Convergence of human and veterinary medicine: leveraging canine naturally occurring neurological disorders to develop regenerative treatments
Kaitlin C. Clark, Ashley Amador, Aijun Wang
In recent years, large animal models of naturally occurring diseases have become increasingly studied, with the rationale that their disease attributes may better recapitulate the pathological features of corresponding human diseases as compared to induced disease models (Hoffman and Dow, 2016). Of the available naturally occurring disease models, the canine is increasingly recognized as a valuable preclinical animal model in translational medicine for numerous human diseases, including cancer, respiratory disease, and inflammatory disease (Kol et al., 2015; Hoffman and Dow, 2016). The canine also frequently suffers from central nervous system (CNS) disorders, such as brain and spinal cord injuries, neurodevelopmental diseases (e.g., spina bifida (SB)), and neurodegenerative diseases (e.g., inflammatory brain disease (IBD)) that have comparable pathological features to human CNS disorders (Hoffman and Dow, 2016; Song et al., 2016). Additionally, canines live in similar environmental conditions as humans and can receive long-term monitored medical care. While research-induced animal disease modeling systems are the standardin vivo
approach to evaluate therapeutics, there are notable limitations, including inconsistency from natural disease in phenotypic heterogenicity, clinical and pathological features, and responsiveness to treatments. Furthermore, effective treatments are extremely underdeveloped for dogs with these conditions, and therefore many animals are left untreated or euthanized. The comparable features of canine CNS to human CNS disorders could allow the advancement of new standard care practices for companion animals, as well as provide critical insights for the development of regenerative medicine therapies for human clinical use.Canine CNS disorders:
Spinal cord injury (SCI):
SCI is a devastating condition that afflicts both human and canine patients. SCI can be acquired via acute injury or be the result of a congenital defect, most notably SB. SCI clinically presents as sensory and motor deficits, and in some cases complete paralysis. In the canine, SCI injuries typically result from acute injury including embolic myelopathy, disc herniation, and trauma which can result in chronic injury leading to degeneration (McMahill et al., 2015). The pathology of SCI is generally regarded as a two-hit hypothesis, in which primary injury results from mechanical trauma to the spinal cord and leads to the secondary injury in which prolonged proinflammatory responses and astrogliosis that results in demyelination and neuronal injury (McMahill et al., 2015). Intervertebral disc disease (IVDD) is an example of SCI that occurs in both humans and canines and results in degeneration and loss of vascularization to intervertebral discs (McMahill et al., 2015; Hoffman and Dow, 2016). The pathological features of canine and human IVDD are comparable and the risk of secondary injury is also analogous. Unlike rodent models, the canine disease can account for variability in clinical manifestations of SCI and the variability of lesion size and locale. Therefore, naturally occurring forms of SCI in the dog, including IVDD, may serve as superior models to evaluate therapeutic intervention strategies for the treatment of various forms of SCI.:
SB
is the most common cause of lifelong childhood paralysis and results from a congenital neural tube defect that exposes the spinal cord to chemical and mechanical trauma and results in paralysis and incontinence (Song et al., 2016). Canine SB clinically presents very similarly to human SB and occurs congenitally in dogs, making them a more relevant model of human SB, compared to induced disease models. English bulldogs, have the highest incidence of naturally occurring SB, making up a third of all canine cases due to inbreeding (Song et al., 2016). There is currently no standard of care treatment available for dogs with SB, and many are euthanized at birth. Therefore, surgical intervention strategies for postnatal repair in canines are being explored. It has been shown that recovery from SCI is substantially higher in neonates compared to adult and young animals. (Boulland et al., 2013; Zuchner et al., 2018). One reason for this is that elevated adaptive plasticity is maintained in the spinal cord after birth, which has led to the introduction of plasticity-augmenting procedures into clinical trials on human SCI patients (Boulland et al., 2013). While this plasticity indicates the potential for a successful regenerative therapy for SB, there are no existing animal models to study postnatal repair of SB. Additionally, for human SB,in utero
intervention strategies are not applicable for all cases, creating a clinical need for improved postnatal treatment strategies. Therefore, the English bulldog serves as a novel model to study postnatal therapy to treat naturally occurring SB in human patients:
IBD
