Tetra-, penta-, and hexa-nor-lanostane triterpenes from the medicinal fungus Ganoderma australe
Lin Zhou, Subiy Akbar, Meng-Xi Wang, He-Ping Chen and Ji-Kai Liu
Abstract: Chemical investigation on the medicinal fungus Ganoderma australe led to the identification of ten new nor-lanostane triterpenes, namely two hexa-nor ones, ganoaustratetraenones A (1) and B (2), five penta-nor ones,ganoaustraldehydes A-E (3-7), and three tetra-nor ones ganoaustrenoic acids A-C (8-10).The chemical structures along with the absolute configurations were determined by extensive spectroscopic analysis of 1D & 2D NMR and HRESIMS data.The postulated biosynthesis pathways of these compounds were proposed.Ganoaustraldehydes A (3)and B (4) showed moderate inhibition against nitric oxide production in RAW264.7 macrophage cells with the respective IC50 values of 32.5, 34.2 μM (the IC50 of positive control pyrrolidine dithiocarbamate was 20.0 μM).
Keywords: Basidiomycete, Ganoderma australe, Nor-lanostane, Structural determination, Anti-NO production
1 Introduction
The genusGanodermacontains more than 80 species of wood decaying fungi mostly distributed in tropical and subtropical areas.Different species ofGanodermahave been used in Traditional Chinese Medicine for thousands of years for the treatment of many kinds of diseases, for example, hypertension, respiratory diseases, gastrointestinal disorders, autoimmune diseases [1].The two species,G.lucidumandG.sinensewere recorded in the recent five editions ofChinese Pharmacopoeiawith the name “Lingzhi” from the year 2000.Numerous studies have shown that polysaccharides and the main secondary metabolites-triterpenoids, are responsible for the biological activities ofGanoderma, such as immunoregulatory, antiviral, hepatoprotective effects [2-7].Besides,meroterpenes (farnesyl hydroquinones), the other main constituent inGanoderma, have attracted much attention in recent years [8, 9].Ganodermatriterpenes are always characterized by structural poly-oxygenations[6].Among the reported structures, the popular positions been oxygenated are C-3, C-7, C-11, C-15, C-23,and C-26.The oxygenations in the side chain sometimes trigger the C-C bond cleavage by retro-aldol reaction or oxidative cleavage to yield nor-lanostane, a minor group ofGanoderematriterpenes, such as C24 (hexa-nor), C27(tri-nor), and C-25 (penta-nor) lanostanes [5, 6].
Ganoderma australeis regarded as an alternative of the official-recognized speciesG.lucidum, and is used as a folk medicine in some ethnic minority areas of Yunnan Province, China.This fungus, however, was chemically under-investigated compared to other easily widely usedGanodermaspecies, such asG.lucidum,G.cochlear, andG.sinense.Previous studies on the secondary metabolites ofG.australehave led to the isolation of some lanostane triterpenes [10-13], meroterpenoids[14, 15], and alkaloids [14].In this study, we would like to report ten new nor-lanostane triterpenes isolated fromG.australe, of which the structures are assigned by extensive NMR and HRESIMS spectroscopic analysis.The new structures are classified into tetra-nor-, pentanor-, and hexa-nor-lanostanes, and are featured by C-6 oxygenation orα,β-unsaturated aldehyde groups, which are unusual modifications in the reportedGanodermatriterpenes, thereby inspiring a biosynthetic proposal of these compounds.
2 Results and discussion
2.1 Structure elucidation of compounds 1—10
Ganoaustratetraenone A (1) (Fig.1), isolated as paleyellow needles, has the molecular formula of C24H32O4,which was determined from the HRESIMS analysis (m/z385.23724 [M + H]+, calcd.for C24H33O4,385.23718) (Additional file: 1).The 1D NMR spectroscopic data of 1 (Tables 1 and 2) displayed six singlet methyls, six methylenes, two methines, four quaternary carbons (sp3hybridized), a pair of nonprotonated olefinic carbons, and four ketone carbonyls.The1H and13C NMR data of 1 showed high similarity to those of the previously described compound 4,4,14α-trimethyl-3,7,11,15,20-pentaoxo-5α-pregn-8-en[16], a semisynthetic product of methyl ganoderate O by alkaline treatment.There was only one difference between the structure of ganoaustratetraenone A and 4,4,14α-trimethyl-3,7,11,15,20-pentaoxo-5α-pregn-8-en, which was revealed by analyzing the NMR spectra.The pivotal HMBC correlation from H3-30 (δH1.27,s) to a methylene atδC32.3 (Fig.2) suggested that C-15 of 1 is a methylene instead of being a ketone group in 4,4,14α-trimethyl-3,7,11,15,20-pentaoxo-5α-pregn-8-en.Therefore, compound 1 was elucidated to be a hexa-norlanostane derivative.

