Plant invasions facilitated by suppression of root nutrient acquisition rather than by disruption of mycorrhizal association in the nativeplant
Jing Chen , Hi-Yn Zhng , Ming-Cho Liu , Mei-Xu Hn , De-Ling Kong ,*
a College of Forestry, Henan Agricultural University, Zhengzhou 450002, Henan, China
b Liaoning Key Laboratory for Biological Invasions and Global Changes, College of Bioscience and Biotechnology, Shenyang Agricultural University,Shenyang, Liaoning 110866, China
A B S T R A C T Invasive species have profound negative impacts on native ranges.Unraveling the mechanisms employed by invasive plant species is crucial to controlling invasions.One important approach that invasive plants use to outcompete native plants is to disrupt mutualistic interactions between native roots and mycorrhizal fungi. However, it remains unclear how differences in the competitive ability of invasive plants affect native plant associations with mycorrhizae. Here, we examined how a native plant,Xanthium strumarium, responds to invasive plants that differed in competitive abilities (i.e., as represented by aboveground plant biomass) by measuring changes in root nitrogen concentration (root nutrient acquisition)and mycorrhizal colonization rate. We found that both root nitrogen concentration and mycorrhizal colonization rate in the native plant were reduced by invasive plants. The change in mycorrhizal colonization rate of the native plant was negatively correlated with both aboveground plant biomass of the invasive plants and the change in aboveground plant biomass of the native plant in monocultures relative to mixed plantings. In contrast, the change in root nitrogen concentration of the native plant was positively correlated with aboveground plant biomass of the invasive plants and the change in aboveground plant biomass of the native plant. When we compared the changes in mycorrhizal colonization rate and root nitrogen concentration in the native plant grown in monocultures with those of native plants grown with invasive plants, we observed a significant tradeoff. Our study shows that invasive plants can suppress native plants by reducing root nutrient acquisition rather than by disrupting symbiotic mycorrhizal associations,a novel finding likely attributable to a low dependence of the native plant on mycorrhizal fungi.
Keywords: Plant invasion Root strategy Mycorrhizal strategy Tradeoff
1. Introduction
Biological invasions greatly reduce biodiversity globally and have substantial economic costs (Van Kleunen et al., 2015; Pyˇsek et al., 2017; Pathak et al., 2019; Bellard et al., 2021; Rodriguez et al., 2021). Understanding the mechanisms by which invasive plants outcompete their native counterparts and spread through new ranges may help control invasions. Invasive plant species often outcompete native plants because invasive plants have a high capacity for resource acquisition. For example, invasive plants usually shift leaf nitrogen (N) allocated for defense to photosynthesis for a greater competitive advantage over other plants (Feng et al., 2009; Miao et al., 2014; Battini et al., 2021;Harkin and Stewart, 2021; Lin et al., 2021; Wang et al., 2022). In addition, invasive plants are increasingly reported to suppress native plants by disrupting symbiotic associations between native plants and other organisms, such as mycorrhizal fungi (Callaway and Ridenour, 2004; Pringle et al., 2009; Wilson et al., 2012;Grove et al., 2017; Liu et al., 2020).
