SARS-CoV-2 getting into the brain; neurological phenotype of COVID-19, and management by nano-biotechnology
Małgorzata Kujawska, Ebrahim Mostafavi, Ajeet Kaushik
Human coronavirus infection getting into the brain:
By February 2022, the severe acute respiratory syndrome coronavirus 2 (SARSCoV-2) infection, causing the coronavirus disease 2019 (COVID-19) outbreak, has infected around 415 million people, and caused ~5.8 million deaths worldwide (WHO, https://covid19.who.int/). As SARS-CoV-2 replicates during the infection, it undergoes genetic mutation to generate variants with varying characteristics and mutation frequencies. The emerging, over time, new variants that differ with transmissibility, immunity, and infection severity pose continuous challenges to established COVID-19 management strategies and regulations. Several SARS-CoV-2 variants such as Omicron (B.1.1.529), Delta (B.1.617.2), UK (B.1.17), South Africa (B.1.351), Brazil (P.1), and New York B.1.525‒B.1.526 were detected worldwide and accelerated severity of COVID-19 pandemic (Figure 1A
; McQuaid et al., 2021).Despite respiratory disorders and several organ damages, clinical evidence is also evident for neurological and neuropsychiatric manifestations associated with the pathogenesis of SARS-CoV-2 infection, including hyposmia, headache, dizziness, ataxia, cerebrovascular injury, hypogeusia, nausea, encephalitis, encephalopathy, vomiting, delirium, psychosis, ischemic stroke, neurocognitive syndrome, acute respiratory distress syndrome, and affective disorders. These symptoms are significant in one-third of the COVID-19 infected population causing severe diseases in both acute (less prominent) and chronic (dominating) phases, as the possibilities of SARS-CoV-2 getting into the brain have been demonstrated. However, the long-term effects of COVID-19, especially neurologic complications, are only beginning to be evaluated, and robust data are lacking. In the present situation, the significance of COVID-19 infection-related neurological complications created new NeuroCovid associated challenges as specific diagnostic, management, and therapeutic strategies are needed to be implemented (Cárdenas et al., 2021). Thus, the plausibility that SARS-CoV-2 can neuroinvade the central nervous system (CNS) and how it affects brain cell functions has raised the demand of detail studies. The careful and critical analysis of COVID-19 patients confirmed SARS-CoV-2 RNA and proteins presence in their cerebrospinal fluid and brain. Although viral loads were relatively low, neurological sequelae in longer-time scenarios have been suggested (Cárdenas et al., 2021; Krasemann et al., 2022). The SARS-CoV-2 exhibits tissue tropism, including neurotropism, as demonstrated recently. In general, spike protein (SP) of SARSCoV-2 interacts with host angiotensin-converting enzyme-2 (ACE2) receptor and the receptorbinding domain to facilitate virus entry and initiate the viral replication process. Additionally, virus entry is also facilitated by other proteins such as integrins, neuropilin-1, and the transmembrane proteases serine 2 and serine 4 (TMPRSS2 and TMPRSS4, respectively) (Krasemann et al., 2022; Mostafavi et al., 2022). The broad distribution within the CNS of ACE2 receptors, which is the main mechanism of SARS-CoV-2 entry into host cells, appears to be critical for the neurotropism of SARS-CoV-2SP again playing an important role (Pacheco-Herrero et al., 2021; Krasemann et al., 2022). Some SARS-CoV-2 mutations appeared to make SP more active, altering the protein structure to increase affinity with host cells. However, how variants affect SARS-CoV-2 neuroinvasion is not clear yet. The TMPRSS2 and neuropilin-1 are also expressed in the central/peripheral nervous systems and the brain, respectively (Krasemann et al., 2022). Four possible pathways for the entry of SARS-CoV-2 to the CNS have been suggested: 1) the hematopoietic pathway in which SARS-CoV-2-infected immune cells cross the blood-brain barrier (BBB) via intercellular adhesion molecule 1) mediated transport with subsequent rupture of the BBB, 2) the virus entry into cerebrospinal fluid via the choroid plexus, 3) transsynaptic viral spreading from trigeminal, gustatory, olfactory, and vagal nerves and 4) through the entry to circumventricular organs which highly express ACE2 and lack the BBB. As the expression of ACE2 receptor and neuropilin-1 has also been detected in retinal cells of the eye and/or visual system, they are also suggested as an entry point for SARSCoV-2 invasion (Pacheco-Herrero et al., 2021).
