critical interval during which most dopaminergic cells die is the first few days after transplantation (Sortwell et al., 2000). The idea of utilizing cells with neurotrophic properties to modify the pathologic brain environment or promote neuroprotective effects has been appearing on the horizon for central nervous system disorders. In the following section, we will review the most relevant studies in which combinations of dopaminergic grafts with other cells with supportive properties have been used as a strategy to overcome the poor survival of transplanted neurons (Figure 3
).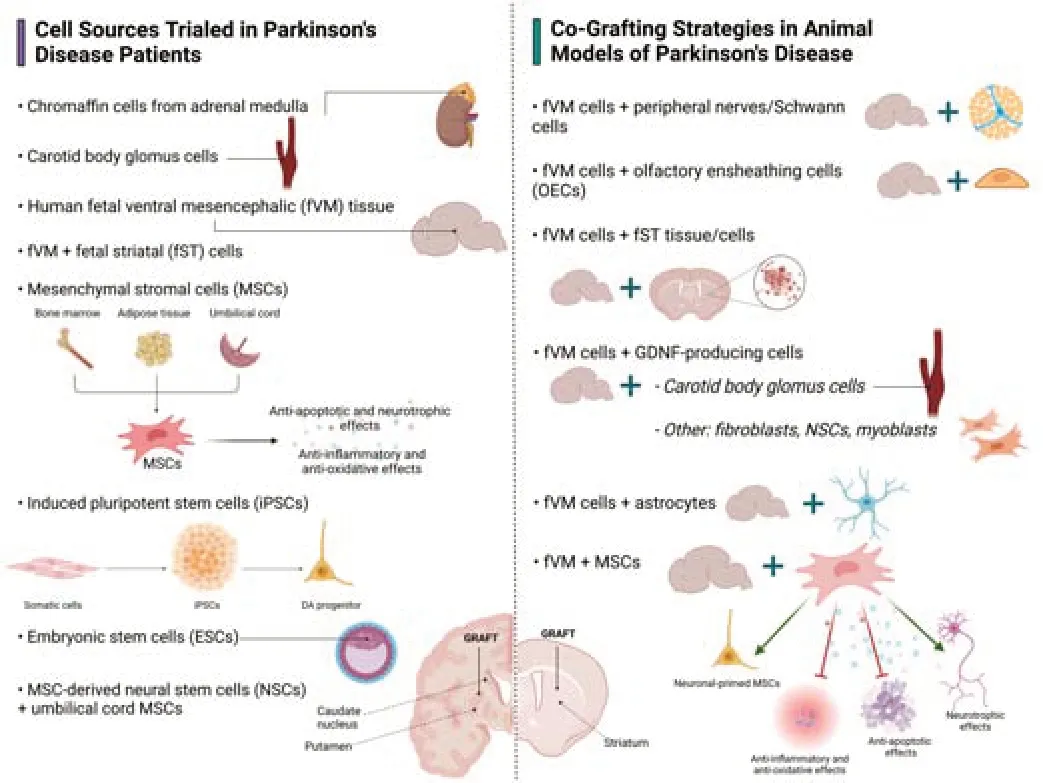
Figure 3 |Cell-based therapies trialed in patients and co-grafting strategies tested in animal models of PD. The left-hand-side of the figure shows cell sources used in clinical trials in PD patients and the right-hand-side shows co-grafting strategies tested in animal models of PD. ESCs: Embryonic stem cells; fST: fetal striatal; fVM: fetal ventral mesencephalic; GDNF: glial cell line-derived neurotrophic factor; iPSCs: induced pluripotent stem cells; MSCs: mesenchymal stromal cells; NSCs: neural stem cells; OECs: olfactory ensheathing cells; PD: Parkinson’s disease. Created with BioRender (https://biorender.com/).
