Developmental exposure to thyroid disruptors: misprogramming of the brain’s stem cells in later life?
Pieter Vancamp, Sylvie Remaud
Introduction: Ever since the discovery of neural stem cells (NSCs) in the adult mammalian brain, scientists have been trying to decipher which signals govern their turnover and lineage commitment to generate neurons and glia. Understanding their role in nervous tissue homeostasis can provide new insights into the etiology of several neurological disorders, and might one day be turned to our advantage to promote endogenous brain injury repair. Others and we have identified thyroid hormone (TH) as a key factor transcriptionally regulating NSC behavior in the largest niche of the adult mammalian brain: the subventricular zone (SVZ).
TH is often considered a classic hormone, of which most is known with regard to its developmental and metabolic actions in vertebrates. However, two revolutions have revived the field. Next to the thyroid axis governing systemic TH homeostasis, tissuespecific TH regulation comprises a second level of control that affects each cell type’s transcriptome independently of one another. Deiodinases, enzymes activating the ‘prohormone’ T4 into the biologically active T3, or inactivating T3, and membrane TH transporters act in concert to control the intracellular availability of T3 able to bind the nuclear TH receptors, determining transcriptional activity. Second, research has revealed alarming connections between continuous, low-dose exposure to thyroid disrupting chemicals and abnormal human brain development, with potential repercussions on laterlife intellectual capacities (Demeneix, 2019). These two new concepts provide the context in which our latest research should be considered.
Over the past years, a general picture emerged of how TH and the ensemble of cellular regulators influence the capacity of adult SVZ-NSCs to generate either new neuroblasts or oligodendrocyte precursor cells (OPCs) in mice. Gain- and loss-of-function experiments first showed that the T3-occupied receptor TRα1 in adult SVZ-progenitors repressedSox2
, a gatekeeper of NSC identity, and stimulated neuronal lineage commitment and neuroblast migration (López-Juárez et al., 2012). Later in 2017, it was shown how the presence of the T3-inactivating DIO3 combined with the absence of TRα1 in adult epidermal growth factor receptor-positive progenitors favored oligodendroglial lineage determination, enhancing the generation of mature oligodendrocytes. The latter are capable to generate new myelin sheaths around axons, and promoted endogenous functional repair in an adult mouse model of demyelination in which mature oligodendrocytes were eradicated (Remaud et al., 2017). In mice null for the two main TH transporters MCT8 and OATP1C1, the resulting cellular hypothyroidism decreased neurogenesis and stimulated OPC fate choice (Luongo et al., 2021). Lastly, the absence of transthyretin or TTR, the T4-distributing protein in the cerebrospinal fluid, altered the adult SVZ-neuron/oligodendroglia output, and therefore affects NSC fate choice too (Vancamp et al., 2019). These data collectively indicate that a lack of T3 in SVZ-progenitors caused by TH regulator deficiency favours oligodendroglial lineage commitment.These observations in adult mice made us question whether TH could also affect the cellular SVZ organization during early life stages. The postnatal SVZ structurally reorganizes and embryonically generated NSCs reactivate to start generating neuroblasts and OPCs at a steady pace throughout adulthood. During this phase, which occurs over the first three weeks after birth in mice, TH serum levels gradually rise and peak at the beginning of the third postnatal week, suggesting TH could influence SVZ remodeling. TH exerts an orchestrating role in other developing brain regions as well, such as the cortex, the cerebellum, and another stem cell niche: the subgranular zone of the hippocampus.
Interestingly, in contrast with hypothyroid insults to the adult brain that are usually reversible, periods with inadequate TH levels during development bare an increased risk of generating irreversible structural abnormalities with functional consequences in later life. Epidemiological data have shown that even subclinical alterations in TH serum levels during pregnancy reduce the cortical grey matter volume in newborns, resulting in a population-level drop of a few IQ points (Korevaar et al., 2016). We hypothesized that TH coordinates postnatal SVZ reorganization at the transcriptome level, and that any disruption of TH action during this window could therefore misprogram NSCs, altering the neuron-oligodendroglial output in adult life with implications for functions that depend on it.
