Melatonin: a multitasking indoleamine to modulate hippocampal neurogenesis
Alejandro Romero, José Ángel Morales-García , Eva Ramos,
Abstract Neurodegeneration affects a large number of cell types including neurons, astrocytes or oligodendrocytes, and neural stem cells. Neural stem cells can generate new neuronal populations through proliferation, migration, and differentiation. This neurogenic potential may be a relevant factor to fight neurodegeneration and aging. In the last years, we can find growing evidence suggesting that melatonin may be a potential modulator of adult hippocampal neurogenesis. The lack of therapeutic strategies targeting neurogenesis led researchers to explore new molecules. Numerous preclinical studies with melatonin observed how melatonin can modulate and enhance molecular and signaling pathways involved in neurogenesis. We made a special focus on the connection between these modulation mechanisms and their implication in neurodegeneration, to summarize the current knowledge and highlight the therapeutic potential of melatonin.
Key Words: adult hippocampal neurogenesis; aging; melatonin; neural stem cell; neurodegeneration; neuroprotection; signaling pathway; therapeutic strategy

Introduction 503 Search Strategy and Selection Criteria 503 Melatonin 503 Neurogenesis-Related Molecular Mechanisms and Signaling Pathways Modulated by Melatonin 504 Neuroprotective Role of Melatonin in Neurodegenerative Disorders and Aging 504 Conclusions 505
Introduction
Neurogenesis is described as the process by which new neurons are formed. During embryonic development and early postnatal weeks, once the nervous tissue has formed and the main neural networks have been developed and established, neurogenesis begins to decline. At this moment, neurogenesis is restricted to two specific areas of the adult brain: the lateral wall of the lateral ventricle and the subgranular zone of the dentate gyrus of the hippocampus. Adult hippocampal neurogenesis describes the generation of new neurons throughout life from adult neural stem cells (NSCs) and the integration of these new neurons into existing neural circuits of the hippocampal formation. Accumulating evidence suggests that adult hippocampal neurogenesis has been implicated in cognitive processes under normal physiological conditions such as learning, memory, mood regulation, and cognitive flexibility. Therefore, adult hippocampal neurogenesis is believed to benefit cognitive functions that enhance survival; indeed, the incorporation of adult-born neurons into the hippocampal circuitry is a remarkable example of plasticity. Adult NSCs are normally in a reversible cell cycle arrest with a low metabolic rate (quiescent state) and high sensitivity to their local signaling environment, and they may be activated by different physiological stimuli such as extrinsic factors (morphogens, growth factors, cytokines, neurotransmitters, and hormones), intracellular factors (transcription factors and epigenetic modulators), and environmental factors like diet or exercise (Surget and Belzung, 2022).
Aging, neurodegenerative disorders, and/or stroke/ischemia have a deleterious effect on the generation of new neurons in brain. In Alzheimer’s and Parkinson’s diseases (AD and PD), the number and maturation of neurons progressively decline as the disease advances. Indeed, a neurogenesis reduction may lead to disease progression, suggesting that therapies focused on enhancing this decline may delay AD/PD onset or reduce symptoms (Moreno-Jimenez et al., 2019; Seki et al., 2019). Adult quiescent NSCs are not activated normally during the development of these pathologies to replace dying neurons. For this reason, a vast amount of research has been performed to understand the molecular mechanisms involved in neurogenesis and to try to stimulate the formation of new neurons in the adult brain. In this context, the endogenous environment is determining neuroregeneration fate and regeneration processes. The intrinsic neurogenic potential and its possible regulation through therapeutic measures present a promising therapeutic approach since many factors, such as neurotrophic support, or endogenous molecules like melatonin, can modulate this process. Given the increasing incidence of neurodegenerative diseases, the promotion of neurogenesis for neural repair is a demanding task. This possible therapeutic approach could result in getting the endogenous factors/molecules to stimulate the adult NSCs and guide the neural precursors to the location of injury to finally replace lost neurons. Due to the technical limitations of human studies, most of our understanding of the functional role of adult hippocampal neurogenesis relies on retrospective analyses using post-mortem tissues andin vitro
animal models.To better understand the physiological roles played by neurogenesis, which functions as a lifelong reservoir of plasticity in the brain, we must unravel the complex interrelationship between the elements that make up neurogenic niches and the dynamics of adult neurogenesis. Currently, some molecules with promising profiles are being studied as potential therapeutic agents to enhance neurogenesis.
Search Strategy and Selection Criteria
The review was performed, including articles from Medline and Web of Science electronic databases updated until January 2022. The terms used for the database search were neurodegeneration AND/OR melatonin AND/OR neurogenesis.