Co-Grafting Strategies: a Review
Co-grafting strategies based on the combination of fVM tissue with cells with neurotrophic properties that could promote the survival of transplanted dopaminergic cells represent a therapeutic option to overcome the typical loss of 80‒95% of the grafted dopaminergic neurons following transplantation. In the 1980s, the first studies exploring the benefits of peripheral nerve transplantation on the survival of dopaminergic grafts were carried out. Schwann cells, the principal glial cell type in the peripheral nervous system, are involved in neuronal survival and nerve regeneration (Ma et al., 2021). Aguayo et al. (1984) demonstrated that transplanted fVM neurons in 6-hydroxydopamine (6-OHDA)-lesioned rats survived, and extended axons guided by sciatic nerve grafts. Different groups also demonstrated that co-grafts of fVM tissue with peripheral nerves or Schwann cells induced behavioral improvements and promoted axonal growth to the striatum compared to fVM grafts in rat models of PD (van Horne et al., 1991; Timmer et al., 2004). However, other studies did not observe neuroprotective effects after co-transplantation of fVM tissue and saphenous nerve in lesioned monkeys (Collier et al., 1994). Olfactory ensheathing cells (OECs) are another type of peripheral glial cells specialized in supporting continuous olfactory nerve fiber growth throughout adulthood. Co-culture of OECs with nigral cells promoted dopaminergic neurite outgrowth (Denis-Donini and Estenoz, 1988). Implantation of cultured OECs in dopamine-depleted rats after 6-OHDA lesioning was not sufficient to promote tissue repair or functional recovery (Dewar et al., 2007). However, the combination of fVM with OECs enhanced the efficacy of grafted neurons in different rat models of PD (Agrawal et al., 2004; Johansson et al., 2005; Weng et al., 2017). Similar results were obtained when dopaminergic neurons isolated from porcine fVM cells or dopaminergic neurons derived from neural stem cells were co-implanted with OECs (Shukla et al., 2009; Weng et al., 2020).
It is well-established that striatal cells exert both tropic and trophic effects that contribute to providing a target for mesencephalic dopaminergic neurons during the development of the nigrostriatal pathway (Barker et al., 2020). To extend these properties to cell transplantation, several works focused on studying the effects of co-grafting striatal cell suspensions with fVM tissue. In a ground-breaking study carried out by Brundin and collaborators, rat fVM cells were grafted alone or in combination with striatal (i.e., a major dopamine target area) or spinal cord (i.e., a non-target area) cells. The results showed that mixed mesencephalic and striatal suspensions gave rise to a greater area of dense dopaminergic innervation into the host striatum than grafts of fVM alone or fVM combined with spinal cord cells (Brundin et al., 1986). Similar results were obtained in subsequent studies, showing increased dopaminergic survival and more robust, long-lasting functional recovery in denervated rodents and monkeys after nigral-striatal co-grafts (Yurek et al., 1990; Costantini et al., 1994; Emgard-Mattson et al., 1997; Sortwell et al., 1998; Sladek et al., 2008). The effects of co-grafting fVM and striatal tissue in a PD patient were also explored and the results showed improved motor function and a significant increase in the uptake of fluoro-L-DOPA detected by positron emission tomography in the co-grafted putamen (i.e., grafts of fVM and lateral ganglionic eminence tissue) compared to the non-co-grafted side (i.e., a graft of fVM tissue alone) (Lee et al., 2003). The discovery of glial cell line derivedneurotrophic factor (GDNF), which is expressed in the rodent striatum, as the most potent neurotrophin that promotes dopaminergic survival quickly, sparked interest in this factor in neuroprotective and cell therapies for PD. Previous studies showed that GDNF rescues dopaminergic neurons in animal models of PD (Winkler et al., 1996). Different approaches to delivering GDNF into the brain, such as viral delivery, genetically modified cells, nanoparticles, or hydrogels, were used to increase the survival, maturation, and functional integration of transplanted dopaminergic cells (Moriarty et al., 2017; Gantner et al., 2020). Despite its potential, clinical translation of GDNFrelated therapies was hampered by safety and efficacy concerns associated with its short half-life, low ability to cross the blood-brain barrier, and side effects derived from long-term delivery. Co-transplantation strategies have been proposed to achieve a sustained release of minimum effective levels of GDNF that could increase its therapeutic benefits. Initial studies showed that co-transplants of fVM and GDNF-secreting