Our recent findings:
Using open access single-cell RNA-Seq data from dissected murine SVZs, and by visualizing single mRNA transcripts with RNAscope, we found a global absence of TH-converting enzymes and -transporters in all SVZ cell types at postnatal day 2 (P2) (Vancamp et al., 2022). However, at P20, just after the peak in TH serum levels,Mct8
,Oatp1c1
,Dio2
,Dio3
, andThra
(encoding TRα2 and TRα1, the most prevalent TH receptor in the brain) were dominantly expressed in NSCs. Expression patterns of TH regulators have always been a strong indication for the intracellular T3 status as well as for what happens at the level of TH-target genes. In our study, they indicated that during and after the TH peak, the majority of NSCs possesses the machinery to autoregulate their intracellular TH concentration, allowing for TH action. In parallel with this increase in TH activity, we observed a burst in SVZ-neurogenesisin vivo
, while OPC numbers stayed more or less the same (Figure 1
).Consequently, blocking TH synthesis by feeding pregnant dams propylthiouracil from embryonic day 15 (E15) to P21 dysregulated several of the tested key TH-target genes at P15 and P21, including the ones encoding the TH regulators, as well as those involved in NSC lineage commitment (Figure 1;
Vancamp et al., 2022).In vivo
analysis showed decreased neuroblast and OPC generation at P21, as well as reduced uncommitted progenitor proliferation. Even more interesting was that in 3-month-old adult mice fed a normal diet for more than 2 months after transient developmental hypothyroidism, neuroblast and OPC generation was still below normal. Related to that were behavioral alterations. Mice rely on olfaction for social interaction and environmental exploration. We found that adult mice developmentally exposed to propylthiouracil were not able to remember a previously presented odor, and that their olfactory bulbs contained fewer neurons, likely caused by an inadequate supply of new neuroblasts from the SVZ. The data reflect the idea of the Barker hypothesis (i.e. developmental origins of health and disease) and prompt the question whether ubiquitous environmental toxicants, which interfere with normal TH homeostasis and to which we are constantly exposed, might evoke similar (irreversible) effects on the brain.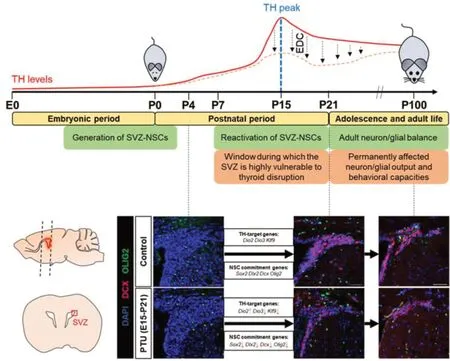
Figure 1 |Overview of the major events in murine SVZ development and the role of thyroid hormones therein.While the SVZ-NSCs are generated during embryonic development, they postnatally reactivate under the influence of genes and extracellular factors. We found that when postnatal TH serum levels peak, intracellular TH action in NSCs increases, coinciding with the onset of SVZ-neurogenesis. Consequently, this window represents a phase when SVZNSCs are particularly sensitive to exposure to endocrine disruptors. Indeed, developmental exposure to the TH-synthesis blocker PTU dysregulated TH-target genes, and permanently disrupted the neuron/oligodendroglia output in the SVZ. As a consequence, young adult mice developmentally exposed but fed a normal diet thereafter, underperformed on olfactory behavior tests that normally depend on proper SVZ-neurogenesis. Scale bar: 50 µm. E: Embryonic day; EDC: endocrine disrupting chemical; NSC: neural stem cell; P: postnatal day; PTU: propylthiouracil; SVZ: subventricular zone; TH: thyroid hormone. Data source: Vancamp et al. (2022) combined with unpublished figures from the authors.