COVID-19 infection is affecting the CNS system:
Besides receptor-mediated entry of SARS-CoV-2 into the CNS, its tropism towards endothelial cells of the CNS triggers the BBB disruption, which also allows the infiltration of virus-carrying leukocytes and monocytes to multiple regions of the brain. In the CNS, SARS-CoV-2 infects neurons, astrocytes, oligodendrocytes, and glial cells through ACE2 and TMPRSS2, triggering a neuroinflammatory response with astrogliosis and microglial activation (McQuaid et al., 2021; Pacheco-Herrero et al., 2021). Simultaneously, systemic infection leads to an overactive and dysregulated immune response known as the cytokine storm. In the situation of COVID-19 infection, the level of plasma inflammatory mediators, including interleukins, chemokines, cytokines, and antibodies is increased, triggering apoptosis of epithelial cells, and leading to vascular leakage, also contributing to the increased BBB permeability. The neuroinflammatory response is known to affect brain function via altering neurotransmitter release, activating cell lysis, inducing apoptosis, and disrupting neuronal transcriptional pathways contributing to neurotoxicity, neuroinflammation, and neurodegeneration. Moreover, recent research has demonstrated that the immune response along with oxidative stress in connection with SARS-CoV-2 replication may increase the beta-amyloid neurotoxicity, a widely believed pathomechanism driving neurodegeneration in Alzheimer’s disease (Chiricosta et al., 2021). The involvement of SARS-CoV-2 infection in proteopathic seed spreading via facilitating intercellular cargo transfer has been demonstrated (Liu et al., 2021) for exploring the process of α-synuclein aggregation (Semerdzhiev et al., 2022) and increasing the hyperphosphorylation of tau protein (Ramani et al., 2020), promoting neuronal degeneration in Parkinson’s disease and Huntington’s disease, respectively.Indeed, patients who suffer from acute respiratory symptoms are found at potentially high risks for long-term residual neuropsychiatric and neurocognitive disorders, including depression, obsessive-compulsive disorder, psychosis, Parkinson’s disease, and Alzheimer’s disease (Figure 1B
). Considering expected pathophysiological mechanisms, the clinical neurological indicators of NeuroCovid are explained as 1) starting from the damage in epithelial cells of the nose and mouth with related hyposmia and hypogeusia, 2) through the occurrence of blood clots in the brain and immune-mediated damages cranial and peripheral nerves with symptoms of fatigue, hemiplegia, aphasia, and ataxia to seizures, encephalopathy, and 3) death due to the cytokine storm damages (Fotuhi et al., 2020).Nano-biotechnology to manage brain function during and post COVID-19 infection:
Largescale and frequent testing is a key step to track COVID-19 infection, precaution, and sensitization to control SARS-CoV-2 transmission and combinational therapies have emerged as the best way to manage severe and lethal forms of COVID-19 outbreak, including its neurological phenotype. As of now, molecular polymerase chain reaction-based SARS-CoV-2 detection (1‒10 viral RNA copies) is still a standard analytical tool to perform selective COVID-19 diagnostics. However, nucleic acid-based diagnostics are time-consuming and challenging logistically. It requires sophisticated laboratories and skilled personnel, limiting COVID-19 diagnostics underprovision and under-performance as our reliance on tests with long turnaround times causes delays in patients receiving test results. In this context, developing sensitive and selective biosensors for rapid detection of SARS-CoV-2 at a low scale in crude