clones of a Schwann cell line enhanced the survival and fiber outgrowth from embryonic nigral cells grafted in 6-OHDA-lesioned rats (Wilby et al., 1999). Carotid body glomus cells are highly dopaminergic and produce GDNF, and their neuroprotective and neuroreparative effects have been demonstrated in rodent PD models (Villadiego et al., 2005) and trialed in PD patients (Arjona et al., 2003). Our group in collaboration with Toledo-Aral’s team observed that the survival of dopaminergic neurons, the graft-derived dopaminergic reinnervation, and the performance of the cylinder test were improved in dopamine-depleted rats that received co-grafts of fVM and cell aggregates obtained from the carotid body compared to control groups that received fVM tissue alone (Rodriguez-Pallares et al., 2012). Other studies obtained similar results when different GDNF-producing cells such as fibroblasts, modified neural stem cells or myoblasts were co-grafted with dopaminergic neurons (Ostenfeld et al., 2002; Jingzhong et al., 2005; Deng et al., 2013; Perez-Bouza et al., 2017). Transplantation of expanded neural precursor cells derived from the developing VM was also explored in animal models of PD (Armstrong et al., 2000; Rodriguez-Pallares et al., 2005). Co-grafting VM-derived precursors with cultured astrocytes resulted in almost complete behavioral recovery and a higher number of tyrosine hydroxylase (TH)-positive cells within the graft, with longer fiber length compared to control grafts (Song et al., 2018). Interestingly, astrocytes exhibited region-specific heterogeneity in their secretory profiles, and the beneficial effects on graft survival and function were more evident when VM-derived precursors were co-implanted in the same suspension with VM-derived astrocytes compared to co-transplants including cortexderived astrocytes. These effects are possibly related to the establishment of a neurotrophic brain environment, as a result of an upregulated expression pattern of neurotrophic factors, trophic extracellular matrix proteins, and antioxidant factors in the striatum after co-transplantation. A reduction in the immune and inflammatory response was also suggested since an increase in the expression of anti-inflammatory markers (interleukin (IL)-1 and IL-10) and the downregulation of proinflammatory markers (Il-1β and inducible nitric oxide synthase) were observed (Song et al., 2018; see for details inAdditional Table 1
).As indicated above, the low survival of dopaminergic neurons during and after grafting is probably caused by a combination of factors beyond growth factor deprivation such as apoptosis, neuroinflammation, oxidative stress, hypoxia, and insufficient vascular supply, and immune rejection, suggesting that a pleiotropic therapy would be advantageous. In this regard, MSCs have broad neurotrophic, anti-inflammatory, and immunomodulatory properties, and have been proposed as a promising cell source for cell therapy in PD.
The Potential of Mesenchymal Stromal Cells for Cell Therapy
MSCs are multipotent cells that possess stem cell-like properties including self-renewal and the capacity to differentiate, under appropriate conditions, into several tissues of mesenchymal origin including cartilage, bone, and fat. MSCs were first isolated from bone marrow (BM) and defined as plasticadherent and colony-forming unit fibroblastic cells. Although BM is the primary source, MSCs can also be isolated from many other sources including adipose tissue, umbilical cord Wharton’s jelly, skin, dental pulp, peripheral blood, and perivascular niches, among others (Andrzejewska et al., 2019; Dabrowska et al., 2021). Interest in MSCs has increased rapidly in recent years due to their widespread availability in the body, high proliferation capacity, few ethical concerns, and low risk of tumor formation. Moreover, their migration and homing abilities (i.e., capacity to preferentially migrate toward damaged tissues), as well as their capacity to regulate the local environment through the release of immunomodulatory and trophic factors, make them an invaluable source for cell therapy (Fricova et al., 2020). MSCs can regulate mechanisms of both innate and adaptive immune responses, through the modulation of cellular responses and secretion of anti-inflammatory mediators. MSCs also participate in angiogenic processes by secreting different growth factors, cytokines, and chemokines. Moreover, the neuroprotective effects of MSCs on damaged or dying nerve cells, as discussed below, are particularly interesting. In addition to direct actions, different studies suggested that MSC-derived products, and particularly MSCderived extracellular vesicles (MSC-EVs), elicited similar biological effects and have emerged as a powerful regenerative tool (Vilaça-Faria et al., 2019).