Melatonin
Melatonin (N-acetyl-5-methoxytryptamine) is an indoleamine secreted mainly from the pineal gland, and it is also synthesized in other organs, such as the brain, immune system (bone marrow and lymphoid cells), lung (resident macrophages), enterochromaffin cells of gastrointestinal tract, retina (Photoreceptors), skin cells, and endothelial cells, among others (Acuna-Castroviejo et al., 2014). It has been suggested as a promising modulator of adult hippocampal neurogenesis, there is increasing evidence about the regulation of melatonin at several levels. Therefore, this perspective focuses on the evidence surrounding melatonin in adult hippocampal neurogenesis and its promising therapeutic profile when administered exogenously.
To date, several preclinical studies point melatonin as a potential tool to modulate neurogenesis (Table 1
). Thein vivo
evidence describes the ability of exogenously administrated melatonin to increase neuronal progenitor cells’ survival in the dentate gyrus, and subgranular zone likely through melatonin receptors type-1 and type-2 -dependent mechanisms (Ramirez-Rodriguez et al., 2009; Sotthibundhu et al., 2010). In this sense, it has been demonstrated that melatonin affords neurogenesis and cell proliferation through a melatonin receptor type-2 receptor-dependent mechanism after ischemic stroke (Chern et al., 2012). This would be a key factor in aged brains to avoid neuronal impairment, pinealectomized mice exhibited neurogenesis disruption alleviated by exogenous administration of melatonin (Motta-Teixeira et al., 2018). Notwithstanding, this activity seems to be effective in short treatments, while a 12-month treatment did not afford neurogenesis (Ramirez-Rodriguez et al., 2012). As it is known, melatonin membrane receptors expression decrease during aging (Sanchez-Hidalgo et al., 2009) which may have a direct impact on the neurogenic regulation of this indoleamine (Mihardja et al., 2020). Therefore, old or senescent rodents show changes in the brain that seem to be involved in this lack of effect after melatonin treatment (Hardeland, 2018).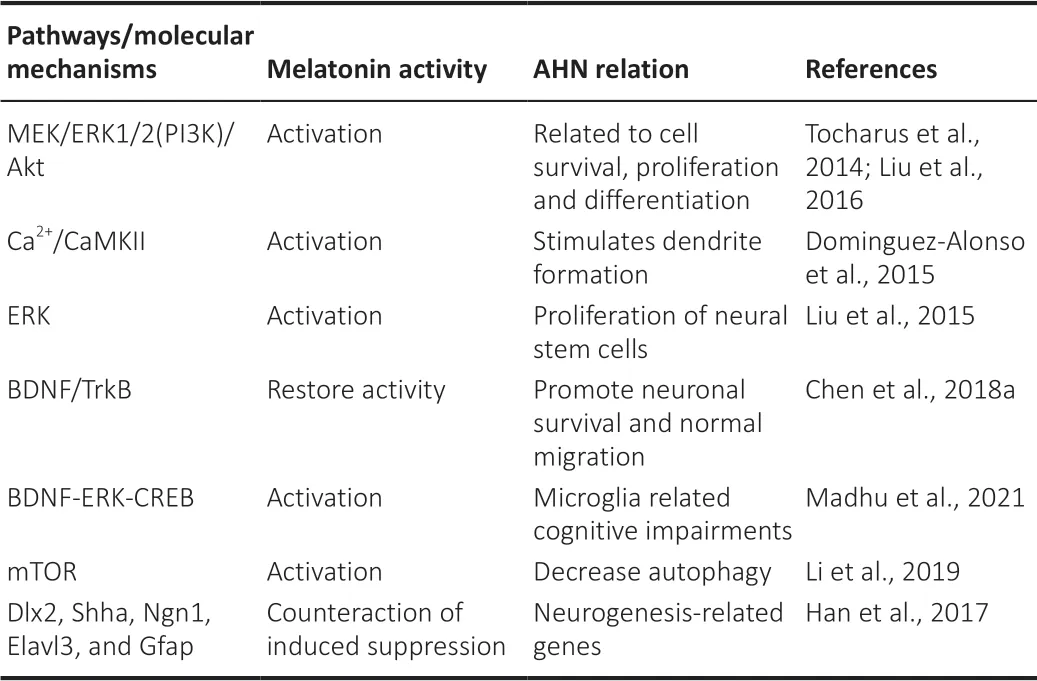
Table 1 |Melatonin activity modulating several pathways and molecular mechanisms related to AHN in animal models
One valuable feature of melatonin is its amphiphilic nature, this allows the molecule to cross morphophysiological barriers reaching neurogenesis restricted areas in the hippocampus. This would be the required first step of a molecule to reach these specific brain regions promoting neurogenesis or survival of neurons. The observed relationin vivo
between melatonin and neurogenesis promotion encourages deeper research to discover underlying molecular mechanisms.Neurogenesis-Related Molecular Mechanisms and Signaling Pathways Modulated by Melatonin
In this regard, several studies reveal that these molecular mechanisms are deeply complex and involve multiple signaling pathways that make melatonin able to achieve newly generated NSCs survival promotion and counteract hippocampal memory impairment. NSCs differentiation and proliferation processes seem to be promoted by melatonin through receptor-dependent mechanisms by mitogen-activated protein kinase/extracellular signalregulated kinase 1/2 and phosphatidyl inositide 3 kinase/Akt signaling upregulation (Figure 1