Table 1 1 H NMR spectroscopic data for compounds 1-5
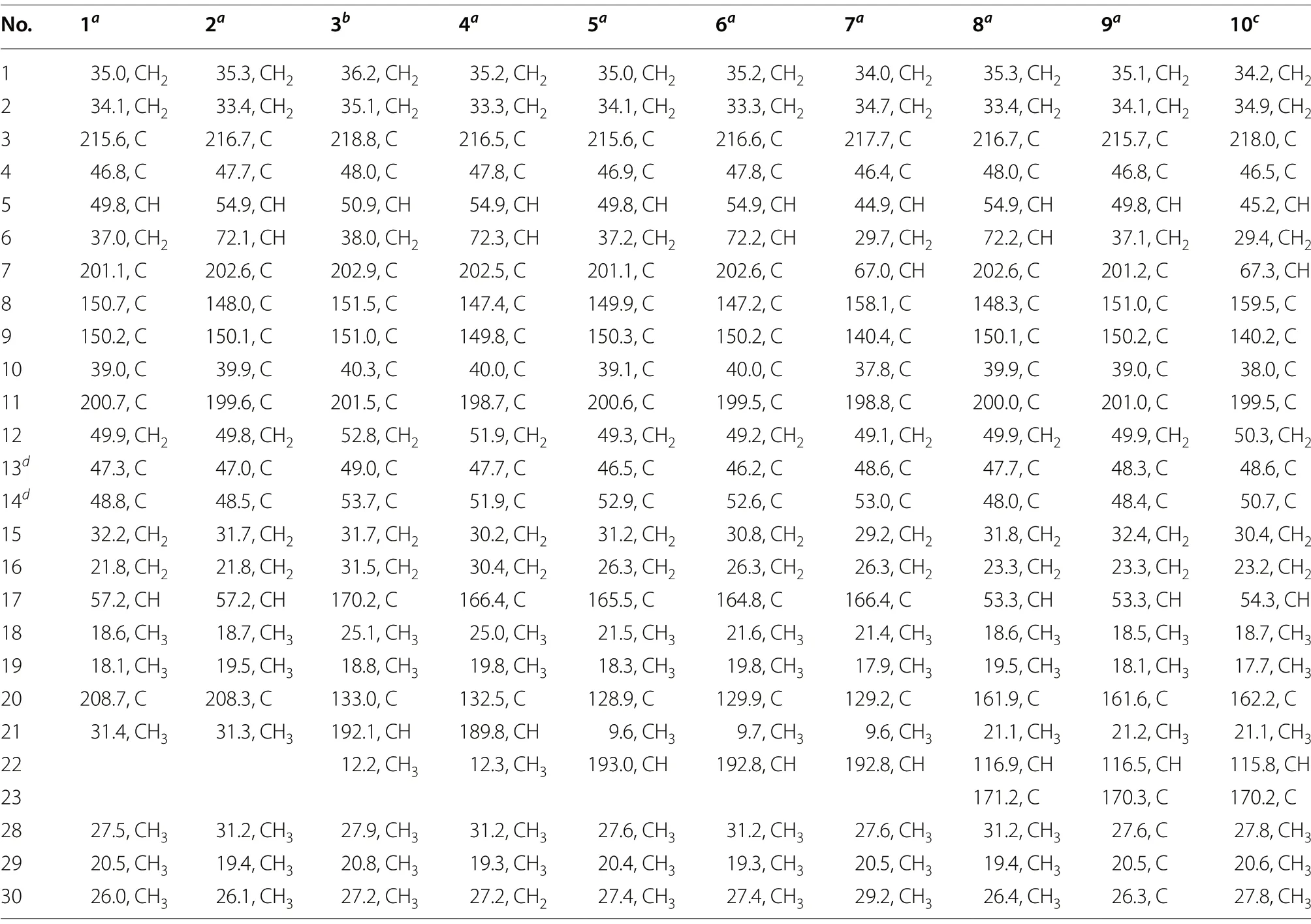
Table 2 13C NMR spectroscopic data for compounds 1-10
Compound 2 (Fig.1) was isolated as a white powder.The HRESIMS analysis of 2 gave a protonated ion peak atm/z401.23224 [M + H]+, corresponding to the molecular formula of C24H32O5(calcd.for C24H33O5,401.23280) (Additional file: 1).The 1D NMR spectroscopic data of 2 (Tables 1 and 2) showed six singlet methyls, five methylenes, three methines including one oxygenated, four quaternary carbons (sp3hybridized), a pair of non-protonated olefinic carbons, and four ketone carbonyls.Comparing the NMR data of 1 and 2 suggested that 2 was a structural congener of 1.Compound 2 differed from 1 by the presence of a hydroxy substituent at C-6, which was confirmed by the chemical shift of C-6 (δC72.1), by the1H-1H COSY correlation of H-5(δH2.30)/H-6 (δH4.42), and by the key HMBC correlations from OH-6 (δH3.68) to C-5 (δC54.9), C-6, and C-7(δC202.6) (Fig.2).The ROESY correlation of H3-19 (δH1.24)/H-6 enabled the assignment of OH-6 asαorientation (Fig.3).Thus, compound 2 was determined to be 4,4,14α-trimethyl-6α-hydroxy-5α-pregn-8-en-3,7,11,20-tetraone, and was trivially named ganoaustratetraenone B.