Mesenchymal Stromal Cells and Neural Repair
Therapies using MSCs rely on their capacity to modify damaged tissue microenvironments and enhance endogenous neural protection and regeneration. MSCs can secrete into the damaged area neurotrophic and anti-apoptotic factors, and cytokines such as insulin growth factor 1, vascular endothelial growth factor, brain-derived neurotrophic factor, fibroblast growth factor 2, and nerve growth factor, among others. MSCs can also promote neural repair through the production of anti-inflammatory cytokines such as IL-10 and transforming growth factor β and by decreasing pro-inflammatory cytokines such as IL-1β, tumor necrosis factor-alpha, or interferon γ (Fricova et al., 2020). Moreover, there is evidence of the capacity of MSCs to counteract oxidative stress and enhance the antioxidant response (Angeloni et al., 2020). Extracellular matrix molecules produced by MSCs have also been demonstrated to promote neural cell attachment, growth, and axonal extension. Pre-treatment of MSCs under defined conditions (i.e., preconditioning) has been shown to modify the secretome of MSCs and improve their efficacy in animal models of different neurological disorders (Baez-Jurado et al., 2019; d’Angelo et al., 2020). Other strategies have been based on the use of genetically engineered MSCs as delivery vehicles to produce specific neurotrophic factors, such as brain-derived neurotrophic factor, or the modification of MSCs to improve their survival and capacity to migrate toward the lesion site, particularly relevant when systemic administrations are performed, or prevent their premature senescence (Ocansey et al., 2020). Significant attention has been paid to the therapeutic potential of MSCEVs to promote neurite growth and remodeling, enhance neurogenesis and angiogenesis and exert neuroprotective effects in damaged neural tissue. Furthermore, MSC-EVs reduce brain inflammation by inhibiting astrogliosis and switching the polarization of macrophages and microglial cells from proinflammatory to pro-repair phenotypes. Treatment with MSC-EVs also reduced the levels of pro-inflammatory cytokines such as tumor necrosis factor-alpha, IL-1β, and IL-6 and increased the levels of anti-inflammatory cytokines IL-4 and IL-10. Overall, MSCs have unique and valuable characteristics for the repair of the nervous system in various diseases including PD (d’Angelo et al., 2020).
Mesenchymal Stromal Cell-Based Therapies for Parkinson’s Disease
Different studies suggest that MSCs can differentiate into neural progenitors from which cells resembling dopamine neurons (i.e., that express the dopaminergic marker TH) can be obtained (Thompson et al., 2019). Neuronal-primed MSCs or dopaminergic cells derived from MSCs have been transplanted in animal models of PD (Delcroix et al., 2011; Park et al., 2012a; Shetty et al., 2013; Wang et al., 2013). However, it remains unclear whether these cells can integrate into the host neural circuits and create new synapses with host neurons. Therefore, most studies have focused on their known ability to produce a wide range of factors. Transplantation of murine BM-MSC into the striatum or the substantia nigra in preclinical models of PD showed significant improvements in behavioral tests and preservation of TH-positive neurons in the substantia nigra and fibers in the striatum (Chen et al., 2017). Dopamine-depleted animals receiving intrastriatal injections of human BMMSCs grafts also showed neuroprotective effects (Blandini et al., 2010; Cova et al., 2010; Xiong et al., 2013). MSCs obtained from other sources such as adipose tissue or umbilical cord have also been tested with similar results (Schwerk et al., 2015; Bagheri-Mohammadi et al., 2019; Wang et al., 2020; Lian et al., 2021). The mechanisms underlying these improvements have been ascribed to increased levels of GDNF and brain-derived neurotrophic factor, reduction of apoptosis and inflammation, or neurogenic effects (Blandini et al., 2010; Cova et al., 2010; Lian et al., 2021). However, other studies showed modest or no improvement after MSCs grafting (Moloney et al., 2010; Sun et al., 2020). In addition to intracerebral grafting, other routes of administration have also been tested as suitable approaches in PD. Systemic administration of MSCs by intravenous or intracarotid injection induced a significant reduction in amphetamine-induced rotational behavior, preserved TH-positive cells in the substantia nigra and dopaminergic striatal terminals, and reduced astrocytic and microglial response in animal PD models (Chao et al., 2009; Park et al., 2012b; Inden et al., 2013; Cerri et al., 2015; Suzuki et al., 2015; Park and Chang, 2020). Although it has been suggested that systemic administered MSCs do not pass the capillary system of the lungs, other studies showed that MSCs have an intrinsic ability to target the brain and migrate to the lesioned substantia nigra after intravenous administration (Chao et al., 2009; Do et al., 2021). Other works have used intranasal infusion as a