Effects of COVID-19 on the liver: The experience of a single center
Valentina Liakina, Ieva Stundiene, Gabriele Milaknyte,Ramune Bytautiene, Rosita Reivytyte, Ron a Puronaite, Gintare Urbanoviciute,Edita Kazenaite
Abstract
Key Words: COVID-19; SARS-CoV-2; Liver enzymes; Complications; Underlying disease; Disease severity
lNTRODUCTlON
In addition to the most common symptoms of coronavirus disease 2019 (COVID-19) - such as fever,dyspnea, sore throat, dry cough, headache, fatigue, restlessness, myalgia, anosmia, dysgeusia, and chest pain with ground-glass opacities seen on radiological investigations[1] - approximately half of patients suffer from gastrointestinal symptoms such as a lack of appetite, nausea, and vomiting[2]. In some cases, gastrointestinal symptoms may precede respiratory symptoms or even occur as the sole symptom of COVID-19[3]. A wealth of evidence suggesting that elevated liver enzymes are also a common finding in COVID-19 pneumonia has already been published[4]. Depending on the population studied,elevated levels of liver enzymes- alanine aminotransferase (ALT) and aspartate aminotransferase (AST)- in the blood have been detected in the range of 14%-76%[5,6]. In patients with severe COVID-19,gamma-glutamyl transferase (GGT) and hypoalbuminemia have also been documented[7]. Although liver injury is often transient and is usually normalized without special treatment in mild cases of disease[8], in severe and critical cases it can be the first sign of life-threatening upcoming events such as acute liver failure[9].
However, the exact triggers of liver damage, how it affects patients, and whether it could predict the course and outcomes of COVID-19 itself remain unclear. To address this issue, this study examines liver enzyme abnormalities in patients hospitalized with COVID-19.
MATERlALS AND METHODS
Study design and participants
This is a retrospective cohort study of patients hospitalized at Vilnius University Hospital’s Santaros Clinics during the 2020-2021 severe acute respiratory disease coronavirus 2 (SARS-CoV-2) viral pneumonia pandemic. The inclusion criteria for patients were as follows: documented SARS-CoV-2 infection, diagnosed according to NIH guidelines[10] based on manifestations of clinical pneumonia;positive real-time reverse transcription SARS-CoV-2 polymerase chain reaction (RT-PCR) test from nasopharynx swab specimens (MagMAX™ Viral/Pathogen II Nucleic Acid Isolation Kit and TaqPath COVID-19 CE-IVD kit, Applied Biosystems); radiologically confirmed viral pneumonia; and age over 18 years.
Thus, exclusion criteria were: Age ≤ 18 years; patients with incomplete medical records, and negative SARS-CoV-2 test from nasopharyngeal swab specimen.
The depersonalized data of 684 patients were analyzed. Patients were assigned to two groups according to the results of liver tests: those with elevated liver enzymes (603 patients), where at least one of four liver enzymes were elevated (ALT ≥ 40, AST ≥ 40, GGT ≥ 36, or alkaline phosphatase (ALP) ≥150; following the norm of hospital laboratory tests) at any point of hospitalization from admission to discharge; or the control group (81 patients), with all four liver enzymes within normal range during hospitalization (Table 1). Depending on the severity of SARS-CoV-2 pneumonia - which was evaluated by radiological observation of lung damage (lung infiltration, pleura infiltration, ground glass opacities), level of respiratory failure (SpO2, respiratory rate), and the overall clinical picture of the case- patients were assigned to the groups of moderate, severe, or critical COVID-19 pneumonia following the NIH COVID-19 disease guide (2022)[11].
Data collection
Depersonalized electronic medical records - including symptoms and clinical characteristics of COVID-19, laboratory and instrumental tests, therapeutic interventions, and outcome data - were collected for each patient. Demographic data included only age and gender. Underlying diseases were also recorded for all patients.
To confirm the diagnosis and evaluate the severity of COVID-19, the following tests were performed for all patients upon admission, and were repeated during treatment as required: throat roentgenogram;SARS-CoV-2 RT-PCR of nasopharyngeal swab; routine hematologic (full blood cell formula) and biochemical blood tests (troponin I, glucose, creatinine, blood urea nitrogen test, ferritin, procalcitonin,lactate, eGFR (CKD-EPI), ALT, AST, ALP, GGT, lactate dehydrogenase (LDH), bilirubin, C-reactive protein (CRP), interleukine-6 (IL-6), blood electrolyte tests (K, Na, Mg, Ca, Cl), coagulation tests (ADTL,Stago prothrombin assay, international normalized ratio, fibrinogen, D-dimers); and urea tests. In particular cases, an arterial blood analysis (pH, pCO2, pO2, HCO3, SBC, ABE, SBE) was also performed,as well as additional instrumental, biochemical, and microbiological tests of blood and urea.
Hepatitis B and C markers, together with human immunodeficiency virus, cytomegalovirus, and Epstein-Barr virus markers as required, were also performed on admitted patients.
Statistical analysis
Quantitative data were presented as mean ± SD and range. Qualitative data were presented as numbers and percentages. The characteristics of the data distribution were evaluated using the Shapiro-Wilk test.Depending on data distribution normality, the difference in continuous variables between the groups of patients with elevated and normal liver enzymes was assessed using the Welch two independent samplet-test or the nonparametric Mann-Witney-Wilcoxon test. Pearson’s chi-squared test was used to compare categorical variables between groups.
