Axonal tuning by GABAA receptor unveils novel tricks from an old dog
Veronica Bonalume, Valerio Magnaghi
In the last years, axonal conductance of action potential trains became a novel subject of study, changing the view of axons, from a static cable-like compartment to a more complex and dynamic system (Debanne et al., 2011). Axonal computation, indeed, is canonically constituted by the action of voltage-gated ion channels, such as the classic Naand Kchannels, but recent studies demonstrated that it can be modulated by the action of other ion channel pumps, and metabolic factors (Byczkowicz et al., 2019; Zang and Marder, 2021). These non-canonical mechanisms have been studied mainly in the central nervous system (Byczkowicz et al., 2019; Kamiya, 2019), and little is known about axonal conductance modulation in peripheral nerve fibers. Interestingly, the peripheral projecting neurons possess a pseudounipolar conformation and exceptionally long axons, an anatomical characteristic that make propagation and tuning of the axonal action potential more easily adjustable. Notably, unmyelinated axons (i.e. C-fiber nociceptors) prevail in all peripheral fibers as the most affected by changes in conduction velocity, since relatively small alterations cause substantial delays in action potential incoming time (Zang and Marder, 2021). Peripheral C-fiber nociceptors, indeed, are defined by specific activity-dependent slowing properties, whereby repetitive firing of nerve fibers results in the progressive slowing of their conduction velocity and concomitant increase in response latency (Gee et al., 1996). Activity-dependent slowing is so preserved to be designated as a signature mark able to discriminate among different functional C-fiber subtypes (Werland et al., 2021).
Gamma-aminobutyric acid A (GABA
) receptor and Na-K-Cl cotransporter type 1 (NKCC1) are important regulators of axonal conduction:
Very recently, Bonalume et al. (2021) characterized axonal GABAreceptors in peripheral nerves. These receptors mediate depolarizing Clcurrents, which in turn modulate peripheral C-fiber nociceptors computation. Thereby, the endogenous GABAreceptor activation represents a novel physiological mechanism able to increase C-fibers’ fidelity, stabilizing their excitability and enabling prolonged firing. Such a noncanonical location and function of tonic GABAreceptor currents may represent an intriguing target of study in several physio-pathological conditions, such as the neuropathies occurring after nerve injury, chronic pain as well as in pain insensitivity. Furthermore, Bonalume et al. (2021) demonstrated that any dysregulation of the Clgradient upstream GABAreceptor fluxes leads to a significant alteration in C-fiber nociceptors’ excitability and conduction velocity. Deepening the comprehension of this innovative pathway may unveil alternative pharmacological targets for pain treatment, besides the GABAreceptor itself, avoiding most of central side effects resulting from its activation. The main Cltransporter engaged in this process is NKCC1. Peripheral neurons possess a high intracellular Clconcentration even in adults, compared to central neurons that gain a low intracellular Clconcentration upon maturation. This reverse gradient results from the physiological predominance of inward Cltransport, mediated by NKCC1, versus the outward transport, mediated by KCC2, being the last faintly expressed in peripheral neurons. Accordingly to the literature, GABAreceptor opening in peripheral axons mediates depolarizing Clcurrents. Bonalume et al. (2021) further demonstrated that NKCC1 activity is dynamically coupled with C-fiber nociceptors firing. The high-frequency stimulation of C-fibers induces a feedforward activation of NKCC1, leading to an increase in Clgradient upon axonal membrane, within a consequent potentiation of GABAreceptor currents. In this context, GABAreceptor serves as a regulator of the “Clconductance”, acting downstream the NKCC1 control. Overall, the mutual activity of these two players elicits tonic depolarizing currents along the peripheral C-fiber nociceptors, able to sustain a prolonged firing, and preventing fast desensitization mediated by the activity-dependent slowing mechanism. The blockade of NKCC1 or the absence of functional GABAreceptor in C-fibers, indeed, causes a strong uprise in the activity-dependent slowing. The increased conduction velocity of axons upon mild depolarization is consistent with previous observations correlating the shift of membrane potential in the subthreshold range to the raise in firing frequency (Carp et al., 2003). Intuitively, such a GABAreceptor-mediated hypothesis of the “acceleration of conduction” in C-fibers can be figured out as follows: during the action potential sustained activity, the NaV channelsmediated current in one axonal site depolarizes the neighboring locations faster whether the axon is already depolarized through the GABA/NKCC1/Clcontrol, rather than in an axon still in a resting state.Where does the GABA come from?