Perspectives for toxicological research:
We identified the SVZ as a brain region vulnerable to developmental thyroid disruption. Of note, perturbation of one hormonal axis can bring the cross-talk with other hormonal pathways out of balance, potentially worsening the phenotype, knowing that SVZ development and function depend on other hormones as well. Hence, it should be additionally tested how chemical disruption of these endocrine pathways affects the above-mentioned processes. That knowledge can promote the SVZ to a region of choice to evaluate whether a chemical substance possesses neurotoxic properties or not, using our well-established read-outs.Trying to identify reproducible read-outs that allow classifying substances as developmentally (neuro)toxic or not is the purpose of recent collaborative efforts such as the ATHENA project (Kortenkamp et al., 2020). For instance, Ramhøj et al. (2021) assessed the effects of perinatal exposure to the TH disruptor and herbicide Amitrole on the rat brain. They analyzed the formation of periventricular heterotopia, i.e. collections of neurons that had wrongfully migrated to the dorsal corpus callosum. Hypothyroid conditions cause multiple large heterotopia to form in rats. Exposure to 50 mg Amitrole/kg/day from E7-P16 lowered T4 serum levels, and resulted in a more than three-fold increase in the number of P16 brain sections that contained large heterotopia (Ramhøj et al., 2021). This neurological endpoint is potentially more reliable and consistent to detect adverse effects of thyroid disruptors than are the standard measurements of T4, T3, and TSH in the blood. What causes the heterotopia in the first place remains unclear, but similar migration defects as observed in TH regulator-deficient mice might be at play (Luongo et al., 2021).
We propose that our read-outs in the mouse SVZ, notably NSC proliferation, the neuron/glia output and the olfactory capacities, can be used in parallel to assess developmental neurotoxicity (Additional Figure 1
). One study already assessed the effects on postnatal SVZ-neurogenesis in mice following oral exposure from E6-P16 to the polybrominated diphenyl ether BDE209, a widely used flame retardant. The authors found reduced NSC proliferation and neurogenesis in the SVZ at P16, and fewer calretinin-expressing interneurons in the olfactory bulbs of those exposed to 100 mg/kg/day. Doses of 20 mg/kg/day were sufficient to hamper neuronal migration and dendritogenesis of the olfactory granule cells (Xu et al., 2018). Further studies should definitely examine the long-lasting consequences of neurodevelopmental toxicity too, by evaluating whether these structural alterations are permanent, and how they affect animal behavior in later life.Currently, we are testing how exposure to low doses of the TH-disrupting and bisphenol A-replacing substance bisphenol F (BPF) during E15-P21 affects SVZ reorganization and adult mouse behavior using the read-outs mentioned above. As is often the case, following restriction or a ban on the use of a synthetic substance, a replacement chemical is rapidly introduced for which no juridical regulation exists. However, structural similarities with the original compound often suggest similar, and in some cases worsened effects on organ development. Unfortunately, it requires years of work to prove that this is actually true.
BPF is a widely used chemical in consumer products such as plastics and epoxy resins. Just last year, epidemiological data have revealed a worrying association between the BPF levels found in the urine of moms in their 10week of pregnancy, and an average drop in IQ points amongst the respective children at the age of 7 years, notably in boys (Bornehag et al., 2021). For the sake of our future generations, we need to find out how exactly these compounds act and perturb (neuro)development (Additional Figure 1)
to construct a better healthregulation policy, and to inform the public about the potential harm of these kinds of endocrine disruptors. The observed sex-specific differences remind us that additional perturbation of sex hormone homeostasis can lead to dissimilar outcomes in boys and girls. Therefore, toxicological studies in animals should include both sexes to evaluate whether a chemical substance poses an equal threat to males and females. For example, knockout of Ttr decreased neurogenesis in the dorsal SVZ of male mice, but less so in females (Vancamp et al., 2019). One can imagine a toxicant obstructing the TTR binding pocket for T4 to induce similar sex-specific effects. Another remark to be made is that the effects of low-dose exposure are often more subtle and require larger sample numbers to bring harmful effects to light, both in animal and epidemiological studies.Furthermore, it should not be forgotten that from the earliest stage of life, we are not exposed to a single compound, but rather mixtures of dozens of chemicals, for example, via the amniotic fluid and breast milk. Substances of various chemical families can have additive or synergistic actions that could aggravate abnormal brain development (Demeneix, 2019; Caporale et al., 2022). In the case of bisphenols, dozens of substitutes for BPA exist without a consequent and clear regulation. The question arises whether developmental exposure to increasing numbers and always-higher concentrations of the components in such mixtures can at least partially account for the increased incidence of neurological disorders with developmental origins observed over the last years. Better testing tools and increased awareness alone cannot account for this tendency.