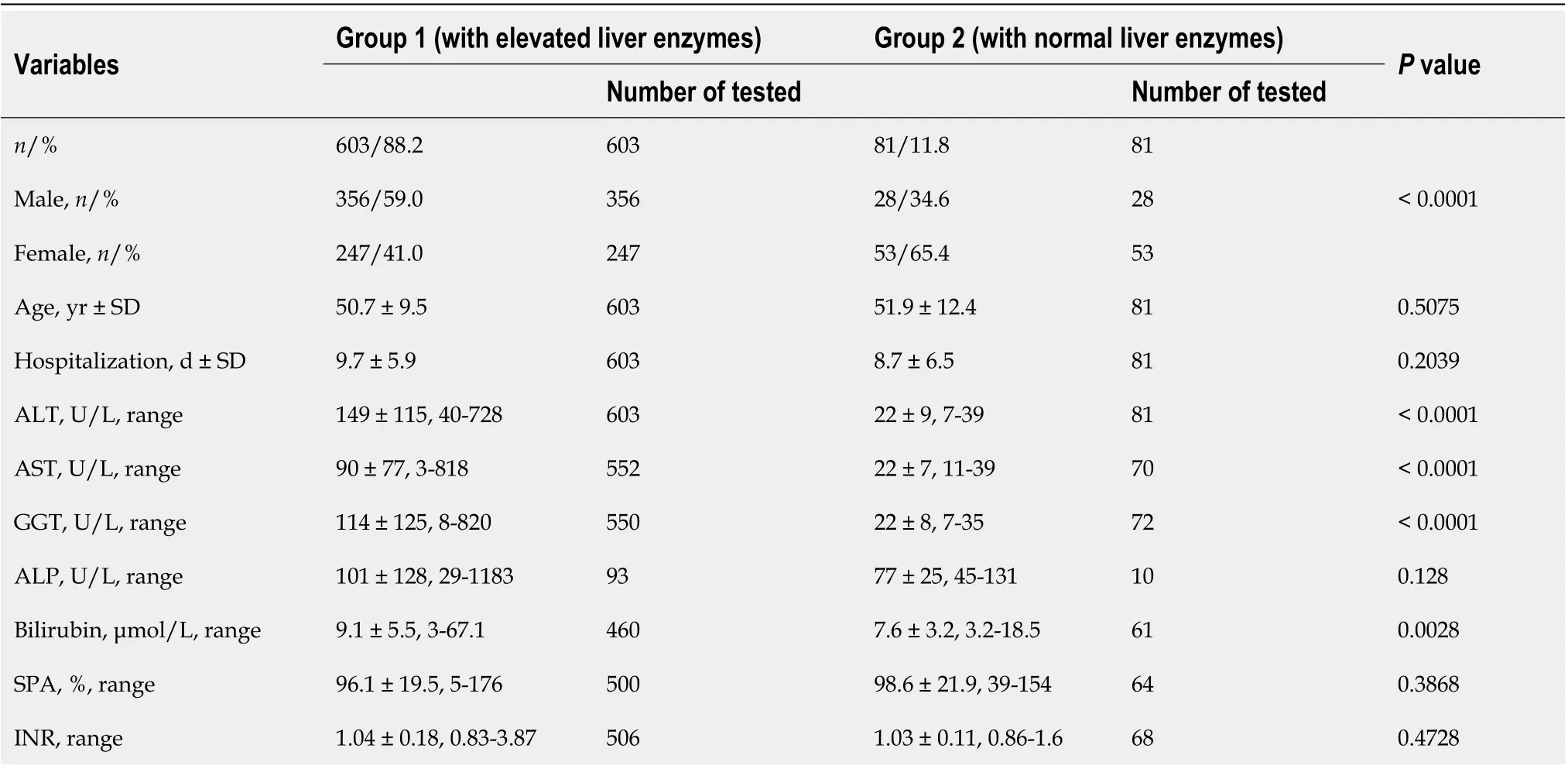
Table 1 Characteristics of the studied patients
Univariate and multivariate logistic regression analysis was performed to assess the likelihood of the cohesion of the variables.
Data were considered statistically significant when thePvalue was < 0.05 for the confidence interval set at 95%. All statistical analysis was performed with the R software, version 4.1.2 (The R Project for Statistical Computing, r-project.org).
RESULTS
Elevated liver enzymes, especially ALT, were detected in the majority of patients hospitalized with SARS-CoV-2 pneumonia (603 out of 684). ALT and AST were elevated by a factor of less than 3 in 54.9%and 74.8% of cases with increased enzyme levels, respectively. Only 9.3% of the cases of elevated ALT,2.7% of AST, and 11.2% of GGT were in concentrations higher than 300 U/L (Table 1).
In most patients (432, 71.6%), elevated ALT in the range of 41-728 U/L was detected on the first day of hospitalization. In almost half (209) of these patients, ALT increased to 80 U/L; in 91 patients, up to 120 U/L; and in only 17 patients did the level of ALT increase to more than 300 U/L (Table 1). ALT tended to rise during hospitalization and pneumonia treatment, and in some cases its level did not recede to the normal range even after SARS-CoV-2 infection recovery and discharge.
AST levels were elevated in 449 (72.1%) patients overall: for 241 (53.7%) of these patients, AST was found to be elevated in the range of 41-351 U/L on the first day of hospitalization; 159 patients had an up to two-fold elevation of AST; 50 patients up to 120 U/L; and only two patients had AST levels over 300 U/L (Table 1). Similarly to ALT, AST levels were prone to increase during the treatment of SARSCoV-2 pneumonia, but for most patients, these levels returned to their normal range by the time of discharge.
GGT levels in the range of 40-820 U/L were detected in 438 (70.4%) of the patients tested, and in 353(80.6%) patients, elevated GGT was detected on the first day of hospitalization. Up to two-fold elevated GGT was detected in 198 patients; 71 patients displayed CGT levels up to 120 U/L; and 25 patients displayed CGT levels over 300 U/L (Table 1). Like ALT, GGT tends to increase during hospitalization and slowly normalizes after patients recover from viral pneumonia.
ALP level was tested for only 103 patients, and most cases 89 (86.4%) were in the normal range(Table 1). Only 7 (6.8%) patients had up to three-fold elevated ALP, and in one patient with a critical course of COVID-19 ALP increased dramatically to 1183 U/L, along with ALT 162 U/L and AST 223 U/L. This patient suffered from acute liver and kidney failure, electrolyte and alkaline-acid imbalance,and sepsis aggravated by resistance to beta-lactam antibiotics, from which they did not recover.
Only 24 patients (3.5%) of the studied cohort had preexisting hepatitis of different etiologies(including two patients with chronic hepatitis C and two patients with chronic hepatitis B), and only two of these patients (8.3%), with hepatitis B in long-term remission, had normal levels of liver enzymes at admission and during hospitalization. The other 22 (91.7%) patients had elevated liver enzymes,including two patients (8.3%) with chronic hepatitis C. Most patients with preexisting liver diseases had a moderate course of COVID-19 pneumonia (20, 83.3%), three (12.5%) had severe cases, and one patient(4.7%) had a critical course of COVID-19. Only one female patient in this group died of COVID-19. No differences in other comorbidities or COVID-19 complications were observed in the remainder of the patients with preexisting liver disease.
Comparison of patient groups with elevated liver enzymes versus normal liver enzymes
When comparing patients who showed signs of liver impairment with those who did not, the main difference was the severity of inflammation. Patients with elevated liver enzymes (Group 1) more often demanded oxygen, and all biochemical inflammation indices were higher than in those with normal enzymes (Group 2). Of the 684 patients studied, 209 (30.5%) required O2supply due to respiratory failure, mostly belonging to Group 1 (Table 2). In addition, these patients required a longer duration of supportive O2due to low blood O2saturation because of severe lung infiltration.
For most patients, Il-6 blood concentration was in the range of 2-626 ng/L. Only one 62-year-old male with a critical course of pneumonia and elevated liver enzymes had an Il-6 as high as 2499 ng/L. This patient died after the manifestation of a cytokine storm (Table 2).
The level of LDH in the blood was in the range of 134 U/L-979 U/L for most patients. Only one patient (a 72-year-old male), who had normal liver enzyme levels during all stages of the disease, had LDH 1304 U/L and died of critical COVID-19.
Patients with elevated liver enzymes had almost double the chance of bacterial complications of viral pneumonia (univariate logistic regression: odds ratio (OR) = 1.73 (1.087-2.789),P= 0.0217). The incidences of other complications were largely similar in both groups (Table 3).
Underlying diseases - including hypertension, the most common - did not substantially prevail in patients with elevated liver enzymes (Table 4).
Analysis of the association of variables with the seriousness of SARS-CoV-2 pneumonia
A moderate course of SARS-CoV-2 pneumonia was diagnosed in 500 (73.1%) patients; a severe course in 148 (21.6%); and a critical course of SARS-CoV-2 pneumonia was diagnosed in 36 (5.3%) patients. There were no significant differences in the distribution of severity between the two groups of patients studied(Table 2).
To clarify which factors predispose a patient toward a more severe course of COVID-19, univariate(Tables 5 and 6) and multivariate (Table 7) analyses were performed.