human fluids at point-of-care (POC) testing has been recommended as the best alternative to manage infection. Advancements in functional nanostructures enable the development of sensitive chips and microfluidic systems for developing POC analytical tools for detecting SARS-CoV-2 at the picomolar level in real samples (Kaushik et al., 2020; Mostafavi et al., 2022). For example, a miniaturized plasmonic immunosensor using functionalized gold nanoparticles conjugated with a monoclonal antibody specific to SP is developed for the detection of SARS-CoV-2 SP femtomole level (Ahmadivand et al., 2021). These POC systems can be very efficient but require extended confirmative testing based on real samples to claim validation and projection for clinical use along with POC diagnostics. Presently, health experts suggest the integration of wireless technology with POC to explore telemedicine as an alternative to manage COVID-19 timely. In this direction, the internet of medical things (IoMT) is emerging as good support to COVID-19 diagnostics tools for generating, sharing, and analyzing bioinformatics needed to monitor and manage the pandemic. Moreover, an optimized IoMT-assisted POC testing can shorten time to treatment decision-making as well as reduce clinic crowding and virus transmission as a result as neuroCOVID-19 management requires a careful short-term and long-term neurological symptoms analysis for therapy optimization. Therefore, IoMT-based COVID-19 diagnostics at POC can spot subtle signs and detect mental illnesses with high accuracy and precision (Figure 1C
; Jain et al., 2021; Mostafavi et al., 2022).Due to emerging new SARS-CoV-2 variant of concerns, COVID-19 is still a challenge to manage as the new variants have different but serious health consequences. This situation raises the demand to re-evaluate the performance of established COVID-19 management strategies, mainly treatment approaches. Stimuli-response nanomaterials have been useful to trap (virus-like particles) and eradicate SARS-CoV-2 (nanomedicine approach for targeted drug delivery) and certainly will be capable of managing NeuroCOVID. In this direction, nanomedicines will certainly be developed to protect against premature degradation of antigens, increased cell membrane, including the BBB, permeability, sustained release, improved drug stability, on-demand controlled drug release, and enhanced immunogenicity; therefore, they can act as carriers and adjuvants. Achieving therapeutic concentrations in target tissues and adjuvant strategies to improve efficacy are significant for lowering the dosing of treatments and vaccines and, therefore, widening their therapeutic and safety window (Tiwari et al., 2021). Based on patient medical profiling and current biomonitoring, artificial intelligence-supported nano-biotechnology can be moreover applied for personalized prevention and treatment, contributing to a population-based cure (Figure 1C
). Additionally, the formulation of pharmacologically relevant therapeutic cargos designed using multiple drugs is in demand not only to eradicate SARS-CoV-2 but also for repairing affected or damaged neurons along with other CNS cells (Tiwari et al., 2021, 2022; Mostafavi et al., 2022). Ongoing efforts also involve development strategies modulating SARS-CoV-2 immunity. Vaccines capable of generating mass immunization still stand priority for controlling the pandemic. Their efficacy related to long-term prevention and stopping transmission in broad populations requires tackling challenges associated with variability within and across individuals, including geographic and age-related differences as well as the evolutionary trajectory of