has been shown to have a similar phenotype as multiple sclerosis (MS) in humans (Vitale and Foss, 2019). The two pathologic features of pediatric and adult MS are demyelination and inflammation caused by immune cell infiltration of the CNS (Bjelobaba et al., 2018). Mouse experimental autoimmune encephalitis is one of the most widely used models for studying MS pathology and therapeutic intervention (Bjelobaba et al., 2018). While this induced model strongly resembles the inflammatory and demyelinated state associated with MS, it does not capture all the features of the naturally occurring disease. Canine IBD can generically be referred to as meningoencephalomyelitis of unknown origin (MUO). Several forms of MUO can be characterized from histopathological findings into several categories including granulomatous meningoencephalomyelitis, necrotizing meningoencephalitis, and necrotizing leukoencephalitis (Andersen-Ranberg et al., 2021). MUO is presumed to be an autoimmune disease with a genetic predisposition (Andersen-Ranberg et al., 2021). It has been shown that there is a central role of MHC II positive cells, T-cells, and macrophages in disease onset of granulomatous meningoencephalomyelitis and necrotizing leukoencephalitis, much like what is seen in human forms of MS pathology (Andersen-Ranberg et al., 2021). Pugs specifically suffer from necrotizing meningoencephalitis, which has been shown to have a strong association of dog leukocyte antigen class II, similar to HLA in human MS (Greer et al., 2010). Necrotizing meningoencephalitis in dogs has a strong autoimmune component and is an aggressive phenotype of disease that has an early and acute onset, which may be more comparable to pediatric MS. Current treatment strategies for canine MUO and human MS manage the inflammatory component of the diseases but cannot address accompanying demyelination and degeneration. Though the heterogeneous nature of MS pathology makes it a difficult disease to study using animal modeling strategies, a novel canine naturally occurring disease model would provide a meaningful approach to assess the function of therapeutics.Mesenchymal stem/stromal cells (MSCs):
MSC-based regenerative therapy has become progressively popular for both human and veterinary patients (Carrade and Borjesson, 2013; Kol et al., 2015). MSCs are known to possess potent immunomodulatory, anti-inflammatory, and regenerative properties through the secretion of bioactive mediators (Carrade and Borjesson, 2013). However, the mechanisms by which canine MSCs elicit these effects on CNS disorders are not yet fully characterized. MSCs may play a pivotal role in neuroregeneration by secretion of neuroprotective, anti-apoptotic, and neurotrophic factors/cytokines as well as other extracellular elements. MSCs may also possess the ability to induce endogenous neuronal growth, promote neurogenesis, encourage synaptic connection from damaged neurons, recruit local oligodendrocyte precursors, stimulate angiogenesis, reduce demyelination and oxidative stress, and regulate neuroinflammation by modulating pathological T, B, natural killer and microglial cell responses (Carrade and Borjesson, 2013; McMahill et al., 2015). In particular, placenta-derived MSCs (PMSCs) are an emerging therapy for disease treatment. The placenta is a unique, fetal-derived tissue that can be reliably obtained to generate PMSCs for the treatment of developmental and perinatal diseases. Compared to adult adiposederived MSCs, PSMCs have a distinctive secretome profile and have more robust immunomodulatory properties, modulating lymphocyte responses by inducing apoptosis (Amorim et al., 2020). Furthermore, the PMSC secretome includes extracellular vesicles, which are membranebound nanovesicles containing proteins and nucleic acids, and can be classified as apoptotic bodies, microvesicles, and exosomes (Zakirova et al., 2020). The PMSC secretome may additionally serve as a potential cell-free therapeutic to treat neurological disease. Collectively, these findings suggest that PMSCs represent a unique stem cell subset and hold significant promise for treatments of canine diseases and disorders.MSCs therapy for canine CNS disorders:
MSCs have immense immunomodulatory and regenerative properties which have been demonstrated in several animal species. Researchinduced disease modeling, using synthetic, genetic, and technical manipulation provides critical preclinical insights for disease study, but has significant limitations modeling the complexity of native pathological features of biological disease. This likely contributes to the high failure rate of rodent disease models transitioning to human phase I clinical trials (Kol et al., 2015). Therefore, naturally occurring companion animal disease may better recapitulate the complex and heterogenous features associated with human disease pathology.Utilizing MSC therapy for the treatment of CNS disorders in the dog has become of high interest in recent years. The findings from these studies can provide critical insights to the use of MSCs and MSC-derived extracellular vesicles as potential drug candidates for human diseases as well. Clinical trials have already been employed in the canine using adult tissue-derived MSCs for several neurologic conditions including, IVDD and MUO (Hoffman and Dow, 2016). It has previously been reported that canine MSCs derived from adult and fetal tissue have comparable functional properties feasible for the treatment of neurological disorders; however, canine PMSCs have superior modulatory properties making them an ideal drug candidate. Investigating the therapeutic functions of canine PMSCs in treating canine neurological disorders will not only benefit canine patients but will also serve as preclinical data to accelerate the development of human counterpart PMSCs as a therapeutic for human patients. MSCs are also currently being evaluated for the treatment of MS, however, the heterogeneous nature of MS pathology poses a large challenge for the development of intervention strategies. Utilizing naturally occurring canine SCI, SB and MUO may better direct treatment plans for future human clinical trials.