The age of patients had almost no influence on the course of the disease, while male patients had forms of COVID-19 that were almost 1.5 times more severe and critical (Table 5). Multivariate logistic regression analysis also confirmed that the male gender was independently associated with more severe COVID-19 (Table 7). Acute kidney failure, but not acute liver failure, was also found to be associated with a more severe course of the disease (Table 5).
Neither the underlying disease that was most frequently presented - primary hypertension - nor less frequent lung diseases, cancers, or obesity were confirmed to be associated with a more severe course of COVID-19. Only heart disease of various etiologies, type 2 diabetes, and hyperlipidemia were prone to aggravate COVID-19 (Table 6). Diabetes and hyperlipidemia, but not heart disease, were also independently confirmed to be associated with more severe COVID-19 (Table 7).
Multivariate logistic regression analysis included age, gender, underlying diseases, and complications of pneumonia. Among the pneumonia complications analyzed, only sepsis, increased respiratory rate,and respiratory failure were independently associated with the severity of COVID-19 (Table 7).
Analysis of patients with liver failure
In our study, five patients, all of whom were male, experienced acute liver failure (0.73%) (Table 8).Septic shock developed in four patients, who did not recover despite all efforts to stabilize their condition. Two patients experienced rapid progression of the disease, and died after three days (a 50-year-old male with angioblastic T lymphoma) and eight days (an 84-year-old male with intracranial abscess, epilepsy, and chronic heart disease) of hospitalization.