This paradigm rises an intriguing question: how would the axonal GABAreceptor be activated without a classical synaptic-like release of GABA? In the mouse sural nerve, Bonalume et al. (2021) demonstrated that the GABAreceptor is endogenously activated under physiological conditions, exhibiting a tonic profile. In fact, different pharmacological approaches (that is a competitive GABAreceptor antagonist, the allosteric agonist allopregnanolone, as well as an NKCC1 blocker) confirmed the effect mediated by the endogenous GABA. Undoubtedly, the release of GABA fromex vivo
desheated nerves has been detected in the concentration range of tens of nanomolar (Bonalume et al., 2021). Altogether, these observations prove that GABA is physiologically released within peripheral nerves, allowing a tonic modulation of axonal excitability and supporting the action potential propagation and tuning, from the periphery to the central nervous system. However, in line of principle, it should not be excluded that other extrasynaptic ligands of GABAreceptors such as glycine and taurine (Le-Corronc et al., 2011) could participate in the modulation of axonal excitability.Surprisingly the novel interpretation of GABAreceptor-mediated actions in peripheral nerves corroborates the illuminated observation by Jessen et al. (1979), that formerly showed the presence of GABAergic neurotransmission in the peripheral nervous system. The scientific challenge, however, to fully understand the role of Cl-mediated currents modulation of axonal conductance, would be to define precisely the local origin of GABA, studying the pathways involved in its homeostasis and the putative triggers for its release. In principle, both neuronal and glial compartments of peripheral fibers could be considered as local sources and reuptake of GABA (Colciago et al., 2020). Both GABAand the metabotropic GABAreceptors are expressed and functional in Schwann cells, whereas their endogenous activation participates in axonal sorting and myelination, likely during the development (Castelnovo et al., 2017). Furthermore, the Schwann cells have been proved to synthesize and release the neuroactive steroid allopregnanolone (Bonalume et al., 2020), which can modulate their fate as well as the peripheral neuronal activity (Meyer et al., 2019) via a fast GABAreceptor activation (Cociago et al., 2020). In this regard, the presence and importance of endogenous tonic GABA in peripheral nerves have been already demonstrated, for peculiar physiological aspects, in those cellular compartments. Interestingly, the concomitant modulation of axonal conduction velocity adds more complexity to the GABAergic puzzle in the peripheral nervous system, emphasizing a more dynamic role for GABA, GABAreceptor, and Clconductance in the context of sustained firing and strong axonal fidelity.
Concluding remarks:
