Biofabrication of nanovesicles for brain diseases
Pasquale Picone, Domenico Nuzzo
Background:
Nanotechnologies promise to improve disease diagnosis and treatment, overcoming the limitations of conventional administrations. In particular, extracellular vesicles (EVs) and artificial vesicles (AVs) are strongly emerging tools in nanomedicine (Leggio et al., 2020). EVs are cell-derived membrane structures secreted after the fusion of endosomes with the plasma membrane (exosomes) or shed from the plasma membrane (microvesicles). EVs are released by different brain cells (neurons, oligodendrocytes, astrocytes, and microglia) and constitute a physiological intercellular communication system. Indeed, EVs can deliver different types of molecules (nucleic acids and proteins), which often influence the phenotype of the recipient cells. They play a physiological role in the central nervous system (CNS), such as development, myelination, regeneration, and synaptic activity (Lai et al., 2012). Due to their content, EVs could therefore constitute an important biomarker for neurodegenerative diseases, represent candidates for therapeutic use, enclosing regulatory molecules, or be considered as vectors for brain drug delivery (Croese et al., 2018).AVs (liposomes and polymersomes) that mimic natural vesicles consist of an aqueous compartment physically separated from the surrounding environment by an artificial membrane (Leggio et al., 2020). EVs and AVs in addition to delivering molecules (RNA and/or proteins) or loading the drugs have the potential to improve the stability and solubility of encapsulated molecules and drugs, promote transport across membranes, and extend circulation times to increase the safety and efficacy of treatments (Leggio et al., 2020).
In this context, the bio-fabrication of cellderived nanovesicles (C-DNv) has been recently produced by technological approach, from cells by using different physical techniques including extrusion through micro-filters, microfluidic, sonication, nitrogen cavitation, and cell bleb-based method (Li et al., 2021). These methods require cellular destruction to obtain nanovesicles with characteristics similar to the cells membranes.
In literature, C-DNv generated are also called exosome-mimetics (Vázquez-Ríos et al., 2021), artificial exosomes (Li et al., 2021), or bioinspired cell-derived nanovesicles (Goh et al., 2017). This different terminology is due to the fact that C-DNs appear to be similar in several aspects to exosomes, but are produced through physical processes such as AVs. The C-DNv are great promise as drug delivery systems (DDS) due to the combined potential benefits of natural and synthetic vesicles. The bio-fabrication of C-DNv is a scalable and efficient alternative to EVs production to obtain a high yield of cell-derived nanosized vesicles (Ilahibaks et al., 2019). CNS disorders, such as neurodegenerative and tumors are among the most serious health problems (more than 600 disorders), degrading the quality of life and causing enormous economic costs. Today, the effectiveness of treatments represents an important and priority challenge for medicine. The presence of barriers, such as the blood-brain barrier (BBB) and blood-cerebrospinal fluid barrier are a major obstacle to delivering drugs to the brain (only small molecules can cross these barriers), reducing the efficacy of various therapies. Recently, it has been identified that membrane-bound vesicles of brain endothelial cells influence the formation of nanotubes involved in the construction of the BBB (Mentor and Fisher, 2021). In addition to crossing the BBB is necessary to target specific cell types such as neurons, astrocytes, oligodendrocytes, and microglia involved in specific brain diseases. Several DDSs, including nanoparticles, extracellular vesicles, and artificial vesicles have been studied for brain drug delivery (Dong 2018; Nuzzo et al., 2021). Vesicles derived from cells and brain tissue have been fabricated recently as DDS. In this review, we analyzed these new systems and their potential as DDS to contrast the brain disorders.