Ganoaustraldehyde A (3) (Fig.1) was obtained as a white, amorphous powder.The 1D NMR spectroscopic data of 3 showed six methyl singlets, six methylenes, one methine, four quaternary carbons (sp3hybridized), two pairs of non-protonated olefinic carbons, three ketone carbonyls, and an aldehyde carbon.The NMR data of 3(Tables 1 and 2) showed similarity to those of ganodernoid A [17], a penta-nor-lanostane triterpenes isolated fromGanoderma lucidum.Analysis of the HMBC,1H-1H COSY, and ROESY spectra of 3 allowed the elucidation of the chemical structure, and also revealed the differences between 3 and ganodernoid A.The key HMBC correlations from H3-30 (δH1.25) to C-15 (δC31.7), from H3-18(δH1.21) to C-17 (δC170.2), and from H3-22 (δH1.74) to C-17, C-20 (δC133.0), and C-21 (δC192.1) (Fig.2) indicated that the C-15 in 3 was a methylene group, C-21 was an aldehyde group, and C-17 and C-20 was a double bond.Therefore, the 2D structure of 3 was elucidated as shown in Fig.1.The key ROESY correlations between the aldehyde proton atδH10.00 and H-12 (δH3.11) (Fig.3)suggested that the C-17-C-21 double bond wasZconfiguration.The above assignment is consistent with the molecular formula of 3, C25H32O4, which was determined from the HRESIMS analysis (ion peak atm/z397.23721[M + H]+, calcd.for C25H33O4, 397.23734) (Additional file: 1).