In the context of this novel interpretation of GABA-mediated depolarizing Clcurrents, it is noteworthy that the peripheral nociceptors are not the unique mature neurons characterized by high intracellular Clconcentration. Somatosensory trigeminal neurons, post-ganglionic sympathetic neurons, and olfactory sensory neurons are equally characterized by high intracellular Cland subsequent outward depolarizing Clcurrents. All these neurons show strong and characteristic desensitizing/adaptive mechanisms, which go together with the inability to hold high-frequency firing for a prolonged period. Interestingly, such a conservative mechanism is physiologically controlled by the presence of opposite currents, which stabilizes fibers, avoiding axonal failure. For instance, Ca-activated Clcurrents (CaCCs) have been proved to modulate repetitive firing in mouse sympathetic ganglion cells (Martinez-Pinna et al., 2018). In this subset of neurons, the Ca-activated Clcurrents serve as a regulator of Clconductance instead of GABAreceptor, acting in a comparable dynamic manner by means of depolarizing currents.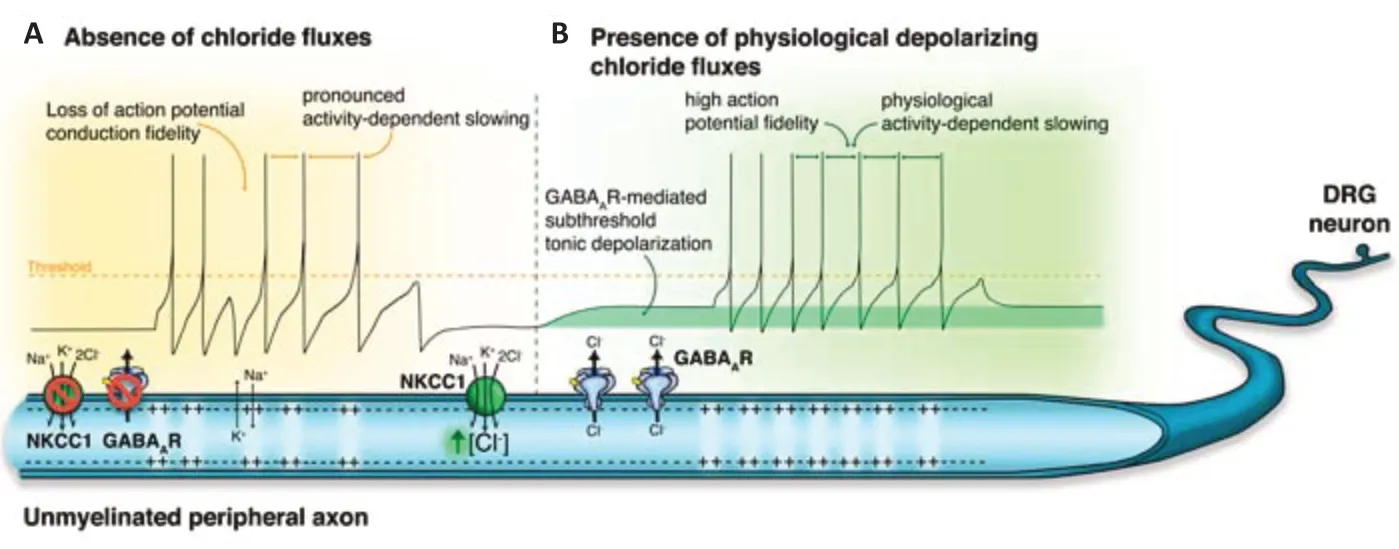
Figure 1 |Modulation of action potential conductance by GABAA receptor-mediated currents. (A) In kinds of Cl‒ dysregulation, such as NKCC1 blockade, GABAA receptor absence or inhibition, the peripheral nociceptor firing is curtailed. Unmyelinated axons (i.e. C-fiber nociceptors) are characterized by excessive slowing and decreases in action potential fidelity. (B) In presence of a high intracellular Cl‒ concentration, mediated by NKCC1 inward currents, GABAA receptor tonic activation induces a subthreshold depolarization that stabilizes excitability, triggering high action potential fidelity and enabling prolonged firing in time. DRG: Dorsal root ganglion; GABAA: gammaaminobutyric acid A receptor; NKCC1: Na-K-Cl cotransporter type 1.
Therefore, the “GABA/Cl” regulation adds further workpieces to the “non-cable-like” theory of axonal function, posing an alternative overview of the axonal computation and focusing on C-fiber unmyelinated axons, which are affected by pain physiopathology. The pharmacologic possibility to modulate the GABA/NKCC1/Clpaves the way to alternative approaches for the treatment of neuropathic and/or chronic pain.
Veronica Bonalume, Valerio Magnaghi
Department of Pharmacological and Biomolecular Sciences, Università degli Studi di Milano, Milan, Italy
Correspondence to:
Valerio Magnaghi, PhD, valerio.magnaghi@unimi.it.https://orcid.org/0000-0002-6903-7042 (Valerio Magnaghi)
Date of submission:
March 17, 2022Date of decision:
May 5, 2022Date of acceptance:
May 18, 2022Date of web publication:
June 2, 2022https://doi.org/10.4103/1673-5374.346489
How to cite this article:
Bonalume V, Magnaghi V (2023) Axonal tuning by GABA receptor unveils novel tricks from an old dog. Neural Regen Res 18(3):533-534.
Open access statement:
This is an open access journal, and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- Notice of Retraction
- Neuroprotective role of Noggin in spinal cord injury
- Combined cell-based therapy strategies for the treatment of Parkinson’s disease: focus on mesenchymal stromal cells
- Lights at night: does photobiomodulation improve sleep?
- β2-Microglobulin exacerbates neuroinflammation, brain damage, and cognitive impairment after stroke in rats
- The lymphatic drainage systems in the brain: a novel target for ischemic stroke?