Disease outcome analysis
Twenty patients died from COVID-19, 85% of whom (17 patients) had elevated liver enzymes. When comparing between patients with elevated and normal liver enzymes, no significant differences were found in mortality rate - 2.8% for those with abnormal liver test results, and 3.7% for those with normal liver test results.
Most of the deceased patients were older males who experienced much more severe SARS-CoV-2 pneumonia, with more life-threatening complications than recovered patients (Table 9). All deceased patients had at least one underlying disease or a combination thereof, and suffered significantly more often from heart disease, hypertension, and urinary tract infections than patients who made recoveries(Table 9). It should be noted that despite the fact that deceased patients were characterized by developing resistance to antibiotics more often than recovered patients, bacterial complications of viralpneumonia were less frequently documented in this group. Only two deceased patients were obese (2,or 10%,vs15, or 2.26%, of recovered patients).
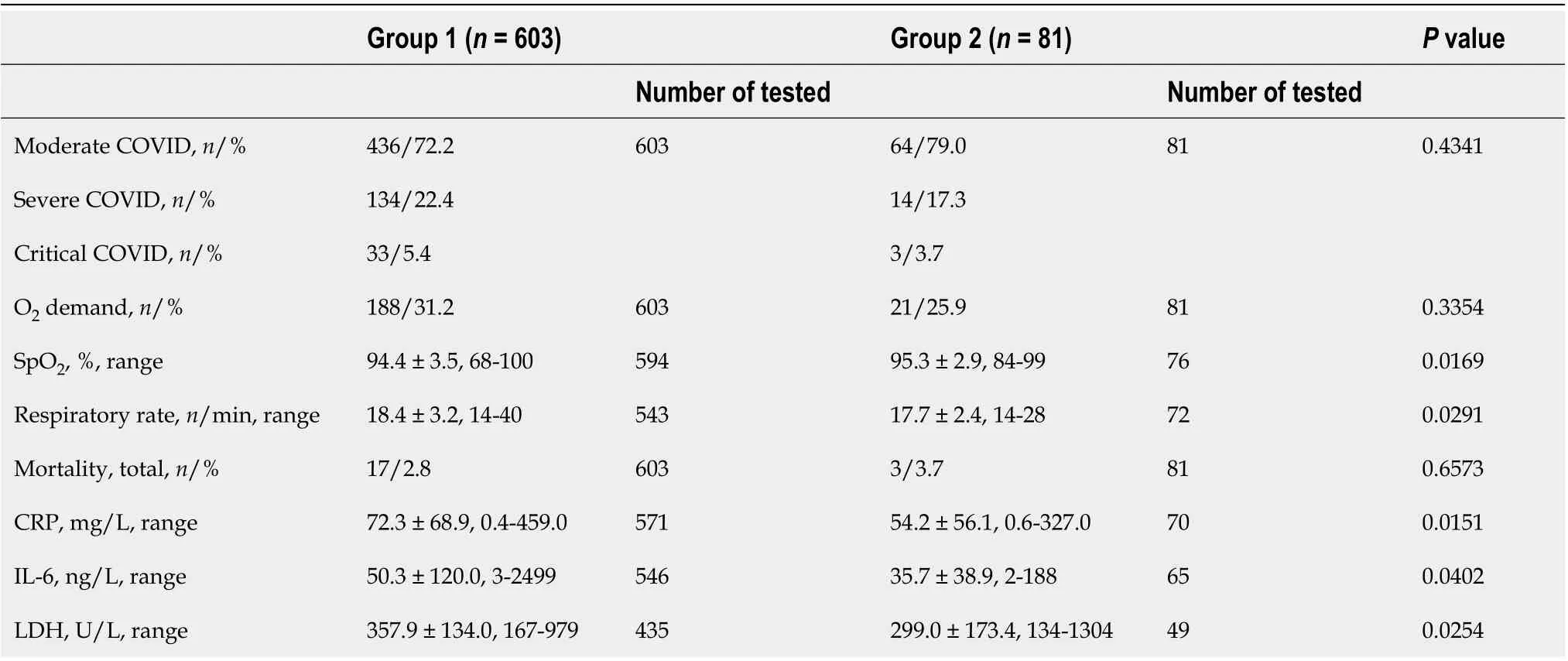
Table 2 Coronavirus disease 2019 pneumonia severity indices of patients with elevated liver enzymes (group 1) versus normal liver enzymes (group 2)
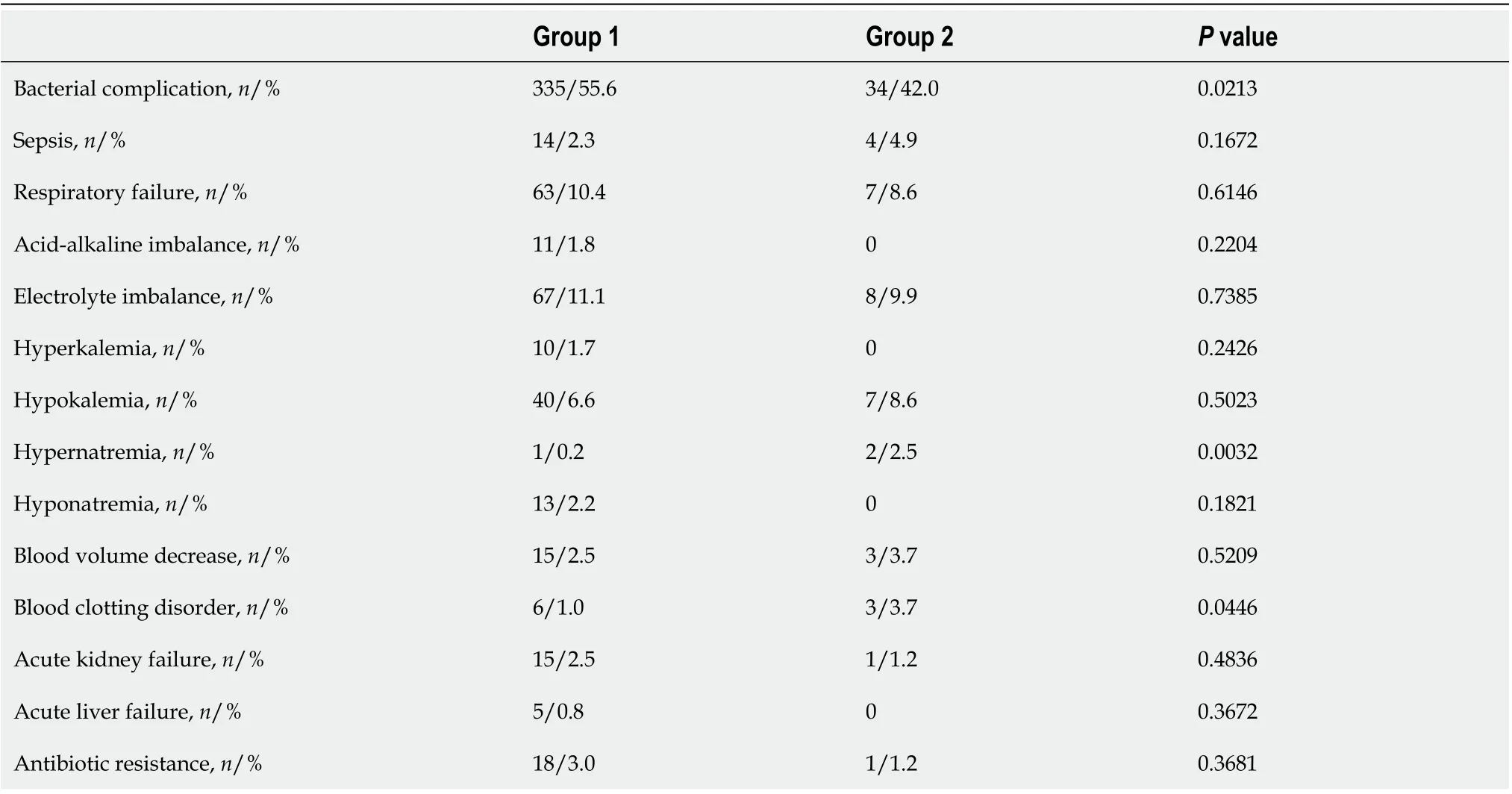
Table 3 Comparison of coronavirus disease 2019 pneumonia complications in patients with (group 1) and without (group 2) elevated liver enzymes
DlSCUSSlON
In our cohort, 88.2% of hospitalized COVID-19 patients had elevated liver enzymes - most often ALT(88.2%) followed by AST (71.6%) and GGT (70.4%). Similar results were found in the Caiet al[7], where 76.3% of hospitalized patients displayed abnormal liver test results. On the contrary, the Hao study reported that 79.2% of hospitalized patients displayed normal liver tests[5].