To assess the clinical feasibility of an MSC product for the treatment of canine neurologic diseases, clinical trials will be necessary. Studies utilizing canine MSCs to evaluate safety and efficacy in the form of a veterinary clinical trial would allow for a new standard of care for these patients. Determining outcomes of these studies will utilize analogous deficits observed in both canine and human patients. For many CNS disorders, ambulatory defects, incontinence, and impaired cognitive functions are typically observed in patients. The need for strategies to improve clinical outcomes for neurodegenerative diseases is warranted in both the veterinary and human medical fields. Large-scale clinical trials evaluating the safety and efficacy of stem cell-based treatments long-term in canines provide unique opportunities to address clinical needs for both companion animals and their human counterparts. A schematic overview of studies utilizingin vitro
models, research-induced disease models, and naturally occurring veterinary disease models to develop regenerative treatments for both veterinary and human patients is shown inFigure 1
. This approach will be a significant step toward translating this cellular therapy to human patients.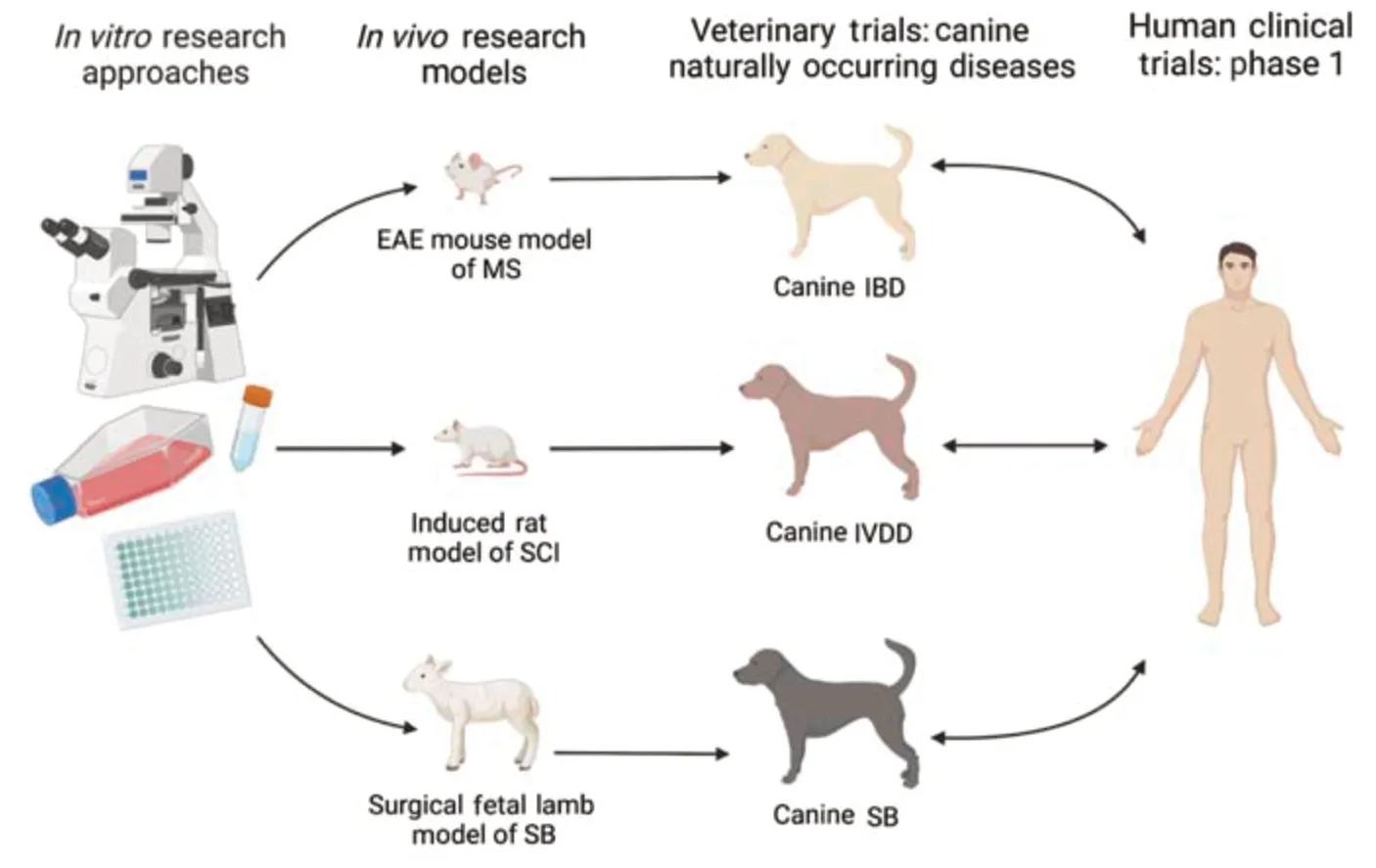
Figure 1 |A schematic overview of studies utilizing in vitro models, research-induced disease models and naturally occurring canine disease models to develop regenerative treatments for veterinary and human patients. EAE: Experimental autoimmune encephalitis; IBD: inflammatory brain disease; IVDD: intervertebral disc disease; MS: multiple sclerosis; SB: spina bifida; SCI: spinal cord injury.
Conclusions:
Findings from these studies would be relevant to companion animal health as these results will provide clinical utility to several populations of dogs that do not have a treatment available in most cases. These animals will typically be euthanized due to the lack of standard care practices and intensive care required for their survival. Due to the immunomodulatory and regenerative properties of stem cells, there is a wide range of pathologies these therapies can be utilized for. PMSCs are a unique subset of cells that may have efficacious therapeutic outcomes for neurodevelopmental disorders due to their potent immunomodulatory and neuroprotective functions. In conclusion, the canine is becoming a significant translational model for