; Tocharus et al., 2014; Liu et al., 2016). In the last decade, several studies with melatonin reveal its unique ability to enhance hippocampal NSCs differentiation and dendritogenesis along its three stages; dendrite formation, enlargement, and complexity. These activities are developed by both receptor and non-receptor mediated mechanisms. The reports also suggest melatonin’s neurogenic activity as a mitochondriatargeted agent (Dominguez-Alonso et al., 2015; Ghareghani et al., 2017). The mechanism by which melatonin stimulates dendrite formation seems to involve the activation of Ca/Calmodulin-dependent kinase II and the translocation of calmodulin to stimulate dendrite formation (Figure 1
; Dominguez-Alonso et al., 2015).The extracellular regulated protein kinases pathway regulation has also been proposed as one of the molecular mechanisms by which melatonin exerts its neurogenic properties stimulating NSCs proliferation in mice (Liu et al., 2015). There are other molecular mechanisms involved in neurogenesis that are also regulated by melatonin, i.e. specific tropomycin-receptor kinase receptors, that activate intracellular signaling of brain-derived neurotrophic factors and promote neuronal survival and normal migration, melatonin administration restores this way induced cognitive impairment (Figure 1
; Chen et al., 2018a). In addition, melatonin has been recently shown to enhance neurogenesis in a model of chronic Gulf War Illness through the brain-derived neurotrophic factor-extracellular regulated protein kinases-cyclic AMP response elementbinding protein signaling pathway mitigating cognitive and mood impairments (Madhu et al., 2021).
Figure 1 |Neurogenic related pathways modulation after melatonin administration.The figure represents the possible neurogenic pathways that may be upregulated (blue: MEK/ERK1/2: mitogen-activated protein kinase/extracellular signal-regulated kinase 1/2; TrK: tropomycin-receptor kinase; BDNF: brain-derived neurotrophic factor; ERK: extracellular regulated; Ca2+/CaMKII: calcium/calmodulin-dependent kinase II; PI3K/Akt: phosphatidyl inositide 3 kinase/protein kinase B; Bcl-2: B-cell lymphoma 2) or down regulated (green: Bax: bcl-2-like protein 4; GSK-3β: glycogen synthase kinase 3 beta; Fas; capase 9, 8, 3) in human after the administration of melatonin, data is depicted according to the preclinical evidence reviewed in the manuscript.
Nonetheless, there is another neuroprotective activity of melatonin in fetal neuropathy recently discovered, it can be also related to NSCs differentiation. These findings suggested that melatonin enhanced NSCs proliferation and self-renewal in embryos of treated mice decreasing autophagy and activating the mechanistic target of the rapamycin signaling pathway (Li et al., 2019). This report is especially relevant in the context of fetal neurodevelopment, in which there are not many therapeutic resources available.
The well-known antioxidant properties of melatonin may additionally assist neurogenesis. In a neurotoxic model of zebrafish exposed to fenvalerate, melatonin reduced oxidative stress and apoptosis diminishing the activity of glutathione peroxidase, Cu/Zn superoxide dismutase, catalase, and malondialdehyde levels. Melatonin attenuated the induced pro-apoptotic gene up-regulation (Bax, Fas, caspase 8, caspase 9, and caspase 3) and upregulates the anti-apoptotic gene Bcl-2. These activities together with the counteraction in the suppression of neurogenesis-related genes (Dlx2, Shha, Ngn1, Elavl3, and Gfap) induced by fenvalerate, restored the abnormalities in zebrafish after fenvalerate exposure (Han et al., 2017).