Most terrestrial plants form symbiotic relationships with mycorrhizal fungi for nutrient acquisition (Kohler et al., 2015;Martin et al., 2017; Steidinger et al., 2019; Tedersoo et al., 2020;Meng et al., 2021; Shi et al., 2021). In this symbiosis, plants help mycorrhizal fungi meet energy requirements by supplying carbon;in return, the fungi provide nutrients to plants through their enormous extrametrical hyphae (Bonfante and Genre, 2010;Delavaux et al.,2017;Jiang et al.,2017;Bisseling and Geurts,2020;Delaux and Schornack, 2021). A previous study has reported that invasive plants can suppress native plants by reducing associations between the roots of native plants and mycorrhizal fungi (Stinson et al., 2006). This is an important mechanism by which invasive plants gain a foothold into new ranges,and it has received a great deal of attention (Vogelsang and Bever, 2009; Lau and Schultheis,2015; Dickie et al., 2017; He et al., 2018; Yu and He, 2021).Despite the importance of this mechanism, it is still yet fully understood (Jordan et al., 2011; Gaggini et al., 2018). For example,mounting evidence has shown a complementary relationship between roots and mycorrhizal fungi in nutrient acquisition (Chen et al., 2016; Duchene et al., 2017; Brundrett and Tedersoo, 2018;Fabianska et al.,2019;Tao et al.,2019).It is likely that native plants can increase root activity when their associations with mycorrhizal fungi are suppressed by invasive plants. However, we know little about whether invasive species with different competitive abilities differ in their influence on mycorrhizal association of the native roots.In this study,we aimed to test whether larger invasive plants are more effective than smaller plants at disrupting mutualistic associations between a native plant and mycorrhizae. We also tested whether native plants counteract reductions in mycorrhizal associations by investing more in root nutrient acquisition. For this purpose,we examined how nutrient acquisition strategies changed in a native plant,Xanthium strumariumL., in response to five invasive plant species of different sizes. Specifically, we measured changes in root N concentration and mycorrhizal colonization rates inX. strumariumgrown with five invasive plant species given that the two traits are closely related to nutrient acquisition by roots(Reich et al., 2008; Kong and Fridley, 2019) and mycorrhizal fungi(Kong et al., 2014; Bergmann et al.,2020), respectively.
2. Materials and methods
2.1. Study site
This study was conducted at the research station at Shenyang Agricultural University(41.61°N,123.44°E).The elevation is 50 m a.s. l. Mean annual temperature is 8.4°C and mean annual precipitation is 721.9 mm.
2.2. Plant materials
We selected five invasive plants,Xanthium italicumMoretti,Ambrosia trifidaL.,Solanum rostratumDunal.,Amaranthus retroflexusL.andGalinsoga quadriradiataRuiz et Pav.,which commonly occur in Liaoning Province and differ greatly in aboveground plant biomass (Fig. 1). Here,X. strumariumwas selected as the native plant because it occurs widely in the field and we have previously described its biological and chemical characteristics in our lab. All invasive and native plants in this study form an association with arbuscular mycorrhizal fungi,exceptA.retroflexus,which is a nonmycorrhizal plant, i.e.,forms no symbiosis with mycorrhizal fungi.Mature seeds of the native and invasive plants were collected in Chaoyang (Liaoning Province), Changchun (Jilin Province) and Harbin(Heilongjiang Province)in October 2016.Seeds of each plant were collected from several individual plants, each separated by a distance of over 20 m,then placed in large envelopes,air-dried,and stored at 4°C for later experiments.

Fig.1. Plant aboveground biomass of five invasive species in monocultures.
2.3. Experimental design
Because germination time varied for each species, seeds were germinated in a greenhouse at different times in April to ensure a relatively equal size of the plants at the initial growing stage.Before sowing, the seeds were disinfected with 0.5% potassium permanganate,and then washed using distilled water before being sown in soil.The seeds of the native plant were sown in monocultures and in mixed plantings with each of the five invasive plants, respectively. In addition, each of the invasive plants was also sown in monocultures. Each the monocultures and mixed plantings was replicated five times.After seed germination,the seedlings in each pot were thinned in mid-May to keep only two individuals in monocultures and four individuals in mixed plantings (two native and two invasive plants). After seedling thinning, the pots were removed from the greenhouse and plants were grown outside until harvest on July 30.
The soils used in the pot study were mixed plantings of forest topsoil and river sand.The forest topsoil was collected from a forest stand near Shenyang Dongling Park.The soil was sieved(2 mm)to remove large clods and plant residues and then air-dried.The river sand was sterilized by a steam pressure cooker at 121°C.After that,the soil and sand were mixed evenly by a mass ratio of 1:1,and then put into pots (28 cm × 22 cm × 20 cm) with each pot filled with 7 kg soil to a depth of 15 cm. The initial physical and chemical characters of the soil were as follows: pH, 6.75; organic matter content, 54.71 g kg-1; total nitrogen content, 2.43 g kg-1; total phosphorus content, 0.060 g kg-1; total potassium content,1.54 g kg-1; available phosphorus, 2.50 mg kg-1; available potassium content, 108.67 mg kg-1; available nitrogen content,182.33 mg kg-1. All chemicals were measured at Analytical &Testing Center, Shenyang Agricultural University.