Cell-derived nanovesicles for brain disease:
To date, few studies have focused on the biofabrication of cells-derived nanovesicles to counteract brain diseases. Dong et al. (2019) proposed neutrophilmembrane-derived nanovesicles to deliver Resolvin D2 (molecule derived from docosahexaenoic acid) to protect mouse brain injury from ischemic stroke. The authors, inspired by the binding of neutrophils to the endothelium during stroke, have generated nanovesicles derived from differentiated HL-60 cells (like neutrophils) by nitrogen cavitation. Nanovesicles specifically bind to inflamed brain endothelium to mitigate neuroinflammation after reperfusion therapy of ischemic stroke (Dong et al., 2019). Nanovesicles derived from macrophage membrane loaded with nerve growth factor were generated by mixing the separated macrophage membrane with nerve growth factor solution by extrusion through the polycarbonate membrane (Xia et al., 2021). The results indicate that nanovesicles effectively delivered nerve growth factor to the spinal cord injury site to exert a neuroprotective (Xia et al., 2021). Wu and collaborators showed that nanovesicles derived from brain-derived endothelial cells, by serial extrusion, were good alternative nanocarrier to exosomes (Wu et al., 2021). In particular, they demonstrated that brain-derived endothelial cells and exosomes showed similar drug-loading capacity (Doxorubicin), BBB-crossing ability, glioblastoma targeting ability, and antitumor effects in various models, but the yield of brain-derived endothelial cells is substantially higher (500-fold) than exosomes (Figure 1A
; Wu et al., 2021).Biohybrid strategies were used for the development of nanosystems by combining synthetic nanoparticles and cellular membranes. Hybrid nanovesicles is an emerging field where the properties of natural vesicles such as tropism, biological barrier penetration, long circulation, low immunogenicity, and high biocompatibility are combined with the advantages of synthetic materials including control of the process of manufacturing, high production, engineering, functionalization, drug loading, and stability. Numerous studies on hybrid nanovesicles have been developed (Li et al., 2021), but very few are present in the scientific landscape for brain applications. In this contest, the cell membrane obtained by different cell types can be used to coat nanoparticles, enhancing the targeting capacity of these carriers. Different studies that use red blood cells or glioblastoma (GBM) membranes for GBM therapy were published (Mendanha et al., 2021). In addition, the extracted membrane can be modified to increase the capacity to cross BBB and to improve the drugs delivery in the tumor site. The manufacturing processes to coat the nanoparticles’ cores with the membrane used coextrusion and sonication, also encapsulating several hydrophobic and hydrophilic drugs (Mendanha et al., 2021).
In 2017, the first work targeting GBM used membranes from red blood cells (cell membranecoated nanoparticles) modified with the DCDX (peptide derived from candoxin) which has a binding affinity toward the nicotinic acetylcholine receptors expressed on the surface of the brain endothelial cells (Chai et al., 2017). The results showed that the nanocarrier carrying doxorubicin presented a superior therapeutic efficacy with reduced toxicity effects (Chai et al., 2017).
In a recent study, Gao et al. (2021) designed a miR155-containing nanogel with erythrocyte membrane-coating functionalized with M2pep and HA2 peptides. The membrane-coated nanoparticle was stable with prolonged circulation time. M2pep peptide modification endows the M2-microglia and macrophage targeting ability and HA2 peptide promotes fusion of membranes of erythrocyte and endosome. miR155 was successfully delivered to the tumor site and entered the cytoplasm of macrophages and microglia and shifted their pro-invasive M2 phenotype to anti-tumor M1 phenotype for GBM immunotherapy (Gao et al., 2021).
The mechanisms by which C-DNv is able to cross the BBB have yet to be fully elucidated. However, as several cell types are able to cross the BBB such as immune cells or cancer cells, C-DNv, originating from cell membranes, could naturally have some cellular membrane surface proteins that could mediate the BBB crossing.
Brain tissue-derived nanovesicles:
Recently, brain tissue has been used as a material for nanovesicles generation with potential biomedical applications. Synaptosomes are subcellular fractions isolated from synaptic terminals that can be prepared by homogenization and gradient centrifugation of brain tissue. Synaptosomes contain synaptic vesicles and mitochondria and are considered a relevant model system for studying human synaptic dysfunction in neurodegenerative diseases. The synaptosome has been proposed as a mitochondria delivery system that could improve the mitochondrial transfer and cellular uptake in neuronal cells (Figure 1B