Neuroprotective Role of Melatonin in Neurodegenerative Disorders and Aging
If we focus on the mechanisms of action described for melatonin related to neurogenesis, we can find that there is a complex regulation of signaling cascades able to regulate self-renewal and differentiation of NSCs, together with an efficient regulation of synaptic plasticity and memory consolidation. Relative to this, deregulation in Wnt/β-catenin signaling pathway is associated with neurodegenerative diseases (Inestrosa and Varela-Nallar, 2014; Singh et al., 2018). It has been shown bothin vivo
andin vitro
that melatonin is capable of increasing the expression of β-catenin in neuronal cells (Jeong et al., 2014), and furthermore activating Wnt/β-catenin pathway downregulating Caspase-3 and Bax, and activating Bcl-2, preventing this way induced apoptotic neuronal cell death (Shen et al., 2017). Interestingly, this ability of melatonin in modulating the Wnt/β-catenin signaling pathway would aid to maintain cellular homeostasis and counteract impaired adult neurogenesis related to neurodegenerative diseases (Figure 1
). Additionally, glycogen synthase kinase 3 beta (GSK-3β) inhibition has been recently related to a Wnt/β-catenin signaling pathway activation, in a PD rat model, improving in this way neurogenesis and gliogenesis enhancing NSCs proliferation, selfrenewal, and cell survival (Singh et al., 2018). Melatonin may modulate GSK-3β protecting neuronal cells against neurodegeneration processes as observed in an AD mice model expressing an amyloid precursor protein human variant (tg2576 mice). These mice develop amyloid plaques and dystrophic neurites, and after treatment with melatonin, the researchers observed a reversion of memory deficits targeting GSK-3β (Peng et al., 2013). This capacity of melatonin in controlling GSK-3 dysregulation takes on special relevance as it is a crucial enzyme that regulates numerous upstream effectors in neurodegenerative diseases and targets amyloid precursor protein contributing to amyloid plaques formation through PI3K/Akt/GSK-3β pathway leading to a learning and memory impairment (Duda et al., 2018).In addition to aging, other factors that may cause neurogenesis impairment, such as the adverse effects of certain drugs. In this context, melatonin has demonstrated in rats its ability to counteract induced neurotoxic effects of valproic acid restoring memory hippocampal neurogenesis impairment, the underlying molecular mechanisms appear to be due to its antioxidant activity (Aranarochana et al., 2021). Other drugs such as methotrexate, used as a chemotherapeutic agent, and scopolamine, an inhibitor of muscarinic cholinergic receptors, are also known to display effects in memory impairment and reduction. In some other studiesin vivo
, melatonin was also able to protect against the reduction of memory or neurogenesis induced by drugs (Chen et al., 2018b; Aranarochana et al., 2019; Sirichoat et al., 2019). Indeed, long-term consumption of psychostimulant drugs such as methamphetamine develops brain function impairment inhibiting cell proliferation and neurogenesis in the hippocampus. Administration of melatonin in mice counteracted neurogenesis and learning and memory-induced dysfunctions in the hippocampus (Singhakumar et al., 2015).In the neuronal cellular environment, the phosphoprotein phosphatases alter phosphorylation/dephosphorylation rate early in neurodegeneration, if this can be counteracted and the homeostasis can be preserved (Arribas et al., 2018). In the cortex and hippocampus of AD patients, there is a substantial drop (about 50%) in Tau dephosphorylation (Gong and Iqbal, 2008). Specifically, phosphoprotein phosphatase 2A is the most susceptible enzyme to decrease, which is a serious problem because it is involved in the cell cycle and regulates numerous signaling pathways. After β-amyloid peptide (1‒42)-induction in the mouse model, melatonin administration improves the neuronal survival and cognitive function increasing phosphoprotein phosphatase 2A expression and attenuating the GSK3β levels (Gong et al., 2018). Recently, it has been hypothesized that there is a direct binding between melatonin and phosphoprotein phosphatase 2A, explaining the observed effects of melatonin modulating phosphorylation/dephosphorylation processes (Arribas et al., 2018).
Conclusions
Given these protective activities, the therapeutic use of melatonin as an adjuvant with conventional drugs can counteract the undesirable effects of these drugs, and enhance the efficacy of the treatments.
The non-toxic nature of melatonin and its wide safety profile are essential when considering this molecule as a promising therapeutic agent. Administration of high-dose melatonin did not increase the number of serious adverse events, nonetheless, other adverse events such as dizziness or drowsiness appear to be increased after melatonin treatment (Menczel Schrire et al., 2021). This safety profile brings up the possibility of safely extrapolating the effective dosesin vivo
to future clinical trials, always considering the therapeutic risk/benefit ratio of melatonin.Therefore, melatonin deserves deeper research and efforts, due to its safety and above described promising ability (Figure 1
) as a potential therapeutic agent promoting neurogenesis and neuron survival in neurodegenerative diseases and aged brain.Author contributions:
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication. AR conceived the idea and he has made substantial contributions to conception, design, supervision. ER and JAMG wrote-original draft preparation and critically revised the manuscript. All co-authors read and approved the final version of the manuscript.
Conflicts of interest:
The authors declare no conflicts of interest.
Open access statement:
This is an open access journal, and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- Notice of Retraction
- Neuroprotective role of Noggin in spinal cord injury
- Combined cell-based therapy strategies for the treatment of Parkinson’s disease: focus on mesenchymal stromal cells
- Lights at night: does photobiomodulation improve sleep?
- β2-Microglobulin exacerbates neuroinflammation, brain damage, and cognitive impairment after stroke in rats
- The lymphatic drainage systems in the brain: a novel target for ischemic stroke?