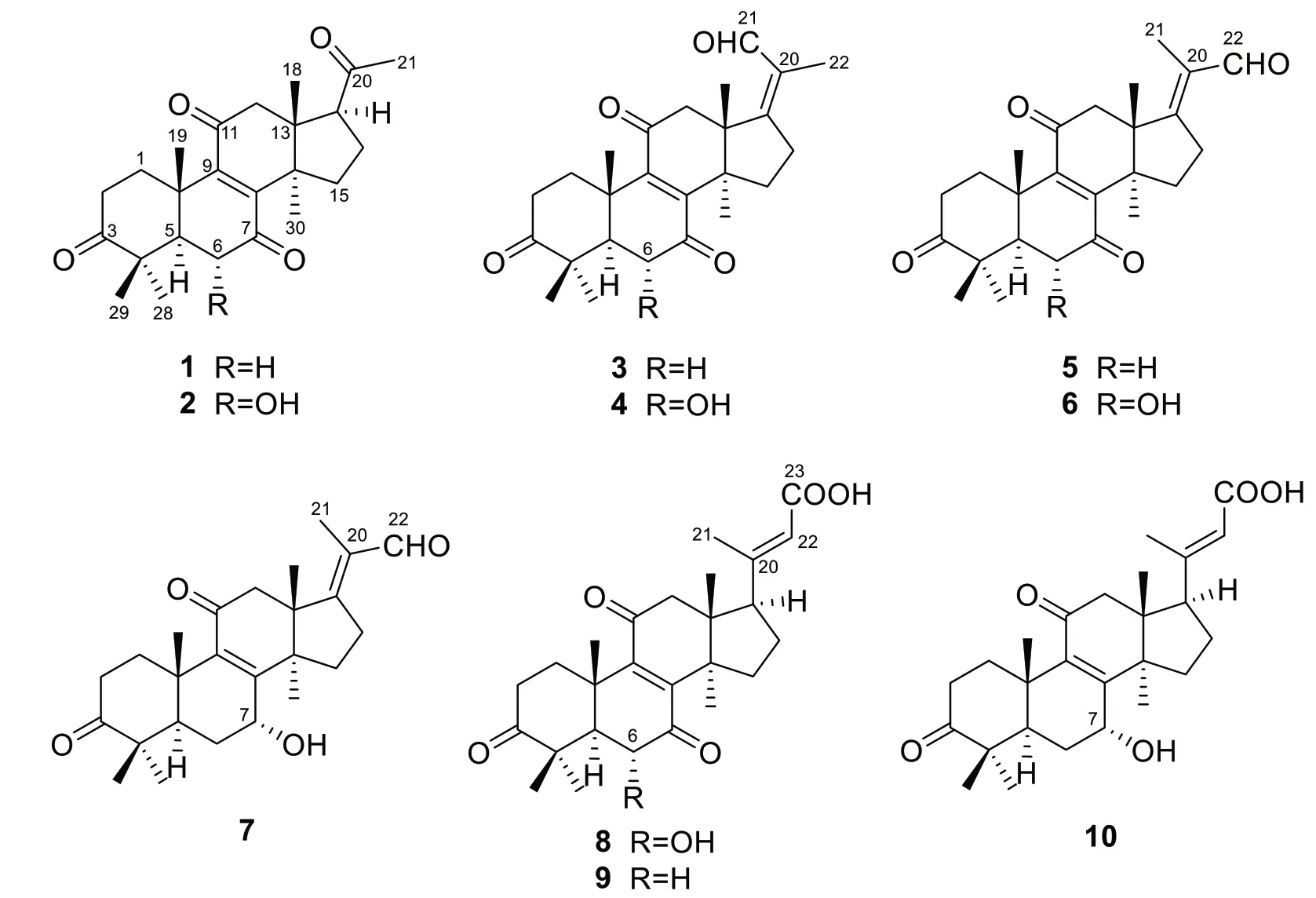
Fig.1 Structures of compounds 1-10
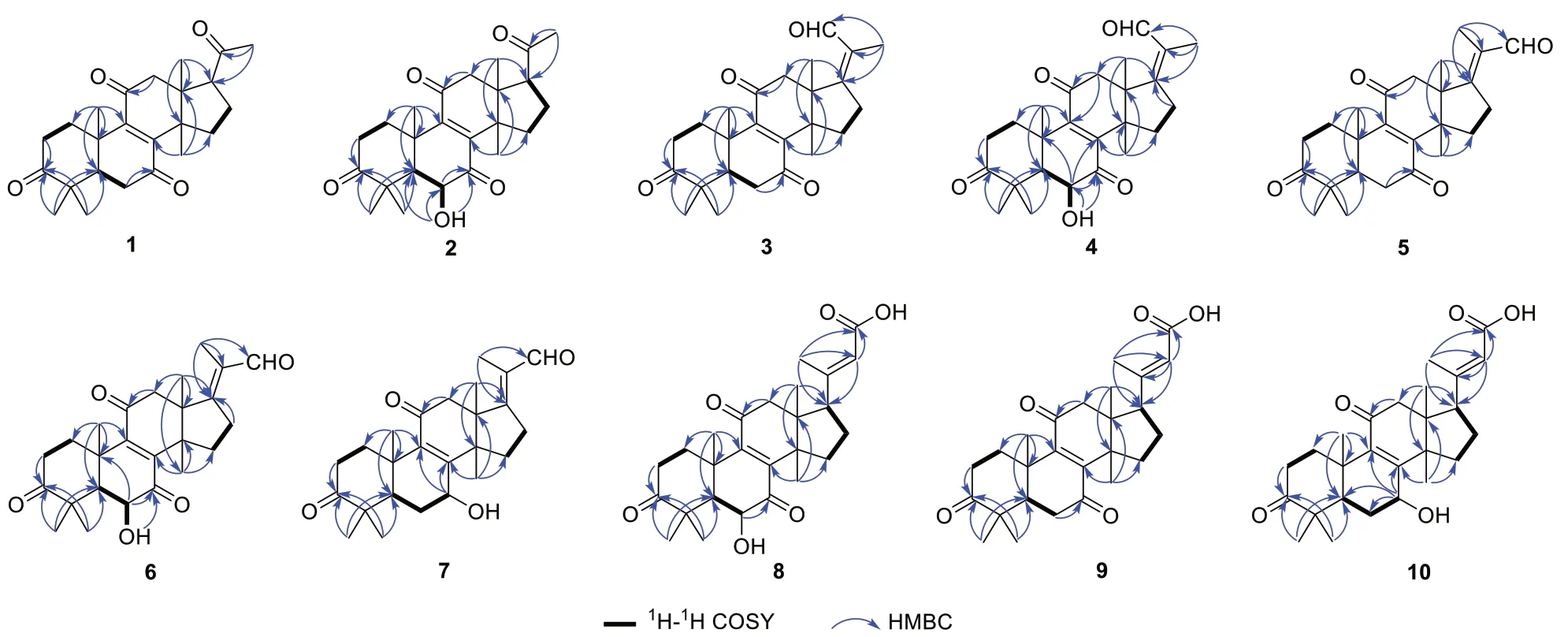
Fig.2 Key 1H-1H COSY and HMBC correlations of compounds 1-10
The white amorphous powder ganoaustraldehyde B (4)(Fig.1) returned a protonated ion peak atm/z413.23221[M + H]+in the HRESIMS analysis, suggesting the molecular formula of C25H32O5(calcd.for C25H33O5,413.23225) (Additional file: 1).The 1D NMR spectroscopic data of 4 (Tables 1 and 2) highly resembled to those of compound 3, indicating the analogous structures of 3 and 4.The signal of an oxymethine atδC72.3 (C-6)in the13C NMR spectra of 4 compared to those of 3,along with the 2D NMR correlations of 4, including the1H-1H COSY correlations between H-5 (δH2.34) and H-6(δH4.47), and HMBC correlations from 6-OH (δH3.69)to C-6 (δC72.3) and C-7 (δC202.5) (Fig.2) suggested that there was a hydroxy group substituted at C-6 in 4.The stereochemistry of the C-17-C-21 double bond was assigned asZconfiguration according to the ROESY cross peaks between H-21 (δH10.03) and H-12 (δH3.08)(Fig.3).The OH-6 was determined to beαconfiguration by the diagnostic ROESY signals of H-6/H3-19 (δH1.25)(Fig.3).Therefore, compound 4 was elucidated as shown in Fig.1.