2.4. Plant sampling and measurement
Plants were harvested on July 30, 2017 when they reached the highest plant biomass. After harvest, we measured aboveground plant biomass of each plant, and root N concentration (Vario EL CUBE, Germany) and mycorrhizal colonization rate for native plants. Mycorrhizal colonization was determined following previous procedures(Trouvelot et al.,1986;Liu et al.,2015b).Briefly,50 randomly selected root segments were washed with distilled water and then soaked in 10% KOH solution for 50 min at 90°C. Afterwards,the roots were soaked in 5%HCl solution for 5 min and then washed with distilled water.The roots were stained in 0.05%acidic magenta solution, flattened on a slide, and then examined for mycorrhizal fungi colonization using a 200 × microscope (Nikon MODEL ECLIPSE NI-U, Japan). The roots were classified into five categories of mycorrhizal colonization,each assigned with a weight of 0.95, 0.70, 0.30, 0.05 and 0.01. The final colonization rate of the native roots was calculated as follows:

wheren5-1are the number of root segments belonging to each of the five categories.
2.5. Data analysis
As a portion of the roots of the invasive and the native plant was used for measurements of mycorrhizal colonization and root N concentration, root biomass could not be measured accurately.Therefore, we used only the aboveground plant biomass of these plants in statistical analyses.The differences of aboveground plant biomass,root mycorrhizal colonization and root N concentration of the native plant,Xanthium strumarium, between its monocultures and mixed plantings with each of the invasive plants were analyzed byttest. Data were transformed when necessary for normal distribution and equal variance.
To assess the effects of the competitive ability of the invasive plants on the native plant, we examined changes in root mycorrhizal colonization and root N concentration of the native plant in monocultures relative to its mixed plantings with the invasive plants. Competitive ability of the invasive plants was represented by the aboveground plant biomass of the invasive plants in monocultures.All analyses were performed in R 4.1.0(R core team)with the significance level set at 0.1.
3. Results
The native species,Xanthium strumarium, had lower aboveground plant biomass, root mycorrhizal colonization rate and root N concentration when grown with most invasive species than when grown in monocultures (Table 1). Two invasive species,G. quadriradiataandS. rostratum, had no effect on aboveground plant biomass or root N concentration of the native species,respectively.

Table 1 Plant traits of the native species, Xanthium strumarium,in monocultures and mixed plantings with different invasive plants.
Aboveground biomass of the five invasive species varied in monocultures, ranging from 0.82 g forG. quadriradiatato 35.75 g forX. italicum. When native species were grown with invasive species, changes in root mycorrhizal colonization rate were correlated with the aboveground biomass of the invasive species(r= -0.57,P= 0.0027, Fig.2a).Root N concentration of the native species was also correlated with above plant biomass of the invasive species (r= 0.74,P= 0.0017, Fig. 2b).
The change of aboveground biomass of the native species was negatively correlated with the change of root mycorrhizal colonization rate(r=-0.38,P=0.0630,Fig.3a).In contrast,the change in aboveground biomass of the native species was positively correlated with the change in root N concentration(r=0.67,P=0.0070,Fig. 3b).
When the native species was grown with invasive species,changes in root mycorrhizal colonization rate were not correlated with changes in root N concentration (r= -0.14,P= 0.6100,Fig. 4a). However, a root-mycorrhizal relationship was observed(r=-0.65,P=0.0088,Fig.4b)when we compared changes in root mycorrhizal colonization and root N concentration of the native species in monocultures relative to its mixed plantings with the invasive species.
4. Discussion
Plants acquire soil nutrients through the root and mutualistic associations with mycorrhizae.Invasive plants have been previously shown to suppress native plant growth by reducing native plant associations with mycorrhizae (Stinson et al., 2006; Tang et al.,2020). In this study, we found that invasive plants inhibit nutrient acquisition of a native plant,Xanthium strumarium, by interfering with roots more than by disrupting mycorrhizal interactions.