SARS-CoV-2 (Machhi et al., 2021).Taken together, during the COVID-19 pandemic, SARS-CoV-2 infection-related neurological phenotypes were increasing continuously and declared as a serious concern. Such neuroCOVID-19 caused during pre-/post-COVID-19 infection implies the appearance of both short-term and long-term neurological sequelae, including neurodegenerative disorders. Management of this neurological phenotype of COVID-19 requires applying appropriate approaches, especially addressing the BBB-associated challenges, both in diagnostics and treatment as well as controlling the pandemic through herd immunity development. High-performance nano-systems offer sophisticated protection, detection, and treatment of SARS-CoV-2 infection, including neurological phenotypes, both at individual and population scales, which seem be a promising strategy for managing COVID-19 in a personalized manner (Figure 1D
).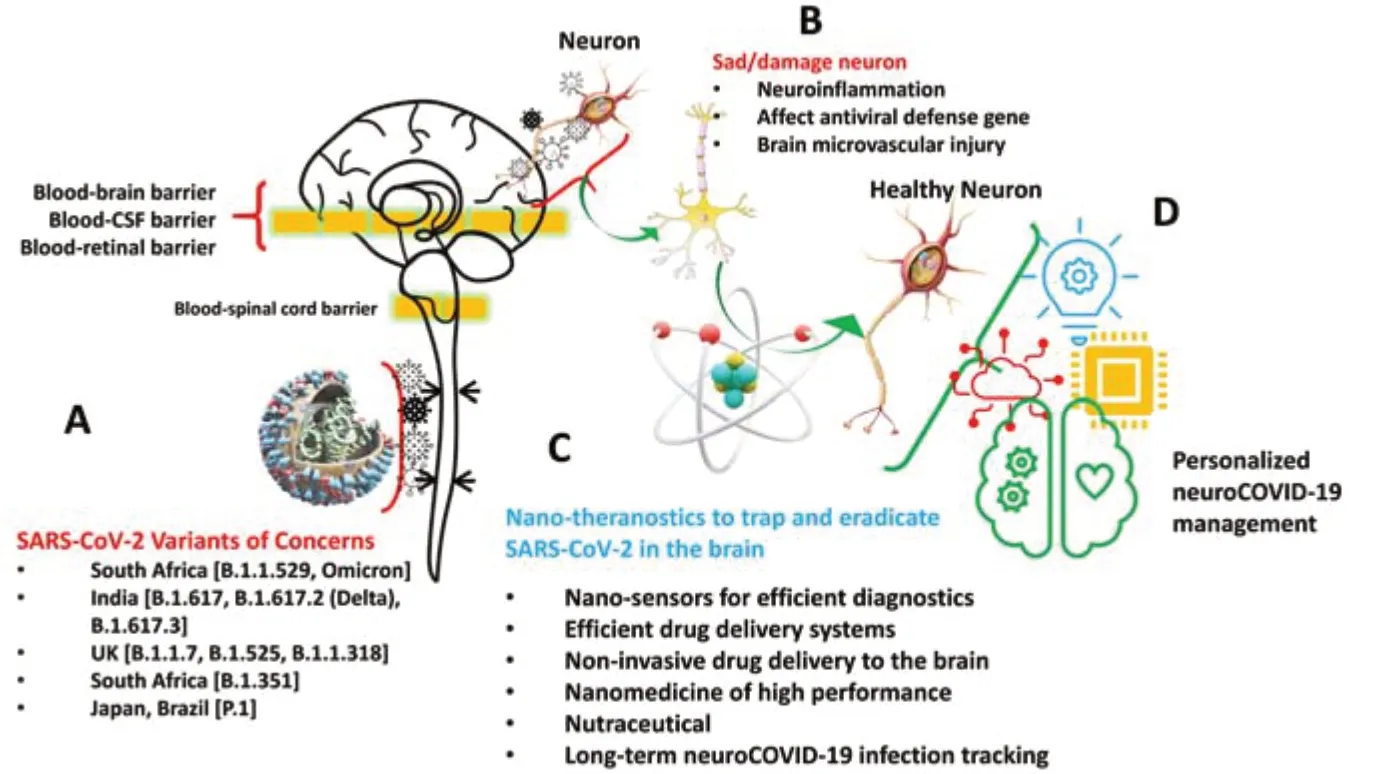
Figure 1 |SARS-CoV-2 getting into the brain. (A) Exploring the possibilities of mutated SARS-CoV-2 getting into the brain via crossing blood-brain, blood-cerebrospinal fluid, and blood-retinal barrier. (B) The interaction of SARS-CoV-2 with the neuronal system can affect brain function to cause cells, tissue, or both damage via causing neuroinflammation, affecting the defense capability of genes, and injuring brain muscle. (C) Proposing nano-theranostics involve efficient diagnostics tools, personalized nanomedicine, natural therapeutics agents, combinational long-term therapies needed to trap and eradicate SARS-CoV-2. (D) An optimized combination of nano-theranostics approaches can manage neuroCOVID-19 in personalized management. COVID-19: Coronavirus disease 2019; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2.
Authors acknowledge respective affiliated institutions for providing facilities and support.
Małgorzata Kujawska, Ebrahim Mostafavi, Ajeet Kaushik
Department of Toxicology, Poznan University of Medical Sciences, Poznań, Poland (Kujawska M)Stanford Cardiovascular Institute/Department of Medicine, Stanford University School of Medicine, Stanford, CA, USA (Mostafavi E)NanoBioTech Laboratory, Health Systems Engineering, Department of Natural Sciences, Florida Polytechnic University, Lakeland, FL, USA (Kaushik A)
Correspondence to:
Małgorzata Kujawska, PhD, kujawska@ump.edu.pl; Ebrahim Mostafavi, PhD, ebimsv@stanford.edu; Ajeet Kaushik, PhD, akaushik@floridapoly.edu.https://orcid.org/0000-0002-5306-9904 (Małgorzata Kujawska)
https://orcid.org/0000-0003-3958-5002 (Ebrahim Mostafavi)
https://orcid.org/0000-0003-4206-1541 (Ajeet Kaushik)
Date of submission:
February 17, 2022Date of decision:
March 11, 2022Date of acceptance:
April 20, 2022Date of web publication:
June 2, 2022https://doi.org/10.4103/1673-5374.346486
How to cite this article:
Kujawska M, Mostafavi E, Kaushik A (2023) SARS-CoV-2 getting into the brain; neurological phenotype of COVID-19, and management by nano-biotechnology. Neural Regen Res 18(3):519-520.
Open access statement:
This is an open access journal, and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:
Dhavalkumar Patel, Texas Tech University Health Sciences Center Jerry H Hodge School of Pharmacy, USA.
- 中国神经再生研究(英文版)的其它文章
- Notice of Retraction
- Neuroprotective role of Noggin in spinal cord injury
- Combined cell-based therapy strategies for the treatment of Parkinson’s disease: focus on mesenchymal stromal cells
- Lights at night: does photobiomodulation improve sleep?
- β2-Microglobulin exacerbates neuroinflammation, brain damage, and cognitive impairment after stroke in rats
- The lymphatic drainage systems in the brain: a novel target for ischemic stroke?