The molecular formula of compounds 5 and 6 (Fig.1)were same with those of 3 and 4, respectively.The 1D NMR data of 5 and 6 (Tables 1, 3 and 2) also exhibited high similarity to those of 3 and 4, respectively, thus indicating that 5 and 6 were the respective structure congeners of 3 and 4.Analysis of the 2D NMR spectra of 5 and 6 enabled us to identify the only difference between these two pairs of compounds, which was the configuration of C-17-C-21 double bond (Fig.2).The diagnostic ROESY correlations of H3-21(δH1.80)/H-12 (δH3.05, 3.10), and H-22 (δH10.00)/H-16 (δH3.12 in 5; 3.19 in 6) (Fig.3)suggested theEconfiguration of C-17-C-21 double bonds of 5 and 6 (Fig.3).The configuration of OH-6 of 6 was determined asαorientation by the ROESY correlation of H-6/H3-19 (Fig.3).Therefore, compounds 5 and 6 were identified as ganoaustraldehydes C and D, respectively.
Compound 7 (Fig.1), a pale-yellow oil, had a protonated ion peak atm/z399.25305 in the HRESIMS analysis (calcd.for C25H35O4, 399.25353) (Additional file: 1).Analysis of the1H and13C NMR spectroscopic data of 3(Tables 3 and 2) revealed that this compound was a structural analogue of 5.The main difference between the two analogues was C-7.In the HMBC spectrum of compound 7, significant correlations from H-7 (δH4.52) to C-8 (δC158.1) and C-9 (δC140.4) were observed (Fig.2).This evidence together with the1H-1H COSY correlation of H-5/H-6/H-7 (Fig.2) suggested that C-7 in 7 was an oxygenated methine, instead of being a ketone group in 5.The exocyclic C-17-C-21 double bond was assigned asEconfiguration by the key ROESY correlations of H3-21 (δH1.79)/H-12 (δH3.04) (Fig.3).The 7-OH was determined to beαconfiguration by the ROESY cross peak signals of H-15β(δH2.14)/H-7 (δH4.52) and the coupling constant of H-7 (t, 3.3 Hz) (Fig.3) [18].Therefore, the structure of 7 was assigned as ganoaustraldehyde E.
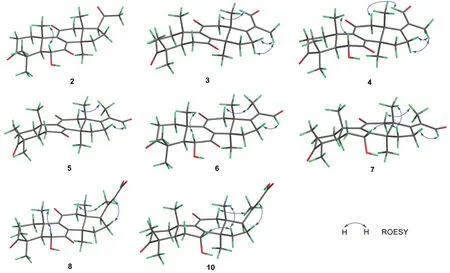
Fig.3 Key ROESY correlations of compounds 2-8, and 10
The 1D NMR data of the compounds 8 and 9 (Tables 3 and 2) each showed 26 carbon resonances with a high resemblance to those of compounds 2 and 1, respectively.Further analysis of the NMR spectroscopic data suggested that 8 and 9 differed from 2 to 1 by the existence of a trisubstituted double bond (C-20, C-22) and a COOH group (C-23), while by the absence of a ketone group (C-20), respectively.These changes suggested that 8 and 9 were two lanostane triterpenes with six carbon degradation (C-24, C-25, C-26, and C-27).The C-23 in 8 and 9 were carboxylic groups which were assigned by the HMBC correlations from H-22 (δH5.77/5.78 in 8/9)and H3-21 (δH2.17/2.18 in 8/9) to C-23 (δC171.2/170.3 in 8/9) (Fig.2), as well as the molecular formulas(C26H34O6for 8, C26H34O5for 9) which were designated by the HRESIMS analysis.The double bond of C-20(22)were assigned asEconfiguration by the key ROESY correlations between H-22 and H-17 (δH2.87/2.85 in 8/9),H-16a, H-16b (Fig.3).Therefore, compounds 8 and 9 were identified as ganoaustrenoic acids A and B (Fig.1),respectively.
Compound 10 (Fig.1) returned a protonated ion peak atm/z429.26361 in the HRESIMS analysis, implying it has the molecular formula of C26H36O5(calcd.for C26H37O5, 429.26410).The molecular formula and the 1D NMR spectroscopic data of 10 (Tables 3 and 2) are reminiscent of those of 9, implying that 10 was also tetra-nor-lanostane triterpene.The only difference between 10 and 9 is located at C-7, which was indicated by a comparison of the NMR data.The key HMBC correlation from the proton atδH4.48 (H-7) to C-5(δC45.2), C-6 (δC29.4), C-8 (δC159.5), C-9 (δC140.2)(Fig.2) suggested that C-7 was a hydroxymethine in 10 instead of being a ketone group in 9.The ROESY correlations of H3-18 (δH0.68)/H-15β(δH2.04)/H-7 (δH4.48), and the coupling constant of H-7 (t, 3.0 Hz) facilitated to determine the orientation of 7-OH asα(Fig.3).Therefore, compound 10 was identified as ganoaustrenoic acid C.