In our experiments,aboveground biomass of the native species was negatively correlated with the change of root mycorrhizal colonization rate, but positively correlated with changes in root N concentration. These findings differ greatly from previous studies that invasive plants suppress native plant growth via a reduction in mycorrhizal interactions (Nasto et al., 2017; Ostonen et al., 2017;Png et al., 2017; Freund et al., 2018; Clausing et al., 2021; de Vries et al., 2021). The native plant used in our study (X. strumarium)has a low mycorrhizal colonization rate (about 16%), and relies mostly on the root for nutrient acquisition. Thus, our contrasting results may be partially explained by the low dependence ofX. strumariumon mutualistic mycorrhizal interactions.
Interestingly,we found that the effect of each of the five invasive species on root nutrient acquisition and mycorrhizal colonization of the native species varied. For example, although invasive plants reduced both root nutrient acquisition and mycorrhizal colonization rate in the native plant, the extent to which invasive plants inhibited mycorrhizal colonization rate wasnegativelycorrelated with the size of invasive plants. Conversely, the extent to which invasive plants inhibited root nutrient acquisition waspositivelycorrelated with the size of invasive plants(Fig.2).These findings do not support our original hypothesis that larger invasive plants cause greater reductions in native plant mycorrhizal colonization rate.The rationale for our initial hypothesis was that larger invasive plants are usually able to outcompete native plants for resources;this great competitive capacity of larger invasive plants reduces native plant growth, causing a reduction in carbon supply, which lowers the ability of native plants to maintain mycorrhizal associations.However,larger invasive plants in our study caused a smaller rather than a larger reduction in mycorrhizal colonization in the native plants.
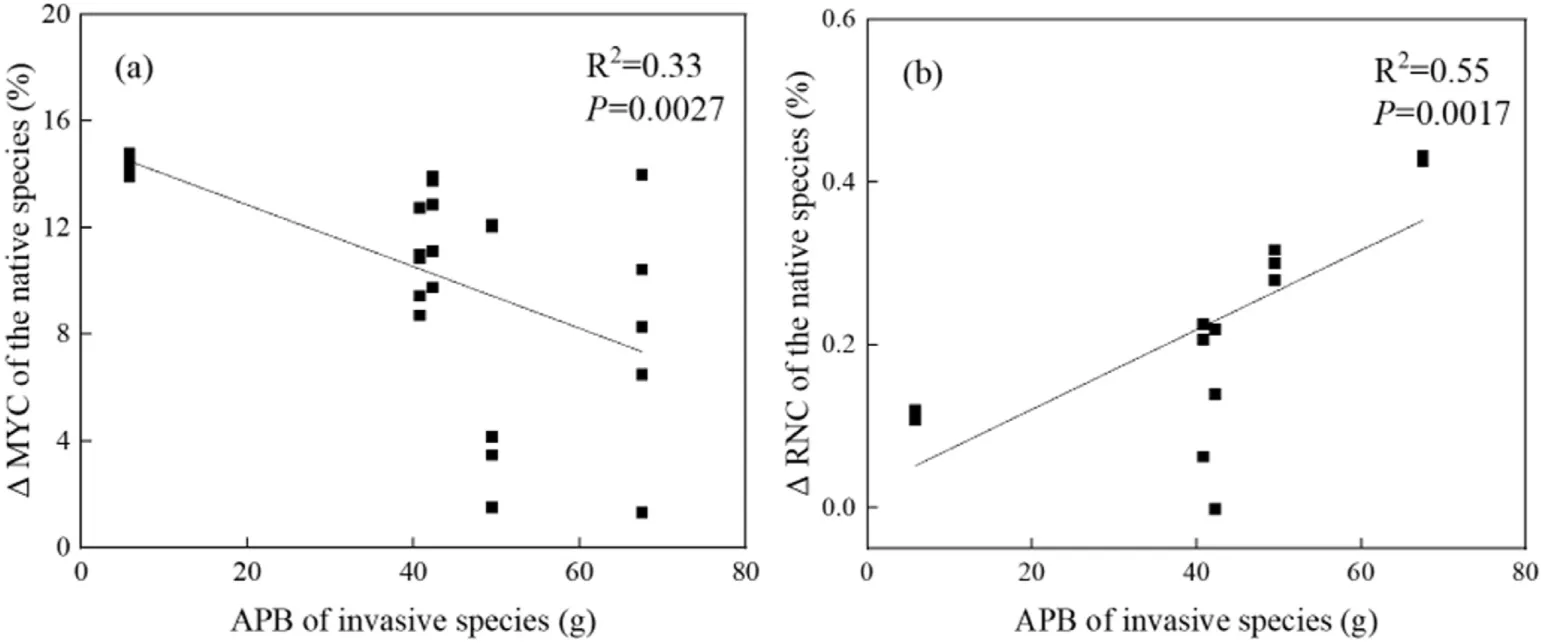
Fig.2. Effects of invasive plant size on mycorrhizal colonization rate(a)and root N concentration(b)in native species, Xanthium strumarium.MYC,mycorrhizal colonization;RNC,root nitrogen concentration;APB,aboveground plant biomass;Δ,the difference of a trait for X.strumarium between its monocultures and mixed plantings with an invasive species.