non-invasive method of administration. Although some authors reported that MSCs administered intranasally reached the brain parenchyma, migrated to lesioned areas, and survived (Danielyan et al., 2011; Salama et al., 2017), others indicated that MSCs could not be detected in the brain (Chartoff et al., 2011). To address these issues, several modification techniques have been proposed to efficiently guide their migration to target sites or improve focal adhesion, which may be of particular interest to improve their viability within the transplanted environment in co-grafting approaches (Ocansey et al., 2020). Another important aspect is the implantation of a sufficient number of MSCs to guarantee a therapeutic effect. Previous studies suggest that MSCs survive for only a short period after transplantation (Chartoff et al., 2011; Bossolasco et al., 2012). The low immunogenicity of MSCs does not ensure that they are fully immune privileged, and they are likely to provoke immune response despite their immunosuppressive properties (Camp et al., 2009). Different strategies have been tested to increase the survival of the transplanted MSCs such as cell encapsulation, a combination of MSCs with microcarriers that release therapeutic proteins, or co-grafting with OECs (Delcroix et al., 2011; Forouzandeh et al., 2021). Other studies have combined MSC transplantation with other therapeutic strategies such as levodopa treatment or exercise to obtain enhanced improvements (Cucarian et al., 2019; Jalali et al., 2021). Other mechanisms underlying the beneficial effects of MSCs have been proposed. Recent data suggest that MSCs can transfer mitochondria to damaged tissue, which seems particularly relevant in PD therapy due to the proposed central role of damaged mitochondria in dopaminergic neurodegeneration (Liu et al., 2014). Interestingly, it has recently been reported that MSC treatment alleviated locomotor deficits and rescued dopaminergic neurons by inhibiting neuroinflammation in a PD model through regulating intestinal microorganisms by cross talk between the brain and gut (Sun et al., 2022; see for details inAdditional Table 2
). As PD pathology involves widespread neurodegeneration beyond the nigrostriatal pathway, MSCs transplantation targeting extra basal ganglia structures could also represent a strategy to alleviate the non-motor symptoms of the disease (Pantcheva et al., 2015).Delivery of neurotrophic factors by MSCs may also serve as a useful tool for the protection and functional stimulation of grafted dopaminergic cells from any source. In fact, co-grafting of fetal porcine neuroblasts and rat BM-MSCs promoted the survival of neuroblasts that when transplanted alone did not survive in the striatum of immunocompetent (i.e., non-immunosuppressed) denervated rats (Leveque et al., 2015). Interestingly, 50% of transplanted rats showed healthy grafts with TH-positive neurons at 120 days posttransplantation and motor recovery was also observed. Detailed analysis revealed a local control of the host immune response in the striatum that received co-grafts, highlighting the immunosuppressive properties of MSCs and their potential utility for the long-term survival of xenogeneic grafts (Leveque et al., 2015). Our group has recently demonstrated that the combination of murine BM-MSCs and fVM cells in the same suspension significantly improves the survival and reinnervation ability of dopaminergic neurons transplanted in a rat 6-OHDA model of PD. However, it is essential to control tightly the density of the transplanted cells or concentration of MSCderived products, since these beneficial effects are only observed when fVM tissue is combined with a low number of MSCs (Rodriguez-Pallares et al., 2021;Additional Table 1
). Interestingly, a conditioned medium derived from MSC (MSC-CM) containing the entire secretome also enhances the survival of dopaminergic grafts (Shintani et al., 2007), suggesting that the therapeutic effects of MSCs are not only based on direct cell-to-cell interactions but that paracrine signaling seems to be a primary mechanism (Mendes-Pinheiro et al., 2019; Fricova et al., 2020).As previously indicated, several mechanisms could be responsible for these effects. Our group observed that classical pathways involved in trophic factormediated signaling, such as MAPK/ERK and PI3K/Akt pathways, were active in dopaminergic cultures treated with MSC-CM. However, lipid removal decreased the viability and reduced the neuroprotective effects of MSC-CM on pure populations of dopaminergic cells, primary mesencephalic cultures (i.e., neuron-glia mixed), and human iPSC-derived neurons (Parga et al., 2018), suggesting that other factors could also be relevant. We demonstrated for the first time that prostaglandin E2, through prostaglandin E2 receptor 2, was largely responsible for the positive effects of the MSC-CM on dopaminergic survival and the cAMP-PKA pathway, used by many prostaglandins to modulate neuroprotection, was active in dopaminergic cultures (Parga et al., 2018).