Since these nor-lanostane are characterized by unusual C-6 oxygenation, andα,β-aldehyde groups, a biosynthetic proposal is postulated as shown in Scheme 1.The key intermediate lanosterol is biosynthesized from squalene, the common precursor of triterpenes.Further oxygenation on multiple sites of the lanosterol leads to the key intermediate A [19, 20].Abstraction of the proton of OH-20 by the retro-aldol reaction (route A) give compound 1, which has been further oxygenated at C-6 position to yield 2.Oxidative cleavage of C23-C24, followed by elimination of the C-20 hydroxy group throughE1cb mechanism to produce 9, which is been further oxygenated or reduced to yield 8 and 10.Alternatively, A undergoes oxygenation at C-22, and oxidative cleavage of C22-C23, and further elimination of C-20 hydroxy group giving compounds 3 and 5.Late-stage oxygenation and reduction of 3 and 5 produce 4, 6, and 7.
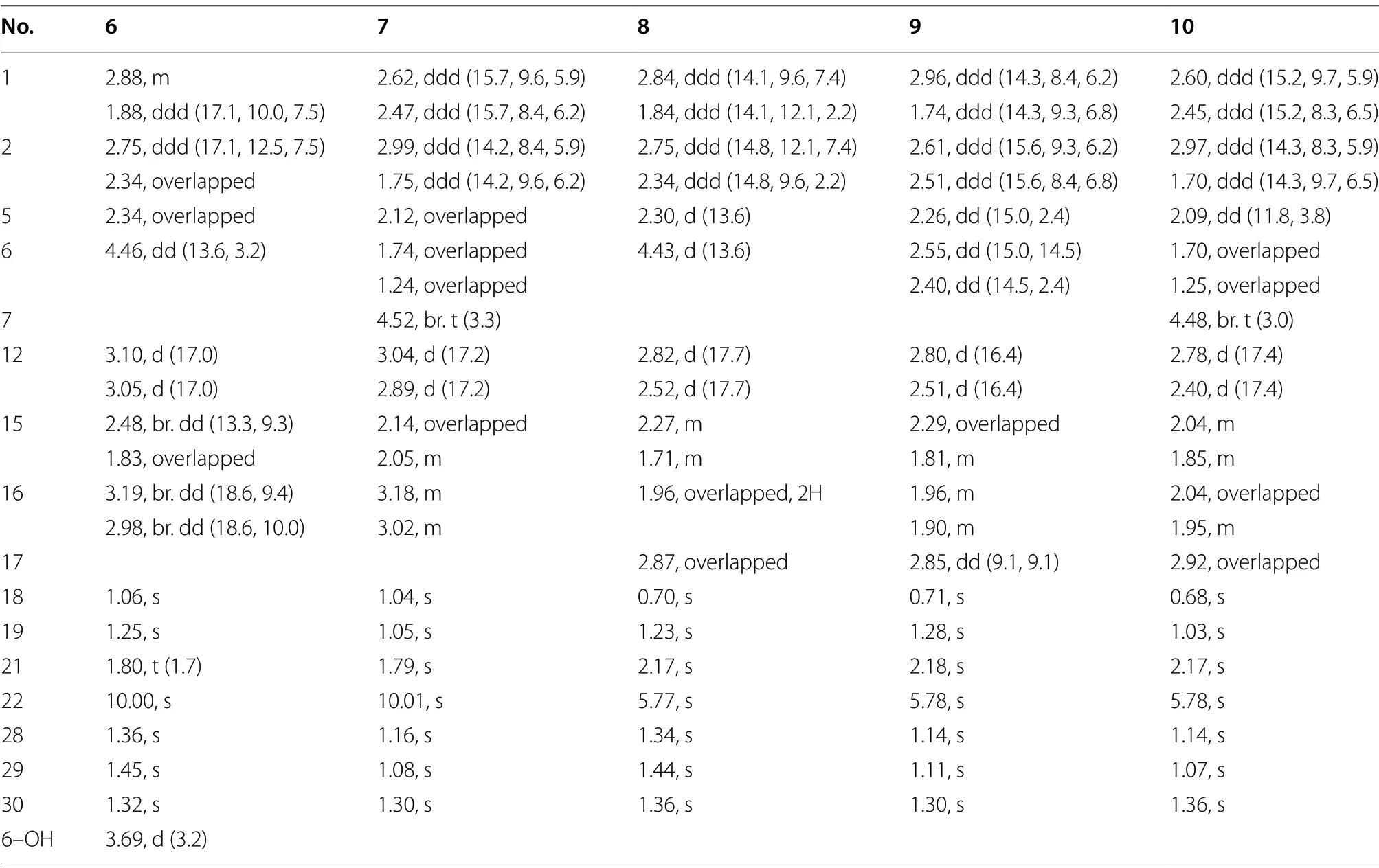
Table 3 1H NMR spectroscopic data of compounds 6-10 (600 MHz, CDCl3)
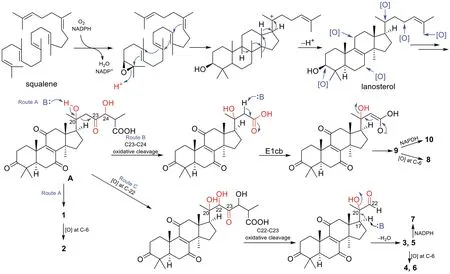
Scheme 1 Postulated biosynthetic pathways to compounds 1-10
2.2 The anti-NO production activity of the isolates
All the isolates were subjected to screening their inhibition against the NO production in murine monocytic RAW 264.7 macrophages.As a result, only compounds 3 and 4 displayed moderate inhibitory activities with IC50values of 32.5, 34.2μM, respectively.