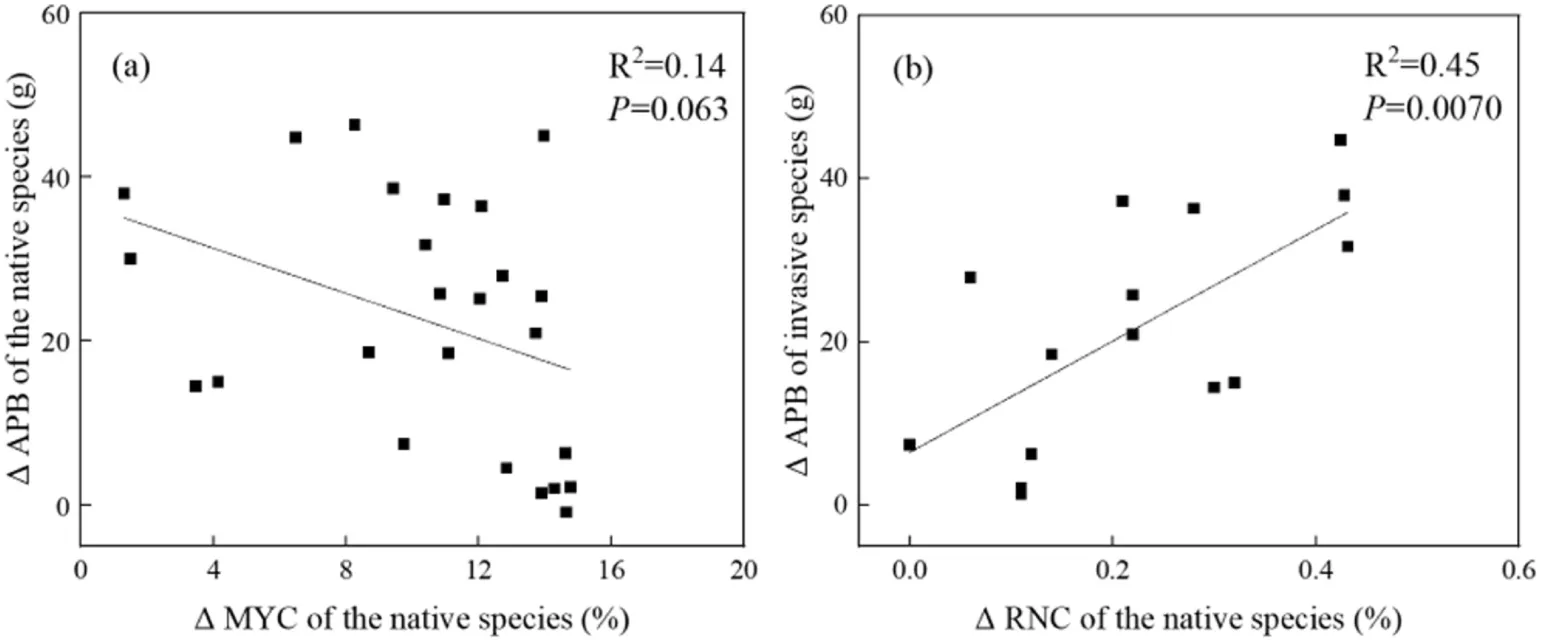
Fig. 3. Relationships of root mycorrhizal colonization (a) and root N concentration (b) with aboveground plant biomass for the native species, Xanthium strumarium. MYC,mycorrhizal colonization; RNC, root nitrogen concentration; APB, aboveground plant biomass; Δ, the difference of a trait for X. strumarium between its monocultures and mixed plantings with an invasive species.