An increase in LDH level was observed in patients with severe forms of COVID-19, and was associated with a poor prognosis. In the acute liver failure group, four patients had elevated LDH in therange of 448-900 U/L. One patient with normal liver enzymes had an LDH of 1304 U/L. These patients died during treatment. Our findings are consistent with data from an Indonesian meta-analysis in which an association between increased LDH levels and mortality was observed (OR = 4.22,P< 0.001)[12].
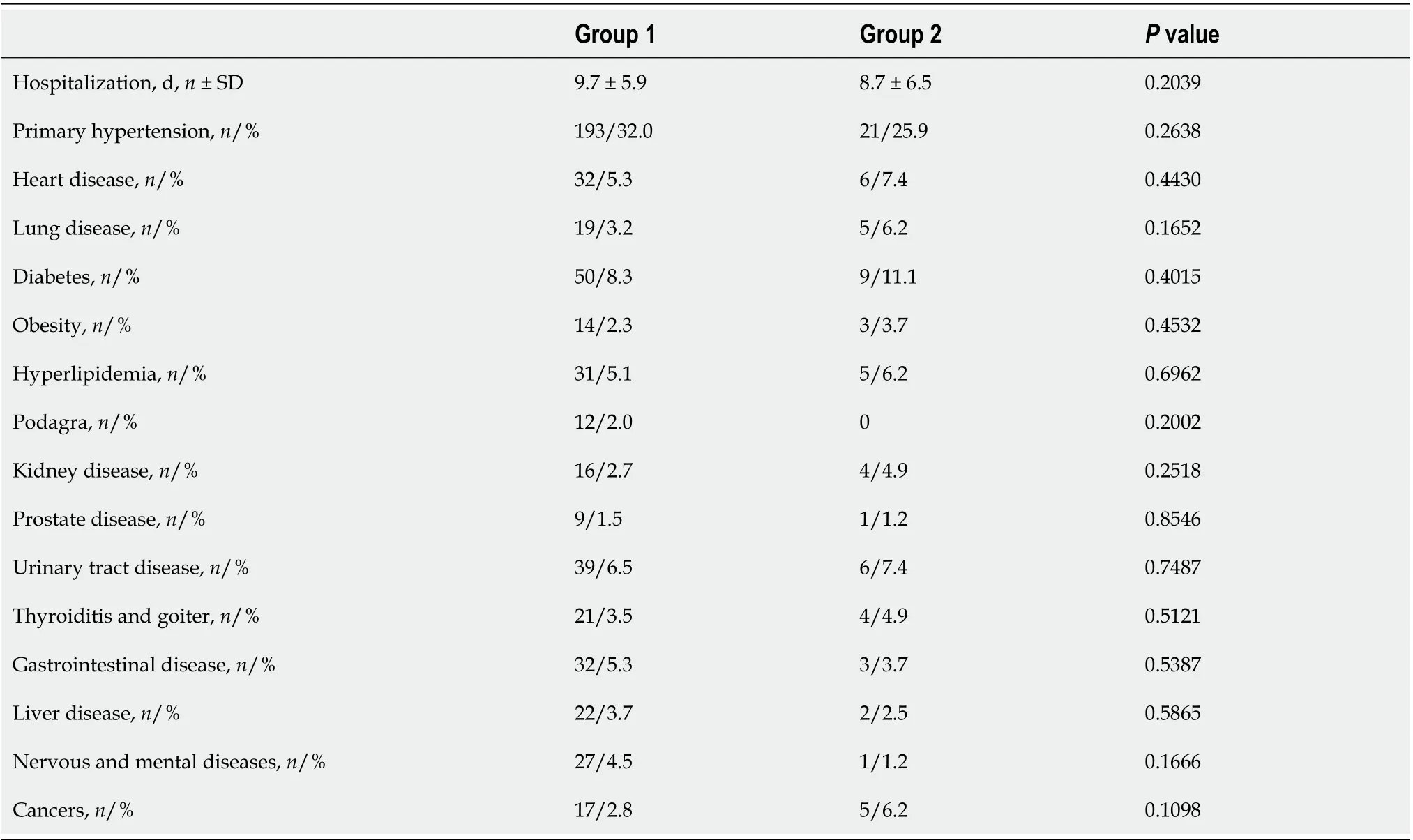
Table 4 Prevalence of underlying diseases in patients with (group 1) and without (group 2) elevated liver enzymes
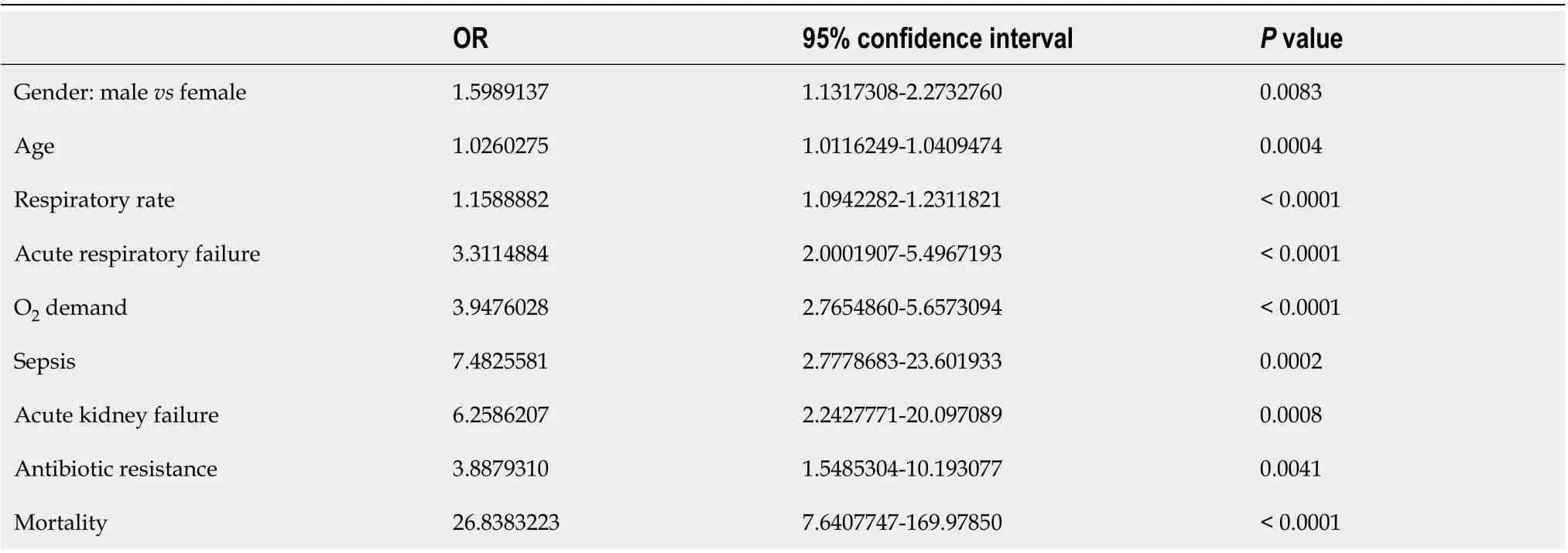
Table 5 The association of the severity of coronavirus pneumonia with the demographic characteristics of patients and critical disease outcomes (data from univariate logistic regression analysis)
It should be noted that the proportion of patients with abnormal liver test results varies between published COVID-19 studies for several reasons. As in our case, some studies are restricted only to hospitalized patients[5,7,13,14], whereas other studies include all positive cases of COVID-19[9].Moreover, the liver test norms that apply in particular hospitals vary, making it somewhat difficult to compare data. In our study, ALT and AST below 40 U/L, GGT below 36 U/L, and ALP below 150 U/L were considered normal for both male and female patients, as in the Caiet al[7], while in other studies the normal limits of liver tests were set lower. In the Hao study, the ALT norm for males was 35 U/L,while for females it was 25 U/L[5]; in the Wishniewska study, ALT and AST norms for males were 41 U/L, and for females they were 32 U/L[15].