3 Conclusions
Ten previously undescribed nor-lanostane triterpenes were isolated from the fruiting bodies ofGanoderma australe.The chemical structures of the compounds were determined with the aid of extensive NMR and HRESIMS spectroscopic analysis.These compounds are featured by tetra-, penta-, and hexa-nor-lanostane scaffold, and by unusual oxidative modifications at C-6.These findings put the diversity of nor-lanostanes ofGanodermaorigin one step forward.
4 Experimental section
4.1 General experimental procedures
An Autopol IV-T digital polarimeter (Rudolph, Hackettstown, USA) was used to measure the optical rotations.A Shimadzu UV-2401PC UV-vis spectrophotometer (Shimadzu Corporation, Kyoto, Japan) was used to record the UV spectra.The Bruker Ascend 500 MHz, Avance III 600 MHz, or Ascend 800 MHz spectrometers (Bruker Corporation, Karlsruhe, Germany) were used to record the one- and two-dimensional NMR spectra.HRESIMS spectra were measured on a Q Exactive Orbitrap mass spectrometer (Thermo Fisher Scientific, MA, USA).Column chromatography (CC) were run on Sephadex LH-20(Amersham Biosciences, Uppsala, Sweden) and silica gel(Qingdao Haiyang Chemical Co., Ltd., Qingdao, China).Medium pressure liquid chromatography (MPLC) was performed on an Interchim PuriFlash 450 chromatography system (Interchim Inc., Montlucon Cedex, France).The diameter and length of the column used for MPLC were 14 and 450 mm, respectively.The column was fill with Chromatorex C-18 silica gel (particle size: 40-75μm, flow rate 40 mL/min, Fuji Silysia Chemical Ltd.,Kasugai, Japan).Preparative high performance liquid chromatography (prep.-HPLC) was performed on an Agilent 1260 liquid chromatography system (Agilent Technologies, Santa Clara, CA, USA).The columns used for prep.-HPLC were Agilent Zorbax SB-C18 (5μm of particle size, i.d.9.4 mm × length 150 mm, flow rate 7 mL/min), and Agilent Zorbax SB C-8 column (5μm of particle size, i.d.9.4 mm × length 250 mm, flow rate 5 mL/min).RPMI 1640 medium (Hyclone, Logan,UT) was used in the anti-nitric oxide production assays.Griess reagent (reagent A and reagent B) was bought from Sigma (Sigma, St.Louis, MO).The plate reader was TECAN Spark 10 M (Tecan Trading AG, Switzerland).
4.2 Fungal material
The fruiting bodies ofGanoderma australewere collected in Tongbiguan Natural Reserve, Dehong, Yunnan Province, China, in 2016, and identified by Prof.Yu-Cheng Dai, who is a mushroom research in Institute of Microbiology, Beijing Forestry University.A voucher specimen ofG.australewas deposited in the Mushroom Bioactive Natural Products Research Group at South-Central Minzu University.
4.3 Extraction and isolation
10 L of mixed solvent CHCl3:MeOH (v/v 1:1) was used to extract the constituents from the grounded and dry fruiting bodies ofGanoderma australe(3.26 kg) (2.5 L × 4 times) at room temperature.The extract was further resuspended in distilled water and partitioned against ethyl acetate (EtOAc) to afford the EtOAc extract (130 g).The EtOAc extract was fractionated on MPLC by using a stepwise gradient of MeOH in H2O (20-100%) to afford seven fractions (A-G).
Fraction D (2.0 g) was separated by Sephadex LH-20(CHCl3:MeOH = 1:1) to afford four subfractions (D1-D4).Subfraction D2 (800 g) was separated by silica gel column chromatography (CC) (petroleum ether-acetone from v/v 15:1 to 1:1) to obtain eleven subfractions(D2a-D2k).Compound 1 (5.2 mg, tR= 12.9 min) was purified from D2k (75 mg) by prep.-HPLC (MeCN-H2O:30-50:50, 30 min, 4 mL/min).Subfraction D4 (610 mg)was subjected to silica gel CC (petroleum ether - acetone from v/v 6:1 to 1:1) and yielded eleven subfractions(D4a - D4k).Subfraction D4d (6.1 mg) was purified by prep.-HPLC (MeCN - H2O: 40:60-60:80, 25 min, 4 mL/min) to yield compound 2 (3.8 mg, tR= 16.5 min).