human disease and may be a better predictor of clinical outcomes in humans, compared to traditional researchinduced disease models, and will provide unique insights into translational therapies for human patients.Financial support for this work was provided by the Center for Companion Animal Health and UC Davis Veterinary Institute of Regenerative Cures (VIRC), School of Veterinary Medicine, University of California, Davis, and grants from the National Institutes of Health (1R01NS115860-01A1, 5R01NS100761-02), and the Shriners Hospitals for Children (85108-NCA-19, 85135-NCA-21). Kaitlin Clark was supported by the Willis W. and Ethel M. Clark Foundation Investment in Community Fellowship, the Lodric Maddox Graduate Fellowship, and the National Center for Advancing Translational Sciences, National Institutes of Health, through grant number UL1 TR001860 and linked award TL1 TR001861. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Kaitlin C. Clark, Ashley Amador, Aijun Wang
Center for Surgical Bioengineering, Department of Surgery, University of California, Davis School of Medicine, Sacramento, CA, USA (Clark KC, Amador A, Wang A)
Institute for Pediatric Regenerative Medicine, Shriners Hospital for Children, Sacramento, CA, USA (Clark KC, Wang A)
Department of Biomedical Engineering, University of California Davis, Davis, CA, USA (Wang A)
Correspondence to:
Aijun Wang, PhD, aawang@ucdavis.edu.https://orcid.org/0000-0002-2985-3627 (Aijun Wang)
Date of submission:
November 19, 2021Date of decision:
January 22, 2022Date of acceptance:
June 17, 2022Date of web publication:
August 2, 2022https://doi.org/10.4103/1673-5374.350195
How to cite this article:
Clark KC, Amador A, Wang A (2023) Convergence of human and veterinary medicine: leveraging canine naturally occurring neurological disorders to develop regenerative treatments. Neural Regen Res 18(3):541-542.
Open access statement:
This is an open access journal, and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- Notice of Retraction
- Neuroprotective role of Noggin in spinal cord injury
- Combined cell-based therapy strategies for the treatment of Parkinson’s disease: focus on mesenchymal stromal cells
- Lights at night: does photobiomodulation improve sleep?
- β2-Microglobulin exacerbates neuroinflammation, brain damage, and cognitive impairment after stroke in rats
- The lymphatic drainage systems in the brain: a novel target for ischemic stroke?