In general, some reviews provide elevated liver enzymes for COVID-19 patients in the range of 14.8%-53%[16], while others consider 50%-78% to be elevated[6].
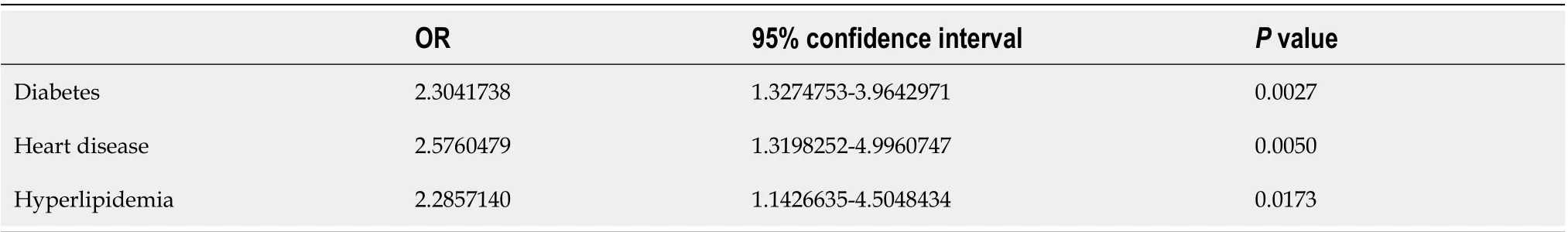
Table 6 The association of the severity of coronavirus pneumonia with underlying diseases (data from univariate logistic regression analysis)
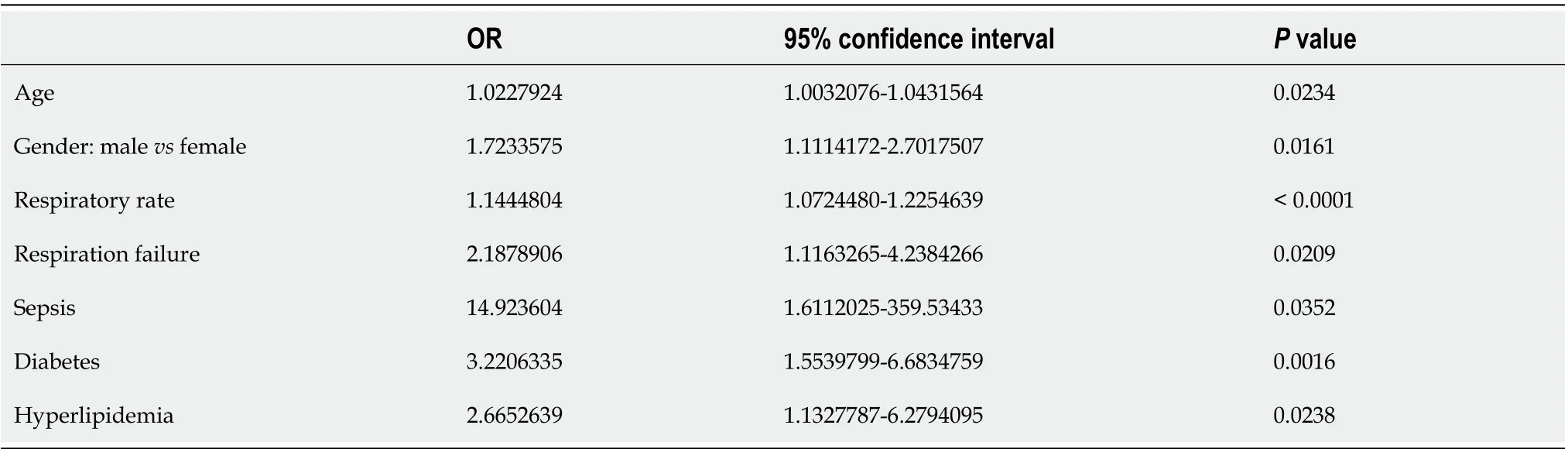
Table 7 lndependent factors associated with the severity of coronavirus disease 2019 (data from multivariate logistic regression analysis)
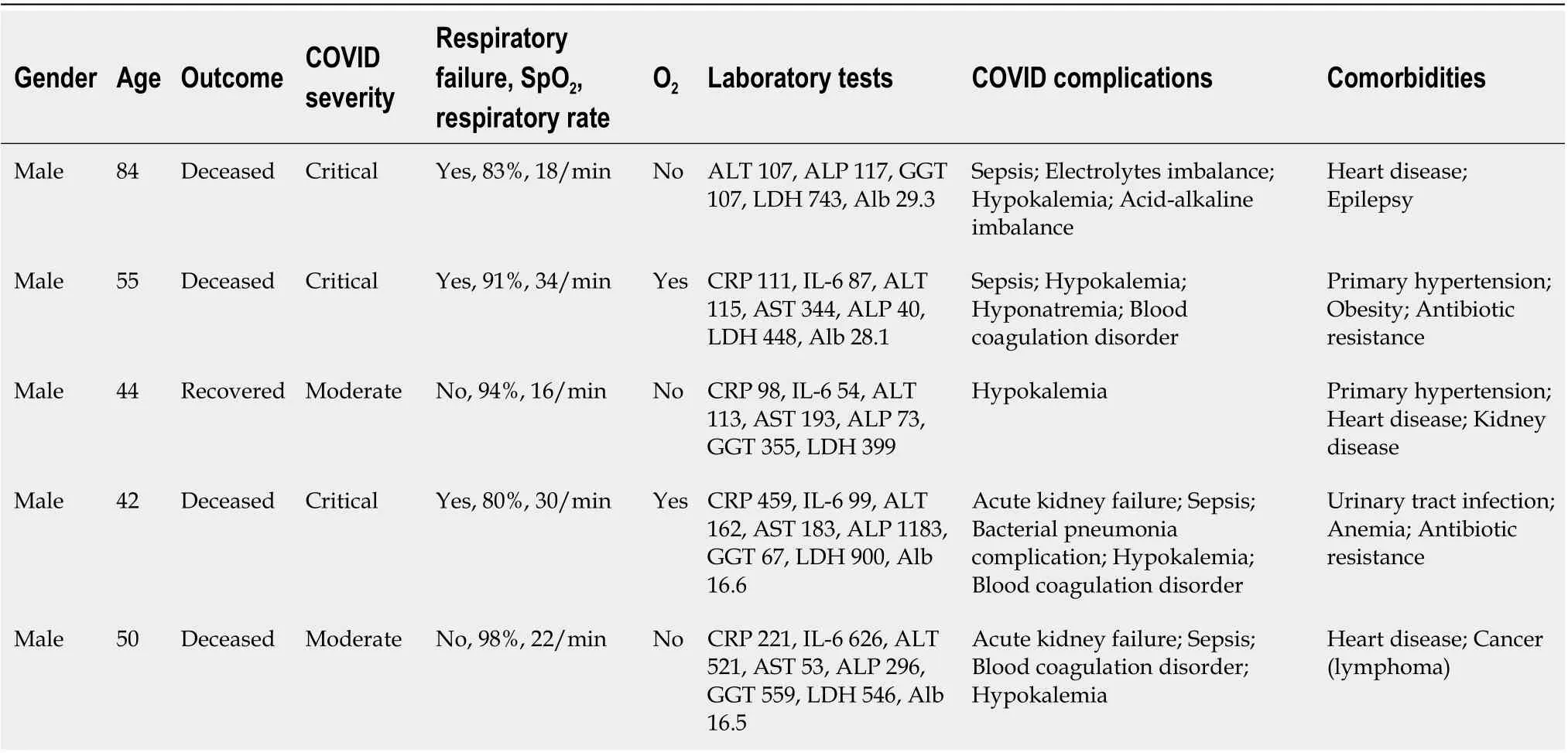
Table 8 Data from patients with acute liver failure
Despite the aforementioned discrepancies, abnormalities in liver tests deserve the attention of clinicians due to the wealth of evidence suggesting that patients with elevated liver enzymes, especially ALT and AST, generally have more severe SARS-CoV-2 pneumonia[6,7,17-20]. In different studies,elevated transaminases are associated with a 2-9-fold increased probability of poor outcomes of COVID-19[19,21].
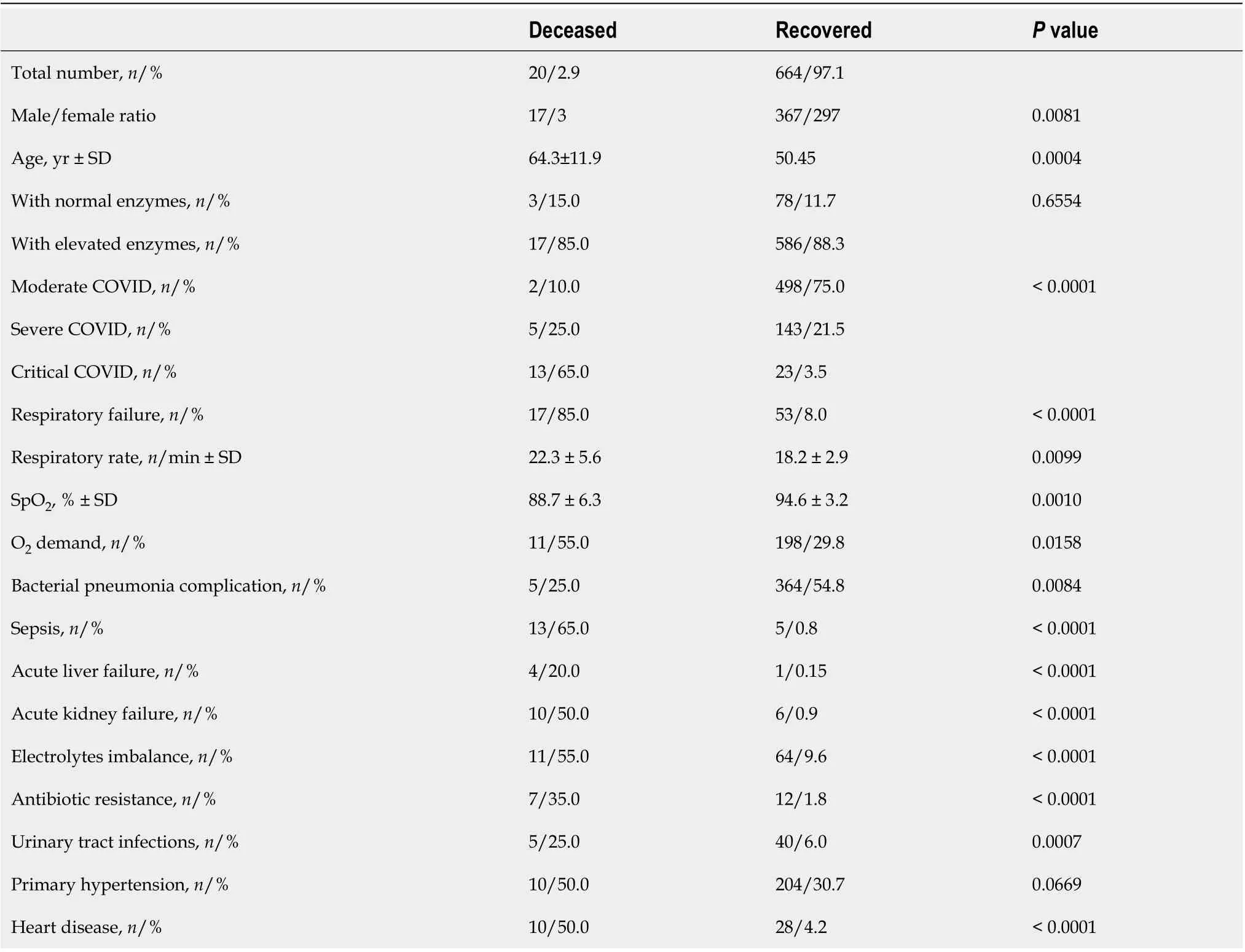
Table 9 Analysis of patients hospitalized with coronavirus disease 2019 depending on the outcome of the disease
In our study, moderate pneumonia was more frequent in patients with normal liver enzymes, while critical pneumonia prevailed in patients with elevated liver test results. Furthermore, the majority of the deceased patients had elevated liver enzymes. Despite this, it should be noted that we have not proved that abnormal liver test results are directly related with the likelihood of more severe COVID-19.