Fraction F (3.6 g) was separated by Sephadex LH-20(MeOH) to afford twenty-six subfractions (F1-F26).Subfraction F26 (280 mg) was separated by column chromatography (CC) on silica gel (petroleum etheracetone from v/v 15:1 to 1:1) to obtain four subfractions(F26a-F26d).Subfraction F26a (48 mg) was isolated on prep-HPLC (MeCN - H2O: 30:70 - 50:50, 30 min, 4 mL/min) to yield compounds 5 (tR= 7.80 min, 1.0 mg), 6 (tR= 6.50 min, 0.8 mg), and 4 (tR= 11.50 min, 1.3 mg).Compound 3 (0.9 mg, tR= 14.4 min) was purified from F26d(11 mg) by prep.-HPLC (MeCN - H2O: 30:70 - 50:50,30 min, 4 mL/min).
Fraction E (1.6 g) was separated by Sephadex LH-20(MeOH) to afford twelve subfractions (E1-E12).Subfraction E5 was separated by CC on silica gel (petroleum ether-acetone from v/v 15:1 to 1:1) to obtain four subfractions (E5a-E5d).Compound 8 (2.4 mg, tR=29.4 min) was purified from E5b (23 mg) by prep.-HPLC[MeCN + MeOH (2:1) - H2O: 50:50, 40 min, 4 mL/min].Compound 9 (2.1 mg, tR= 20.0 min) was purified from E5c (42 mg) by prep.-HPLC [MeCN + MeOH(2:1) - H2O: 40:60-60:40, 25 min, 4 mL/min].Compound 10 (6.5 mg, tR= 13.5 min) was purified from E2e (210 mg)by prep-HPLC (MeCN-H2O: 30:70 - 50:50, 25 min, 5 mL/min).Compound 7 (1.8 mg, tR= 20.0 min) was purified from E2g (13 mg) by prep.-HPLC (MeCN-H2O: 50:50-70:30, 25 min, 4 mL/min).
4.4 Spectroscopic data of compounds
4.4.1 Ganoaustratetraenone A (1)
4.4.2 Ganoaustratetraenone B (2)
4.4.3 Ganoaustraldehyde A (3)
4.4.4 Ganoaustraldehyde B (4)
4.4.5 Ganoaustraldehyde C (5)
4.4.6 Ganoaustraldehyde D (6)
4.4.7 Ganoaustraldehyde E (7)
4.4.8 Ganoaustrenoic acid A (8)
4.4.9 Ganoaustrenoic acid B (9)
4.4.10 10 ganoaustrenoic acid C (10)
4.5 Nitric oxide production in RAW 264.7 macrophages
The murine monocytic RAW 264.7 macrophages were cultured in RPMI 1640 medium 10% Fetal Bovine Serum (FBS).Firstly, the DMSO stocks of the compounds were made, and further been serially diluted in media to make different concentrations of stocks.The cell mixtures were distributed into 96-well plates(2 × 105cells/well) and preincubated in a humid atmosphere with 5% CO2for 24 h at 37 °C.Then different concentrations of stocks of the test compounds were added into each well, the maximum concentration of the test compound was 25μM.Then the lipopolysaccharides (LPS) were added to each cell (final concentration 1μg/mL), and continued to incubate for another 18 h.After adding 100μL of Griess reagent to 100μL of each supernatant from the LPS-treated or LPS- and compound-treated cells in triplicates, and incubated for another 5 min, the nitric oxide (NO) production of each cell was assessed by measuring the absorbance at 570 nm.Pyrrolidine dithiocarbamate (PDTC) was used as the positive control (IC5020.0μM).
Supplementary Information
The online version contains supplementary material available at https:// doi.org/ 10.1007/ s13659- 022- 00356-x.
Additional file 1.The NMR, HRESIMS spectra of compounds 1-10
Acknowledgements
The authors thank the National Natural Science Foundation of China (grant numbers 21961142008, 22177138) for fundings.The authors thank the Analytical & Measuring Center, School of Pharmaceutical Sciences, South-Central Minzu University for the spectra measures.
Author contributions
LZ and SA carried out the experiments, analyzed the results, and wrote the manuscript, they contributed equally to this work; MXW screened the biological activities; HPC analyzed the results and amended the manuscript; JKL designed and checked the whole manuscript.All authors read and approved the final manuscript.
Declarations
Competing interests
The authors declare that there are no conflicts of interest associated with this work.
 Natural Products and Bioprospecting2022年5期
Natural Products and Bioprospecting2022年5期
- Natural Products and Bioprospecting的其它文章
- Apatite phosphate doped by cobalt as hight efficient catalyst of multi-component synthesis of therapeutic spiropyrimidine compound
- Therapeutic roles of plants for 15 hypothesised causal bases of Alzheimer’s disease
- Beauty of the beast: anticholinergic tropane alkaloids in therapeutics
- Mulberry Diels-Alder-type adducts:isolation, structure, bioactivity, and synthesis
- Arctostaphylos uva‑ursi L.leaves extract and its modified cysteine preparation for the management of insulin resistance:chemical analysis and bioactivity
- New seco-anthraquinone glucoside from the roots of Rumex crispus