The latest data from the Swedish SELMA cohort study seem to indicate so, as early-life exposure to endocrine disruptor mixtures found in pregnant women were associated with an increased risk for a delay in language development of exposed children (Caporale et al., 2022). The authors provided mechanistic explanations by exploring multiple endpoints in animal models and human pluripotent stem cell-derived brain organoids. Several genes implicated in autism spectrum disorder were dysregulated following exposure to the mixture that was composed of the chemicals linked to the language delay. In zebrafish andXenopus laevis
, two OECD-validated models, the TH-pathway was identified as a key target of the mixture, perturbing neurodevelopmental gene expression and inducing abnormal behavior (Caporale et al., 2022).Future challenges:
To understand and recognize the threat of real-life chemical exposure, we are in need of covenant exposure protocols testing environmentally relevant doses using appropriatein vitro
,ex vivo
, andin vivo
models with each their strength, and unambiguous read-outs that allow a clear-cut interpretation of the toxicity of a compound or a mixture. This will facilitate uniformity and scientific consensus in toxicological studies, allowing for easier classification of compound toxicity, and accelerating research to identify neurotoxic traits of many chemicals yet to be tested.We hope that our study has provided the next step in this challenging quest, by offering fundamental knowledge on how and when TH normally acts on the SVZ, a less well-understood cell population of the brain, and how and when endocrine disruptors could therefore adversely affect this niche. In addition, a similar approach holds important value for other stem cell niches such as the TH-responsive hypothalamic tanycytes, and the subgranular zone in which local TH signaling appears paramount for correct adult neurogenesis as well (Mayerl et al., 2022). More research on how and when thyroid disruptors act on these stem cells could unveil links between environmental pollution and illnesses with a particularly high prevalence in industrialized countries, such as cognitive and metabolic diseases.
A multi-disciplinary approach, from experimental models to data collection and analysis in large human cohorts (Caporale et al., 2022) combined with the newest techniques that allow for single cell-level phenotyping, should give a clearer picture not only within specific target tissues such as the NSCs in the SVZ, but in the whole brain and body for that matter. Only by those means, will we be able to inform policy bodies, increase awareness, and establish a legal framework to protect those yet to be born.
PV and SR are supported by the European Union’s Horizon 2020 contract ATHENA (grant No. 666869) and ENDpoiNTs (grant No. 825759). PV was
additionally supported by the European Thyroid Association (ETA) and the Fondation pour la Recherche Médicale (FRM grant No. SPF201909009111).
Pieter Vancamp, Sylvie Remaud
Laboratory of Molecular Physiology and Adaptation, CNRS UMR 7221, Department Adaptations of Life, Muséum National d’Histoire Naturelle, Paris, France
Correspondence to:
Sylvie Remaud, PhD, sremaud@mnhn.fr.https://orcid.org/0000-0002-0230-2833(Sylvie Remaud)
Date of submission:
March 24, 2022Date of decision:
April 23, 2022Date of acceptance:
May 7, 2022Date of web publication:
June 2, 2022https://doi.org/10.4103/1673-5374.346053
How to cite this article:
Vancamp P, Remaud S (2023) Developmental exposure to thyroid disruptors: misprogramming of the brain’s stem cells in later life? Neural Regen Res 18(3):527-528.
Availability of data and materials:
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Open access statement:
This is an open access journal, and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Additional file:
Proposed chain of events in the mouse SVZ and related behaviors following developmental exposure to thyroid disrupting compound(s).
- 中国神经再生研究(英文版)的其它文章
- Notice of Retraction
- Neuroprotective role of Noggin in spinal cord injury
- Combined cell-based therapy strategies for the treatment of Parkinson’s disease: focus on mesenchymal stromal cells
- Lights at night: does photobiomodulation improve sleep?
- β2-Microglobulin exacerbates neuroinflammation, brain damage, and cognitive impairment after stroke in rats
- The lymphatic drainage systems in the brain: a novel target for ischemic stroke?