The broader field of research has no consensus on the pathological mechanism by which SARS-CoV-2 infection damages the liver because the histological view of liver injury rarely presents. In the Caiet al[7], postmortem histological liver analysis showed neither lesions of the lobular architecture nor portal tract infiltration - only slight vesicular steatosis, watery degeneration of hepatocytes with minimal plasma cells, and neutrophil infiltration of hepatic sinuses[7]. Such alterations prompted the formulation of a hypothesis of ischemic/hypoxic hepatitis due to altered O2blood saturation, and cardiac failure in critically affected cases[22].
However, the pattern of increase in transaminase in patients with COVID-19 was different from hypoxic hepatitis, suggesting that this was not the case. ALT and AST were elevated by a factor of less than 3 in 54.9% and 74.8%, respectively, of cases of abnormal liver test results in this study. Only 9.3% of the cases of elevated ALT, 2.7% of AST, and 11.2% of GGT were in concentrations greater than 300 U/L.Other studies also emphasize that transaminases rarely increase by a factor of more than 2-3 in COVID-19 patients[19,23].
It should also be borne in mind that elevated transaminases do not always originate exclusively from the liver; therefore, other causes such as myositis, ischemia, and cytokine release syndrome must be excluded to draw a definitive conclusion regarding liver injury in COVID-19 patients[24]. ACE-2,responsible for the virus entry receptor, is expressed not only in respiratory tract epithelium cells but also in vascular endothelium, cardiovascular tissue, renal tissue, and intestinal epithelia, which is why the possibility for the entry and replication of SARS-CoV-2 theoretically exists in practically all vasculated tissues of the human body[25]. Histopathological analysis of autopsies confirmed inflammatory infiltration of the lamina propria; epithelium of the digestive tract, skin, and kidney blood vessels; features of viral myocarditis; and hypoxic brain injury[26]. Furthermore, other research has established that SARS-CoV-2 can persist in the intestines of infected subjects even longer than in the respiratory tract[27-29].
Abnormal liver test results are more likely to reflect the severity of COVID-19 in general than a particular liver injury[21]. A comprehensive meta-analysis of 35 studies with more than 10000 total participants concluded that COVID-19, despite its severity, has a minor impact on the liver[4]. We agree with this conclusion.
Patients with elevated liver enzymes also displayed increased indices of inflammation, such as CRP and IL-6 levels. Elevated serum levels of CRP, IL-1, IL-6, and the tumor necrosis factor were reported in several other studies, and this was associated with a non-favorable course of liver injury[30].Additionally, the bacterial complication of SARS-CoV-2 pneumonia, which contributes to the systemic inflammatory response, was diagnosed more frequently in patients with increased liver enzymes (OR =1.73,P= 0.0217).
In our study, resistance to antibiotics, most often beta-lactams, was found to be associated with more severe COVID-19 (OR = 3.89,P= 0.004) and was documented more frequently in deceased patients. This issue is rarely discussed in COVID-19-related studies and needs more careful evaluation, as only very few studies with a small number of patients have been published[31,32]. Although concerns that the pandemic has led to an increase in antibiotic resistance due to self-medication of this viral infection have already been raised[33,34], it is necessary to elucidate how resistance to antibiotics modulates COVID-19 itself as very little comprehensive analysis has been published so far.
It also appeared that male patients in our cohort were more likely to have elevated liver enzymes than female patients. Furthermore, the male gender was confirmed as an independent factor associated with more severe COVID-19. This finding is consistent with previous data which highlights the male gender as one of the indicators of liver affliction[7,35]. With some disagreements, the protective effect of estrogen is often mentioned in relation to liver diseases[36], but the specific reasons that males suffer from more severe COVID-19 should be elucidated in more studies involving not only clinical but also detailed epidemiological data.
Weber and co-authors reported that only rare cases of acute liver failure were diagnosed in infected patients[37]. Our findings are in line with this: only five (0.72%) patients experienced acute liver failure,all of whom were male. In four of those patients, sepsis developed, leading to death. Males also prevailed among the total number of deceased patients (20, or 2.9%), most of whom (17, or 85%)displayed abnormal liver test results. Other similar results have been posted elsewhere which suggest that the majority (58%-78%) of deceased COVID-19 patients had liver injuries[38,39]. However, a comprehensive meta-analysis of 158 studies involving 78798 patients drew the conclusion that elevated liver enzymes, despite being a common finding in COVID-19 patients, had no effect on mortality or the critical course of the disease[20].
It should be emphasized that none of the patients in our study who experienced liver failure during the course of COVID-19 had an underlying liver disease. Furthermore, there was no association of elevated liver enzymes with preexisting liver disease.
Overall, 3.5% of patients had underlying liver disease, the majority of whom (91.7%) exhibited abnormal liver test results during the course of COVID-19. Only 12.5% of these patients had severe COVID-19, and one female died of critical COVID-19.
Although chronic comorbid liver diseases are reported in 2.6%-11% of patients[19], it seems that in most cases this does not influence COVID-19-associated liver injury, severe COVID-19 infection, or poor patient outcomes. This was confirmed in our study, was also shown in a nationwide matched cohort study[40], and has been approved in several reviews[19,41]. However, in one publication with 99 patients, preexisting hepatitis B infection was reported as a condition for more severe COVID-19 infection compared to patients without hepatitis B[42]. It is assumed that the immunosuppressive effect of the SARS-CoV-2 virus may lead to the reactivation of the hepatitis B virus[43]. We could neither confirm nor deny this, as our cohort included only two patients with chronic hepatitis B in remission and two patients with chronic hepatitis C.
There is an opinion that the SARS-CoV-2 virus itself could be responsible for liver injury during COVID-19, but the histopathological mechanism remains uncertain[41]. In the liver, the majority of abandonment of ACE-2 expression is determined in cholangiocytes, but in patients with COVID-19, the cytopathic, not cholestatic, profile of elevated enzymes prevails. On the other hand, the level of expression of ACE-2 receptors in hepatocytes is believed to be regulated by the virus. There may also be additional ACE receptors or co-receptors[44]. Thus, we agree with the conclusion that COVID-19-associated liver injury usually occurs as a result of the progression of COVID-19 itself[45].