; Picone et al., 2021b). The strategy to replace or supplement damaged mitochondria in the cells with mitochondrial dysfunction is called mitochondrial transplantation. Mitochondrial transplantation has been proposed as a potential therapy for several neurodegenerative diseases (Espino De la Fuente-Muñoz et al., 2020; Picone et al., 2022). Synaptosome-mediated mitochondrial transplantation could be applicable for the treatment of many brain diseases characterized by mitochondrial dysfunction such as Alzheimer‘s Disease, Parkinson‘s disease, etc.Nanovesicles produced from the myelin brain, as a new potential carrier with an enhanced tropism for the brain tissues (from brain to brain), have been biofabricated (Picone et al., 2021a). In specific, the myelin nanovesicles are produced with an easy, efficient, cost-effective, and reproducible production protocol. They have high stability, and cytocompatibility, are able to load the drugs and cross a BBB model. The ability of myelin nanovesicles to cross the BBB could be due to the presence of proteins (transferrin, apolipoprotein E, and glutathione) which are known to promote the crossing of nanosystems through the BBB. In addition, are able to target specific brain regions, such as white matter, and preferentially interacting with microglial cells (Figure 1B
; Picone et al., 2021a).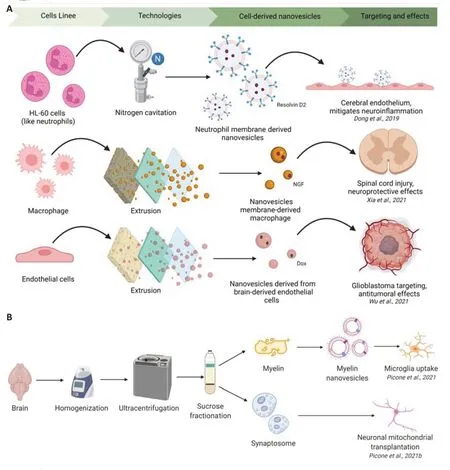
Figure 1 |Schematic representation of the procedure used for cell-derived nanovesicles fabrication and their brain potential applications (A) and brain tissue-derived nanovesicles fabrication and their potential applications (B). Created with BioRender.com.
Nanovesicles tools for brain diseases, potentiality and limits:
Articles on the biofabrication of nanostructures generated by cells or brain tissue represent great potential in the field of treatment of neuronal pathologies. The nanovesicles biofabricated could be used as brain drug delivery systems or could naturally transport molecules with therapeutic effects. Such systems could overcome the most important challenges related to the delivery of drugs/molecules to the brain. One of the main obstacles is the presence of BBB and the occurrence of side effects due to the dispersion of drugs that fail to enter the CNS. Furthermore, it is important to administer therapeutic agents to CNS-specific regions, without affecting other CNS areas to avoid further damage. Therefore, formulations able to bypass physiological barriers selectively and targeted to specific regions and cells (neurons, microglia, or astrocytes) are essential factors for the development of effective therapy for the treatment of brain disorders. Furthermore, further problems are related to the solubility, stability, and consequently bioavailability of drugs.Brain delivery systems can be an exciting and promising platform for overcoming the problems mentioned above. In this scenario, recently the cell or tissue derived-nanovesicles have shown great potential. In fact thanks to their nature and being similar to EVs they can present selective trophism, penetration of the biological barrier, long circulation, low immunogenicity, and high biocompatibility and being produced with physical means such as AVs they can be produced with high yields, engineered, functionalized and loaded with drugs of different nature (lipophilic and hydrophilic).
However, there is an urgent need to improve the extraction protocols, and the reproducibility and characterization of the processes. All this anticipates the need for intense research activity in the next years. It is foreseeable that these vesicles and their potential applications will undergo an increasing interest in the scientific community thanks to their potential which to date has not been fully explored.
Pasquale Picone, Domenico Nuzzo
Istituto per la Ricerca e l’Innovazione Biomedica, CNR; Dipartimento di Scienze e Tecnologie Biologiche Chimiche e Farmaceutiche, Università di Palermo, Palermo, Italy
Correspondence to:
Nuzzo, PhD, domenico.nuzzo@irib.cnr.it; Pasquale Picone, PhD, pasquale.picone@irib.cnr.it. https://orcid.org/0000-0002-4325-417X (Domenico Nuzzo)https://orcid.org/0000-0001-7127-2183 (Pasquale Picone)
Date of submission:
January 13, 2022Date of decision:
February 24, 2022Date of acceptance:
April 27, 2022Date of web publication:
June 2, 2022https://doi.org/10.4103/1673-5374.346473
How to cite this article:
Picone P, Nuzzo D (2023) Biofabrication of nanovesicles for brain diseases. Neural Regen Res 18(3):525-526.
Open access statement:
This is an open access journal, and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- Notice of Retraction
- Neuroprotective role of Noggin in spinal cord injury
- Combined cell-based therapy strategies for the treatment of Parkinson’s disease: focus on mesenchymal stromal cells
- Lights at night: does photobiomodulation improve sleep?
- β2-Microglobulin exacerbates neuroinflammation, brain damage, and cognitive impairment after stroke in rats
- The lymphatic drainage systems in the brain: a novel target for ischemic stroke?