Based on these promising results, several groups have conducted clinical trials using autologous or allogenic MSCs isolated from various sources for PD treatment (Fricova et al., 2020). In a pilot, open-label study in 2010, autologous BM-MSCs were transplanted unilaterally into the sublateral ventricular zone in seven PD patients. This preliminary study suggests the safety of this novel therapeutic approach and its potential beneficial effects, since three patients showed improved symptoms (Venkataramana et al., 2010). Two years later, the same research group conducted another openlabel study using allogenic BM-MSCs implanted bilaterally into the same area with similar results (Venkataramana et al., 2012). Currently, several clinical trials are in progress with a highly variable setup (ClinicalTrials.gov identifiers NCT02611167; NCT03550183; NCT04506073; NCT04928287; NCT04995081), including a study of co-transplantation of MSCs and MSC-derived neural stem cells in PD patients (ClinicalTrials.gov identifier NCT03684122). The experience gained in previous trials should guide the future directions and emphasize the need for further studies to understand the mechanisms underlying the therapeutic effects of MSCs and standardize experimental designs and protocols to achieve reliable and effective cell-based treatments for PD.
Conclusions and Future Perspectives
Over the decades, cell-based therapies have shown their potential in the treatment of PD. Although prematurely conducted trials in the past have cast doubt on the efficacy of these emerging approaches, human fVM tissue continues to be the gold standard and has undoubtedly paved the way for considering the clinical translation of transplantation of dopaminergic neurons obtained from different sources. The number of healthy dopaminergic cells needed for functional improvements in PD patients is around 40,000‒80,000 neurons, which requires readily available cells that contribute to permit the large-scale application of these approaches. Stem cells offer a clear advantage in this regard, and significant progress in obtaining sufficient dopaminergic cells from different expandable sources has been achieved in recent years. However, cell therapy for PD has underlying problems that affect transplanted cells regardless of the source of dopaminergic neurons. Although further studies are necessary, combinations of cell-based strategies, such as the cotransplantation approaches addressed in this review, or combination of cells with pharmacological treatments, deep brain stimulation, or potential new immunological therapies could improve the current results and help to slow or even stop the progression of the disease. Genetic manipulation of the cells may also be another beneficial strategy that could contribute to improving the survival, integration, and functionality of grafted cells. Emerging approaches in neural repair involve the development and application of tissue engineering techniques, such as the use of biomaterial or cellular scaffolds, or patientspecific constructs, that could influence the behavior of native or transplanted cells and promote survival, differentiation, organization, and reconstruction of degenerated circuitry. Further research is necessary to understand the mechanisms underlying the functional recovery of successful transplants and to achieve greater benefits for the millions of patients worldwide affected by PD.
Author contributions:
Manuscript conception and writing: JRP and JLLG; manuscript correction and editing: JAP and MGG. All the authors approved the final version of the manuscript.
Conflicts of interest:
The authors declare no conflicts of interest.
Availability of data and materials:
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Open access statement:
This is an open access journal, and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the
identical terms.
Additional files:
Co-grafting-based strategies in Parkinson’s disease.
Mesenchymal stromal cell-based strategies in Parkinson’s disease.
- 中国神经再生研究(英文版)的其它文章
- Notice of Retraction
- Ataxia-telangiectasia mutated plays an important role in cerebellar integrity and functionality
- The lymphatic drainage systems in the brain: a novel target for ischemic stroke?
- Lights at night: does photobiomodulation improve sleep?
- β2-Microglobulin exacerbates neuroinflammation, brain damage, and cognitive impairment after stroke in rats
- Neuroprotective role of Noggin in spinal cord injury