We did not find any differences between the groups of patients with and without elevated liver enzymes concerning the prevalence of underlying diseases. Therefore, we cannot predict nor draw any conclusions regarding how underlying diseases contribute to liver damage during COVID-19. However,patients with diabetes, heart diseases of various etiologies, and hyperlipidemia, but not obesity, have an increased likelihood of suffering from more severe SARS-CoV-2 pneumonia. Furthermore, diabetes and hyperlipidemia were independently confirmed to be associated with more severe COVID-19 (OR = 3.2,P= 0.0016, and OR = 2.7,P= 0.0238, respectively). Thus, concomitant pathology appeared to be more likely to affect the severity of COVID-19 than the probability of liver damage in our study. Many studies also mention cardiovascular and renal diseases, diabetes, obesity, and hypertension as factors that worsen the course of COVID-19[20].
In summary, elevated liver enzymes are often found in patients with COVID-19. This may be due to a variety of factors, including the effects of medications used to treat the disease, concomitant liver pathology, and the influence of the virus itself. In the cases that we examined, there were significantly more patients with hepatic impairment than with normal liver function, and hepatic-impaired patients had both a higher risk of bacterial complications and a more severe course of viral pneumonia, with increased oxygen demand. This is most likely due to the effects of the SARS-CoV-2 virus, which causes more damage not only to the respiratory system but also to other organs, including the liver, due to more pathogenicity and increased cytopathic aggression which causes a greater storm of cytokines.
Because we only examined inpatients with moderate to severe and critical COVID-19, we cannot compare the frequency of hepatic impairment in mild cases of COVID-19.
Limitations of the study
We must consider several limitations of our study. Because we studied only patients with COVID-19 who needed hospitalization, and outpatients were omitted, the groups of patients differed in size by a factor of almost 8: the group with elevated liver enzymes consisted of 603 patients, and the group with normal liver enzymes consisted of 81 patients. This circumstance perhaps prevented us from statistically proving an association between liver impairment and severity of COVID-19 course - although direct damage of the liver tissue by the SARS-CoV-2 virus itself should be proved histologically, since elevated transaminases do not entirely arise from damage to the liver tissue.
Our study featured no groups with asymptomatic or mild COVID-19; their liver enzymes would likely have been in the normal range. Thus, the group with normal enzymes could be larger. This circumstance is also necessary to consider when evaluating data on the impact of preexisting diseases on COVID-19 severity. We also have not followed up with patients after discharge, which is why we do not know how quickly signs of liver impairment are resolved after the recovery of patients from COVID-19 pneumonia.
CONCLUSlON
Despite a wealth of published data analyzing liver tests in COVID-19 patients, it is still difficult to draw inferences not only about the cause of such an effect of the SARS-CoV-2 virus infection on the liver, but also about the prevalence of elevated liver enzymes in such patients.
In summarizing our results, we can conclude that liver impairment allows a more severe inflammation to be predicted, with a higher risk of bacterial complications and worse outcomes in patients with SARS-CoV-2 pneumonia. Because several drugs with potentially hepatotoxic effects are used in severe cases, patients with more aggressive forms of COVID-19 should have their liver enzymes monitored regularly; their results should be considered when selecting a treatment to avoid further hepatic impairment or even insufficiency.
ARTlCLE HlGHLlGHTS


Research results
In total, 88.2% of patients with SARS-CoV-2 infection produced abnormal liver test results. Alanine aminotransferase and aspartate aminotransferase levels were elevated by a factor of less than 3 in 54.9%and 74.8% of cases with increased enzyme levels, respectively. Patients in Group 1 had almost double the chance of bacterial viral pneumonia complications, required oxygen supply more often, and displayed higher biochemical inflammation indices than those in Group 2. Like in other research, our patients rarely experienced acute liver failure. The majority of the deceased patients had at least one underlying disease or a combination thereof, and most were male. Alongside male gender and older age, diabetes and hyperlipidemia, but not obesity, were confirmed as independent factors associated with more a severe COVID-19 infection in our cohort.
Research conclusions
In our study, the presence of liver impairment allows us to predict a more severe inflammation with a higher risk of bacterial complication and worse outcomes of COVID-19. Therefore, monitoring liver enzyme levels should be a part of the qualitative care of patients with SARS-CoV-2 pneumonia.
Research perspectives
To find out more precisely the sources of increased liver enzymes in patients with COVID-19, it would be beneficial to elucidate whether the SARS-CoV-2 virus can enter and replicate in hepatocytes. For this purpose, an experimental study on the cell line of the liver origin or virus detection in hepatocytes during a histological analysis of autopsies could be promising.
ACKNOWLEDGEMENTS
We are grateful to the collaborators of the Centre for Informatics and the Development of Vilnius University Hospital’s Santaros Clinics for their valuable assistance in the data collection process. Our special thanks go to Ulbinas P, whose patience and competence allowed us to perform impersonalized data extraction in the most efficient and relevant manner.
FOOTNOTES
Author contributions:Puronaite R, Milaknyte G, Reivytyte R and Urbanoviciute G performed raw data collection;Liakina V, Stundiene I, Bytautiene R and Kazenaite E revised collected data for relevance and sufficiency; Liakina V,Stundiene I, Milaknyte G and Bytautiene R wrote the manuscript draft; Liakina V edited draft and prepared the final version of the manuscript; Kazenaite E revised manuscript for important intellectual content.
lnstitutional review board statement:The study was reviewed and approved by the Vilnius Regional Biomedical Research Ethics Committee and by Institutional Review Board (approval No. 2022/2-1411-882).
lnformed consent statement:Patients were not required to give informed consent to the study because the analysis used anonymous data that were obtained after each patients agreed to treatment by written consent.
Conflict-of-interest statement:All the authors report no relevant conflicts of interest for this article.
Data sharing statement:No additional data are available.
STROBE statement:The authors have read the STROBE Statement-checklist of items, and the manuscript was prepared and revised according to the STROBE Statement-checklist of items.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:Lithuania
ORClD number:Valentina Liakina 0000-0001-8685-1292; Ieva Stundiene 0000-0002-2569-3638; Gabriele Milaknyte 0000-0001-9343-6345; Ramune Bytautiene 0000-0002-6071-9688; Rosita Reivytyte 0000-0002-9140-227X; Roma Puronaite 0000-0001-7571-4989; Gintare Urbanoviciute 0000-0001-8392-3019; Edita Kazenaite 0000-0002-7127-1399.
Corresponding Author's Membership in Professional Societies:Lithuanian Society of Immunology; Lithuanian Society of Gastroenterology; European Association of the Study of the Liver.
S-Editor:Gao CC
L-Editor:A
P-Editor:Gao CC
 World Journal of Gastroenterology2022年39期
World Journal of Gastroenterology2022年39期
- World Journal of Gastroenterology的其它文章
- All journals should include a correspondence section
- Risk factors for small intestinal adenocarcinomas that are common in the proximal small intestine
- Receptor of advanced glycation end-products axis and gallbladder cancer: A forgotten connection that we should reconsider
- Serum metabolic profiling of targeted bile acids reveals potentially novel biomarkers for primary biliary cholangitis and autoimmune hepatitis
- Immune checkpoint inhibitor-mediated colitis is associated with cancer overall survival
- SARS-CoV-2-induced liver injury: A review article on the high-risk populations, manifestations,mechanisms, pathological changes, management, and outcomes