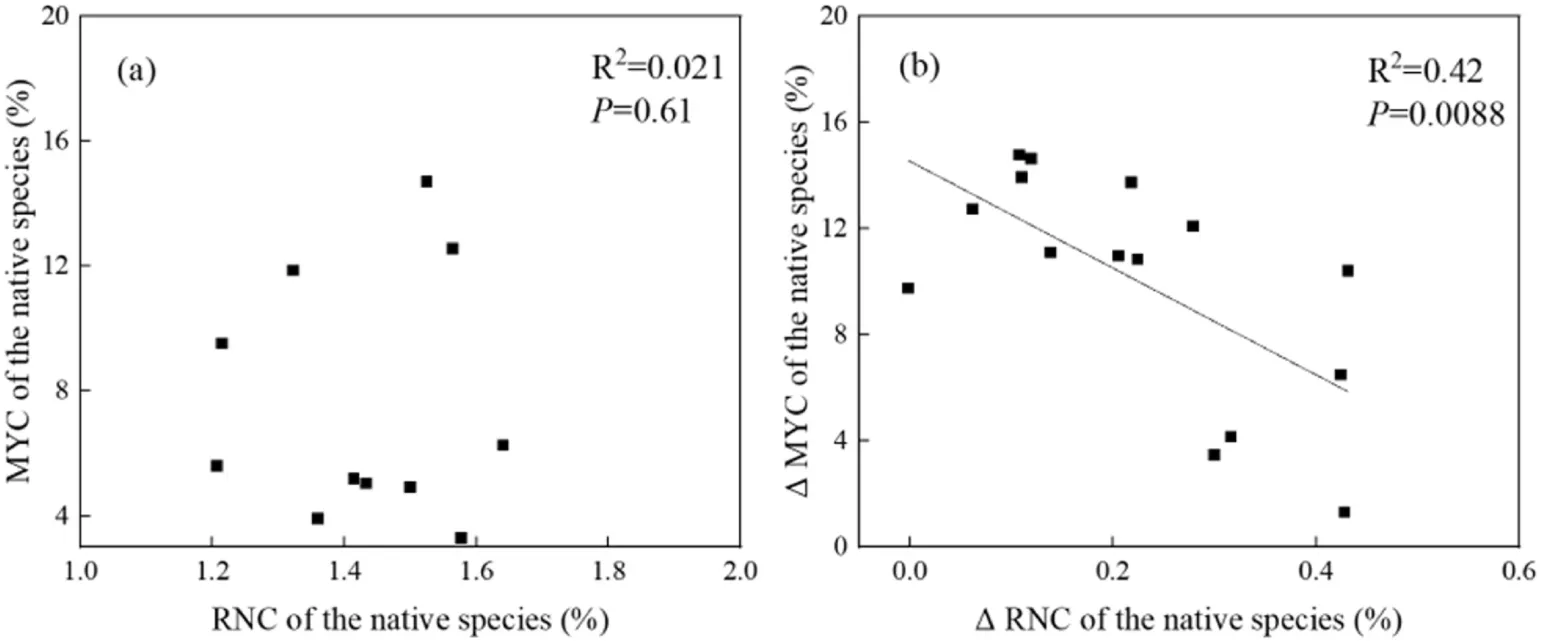
Fig.4. The relationship between root mycorrhizal colonization(MYC)and root N concentration(RYC)of the native species, Xanthium strumarium.(a)and(b) uses the trait data of X. strumarium growing in mixed plantings. MYC, mycorrhizal colonization; RNC, root nitrogen concentration; APB, aboveground plant biomass; Δ, the difference of a trait for X. strumarium between its monocultures and mixed plantings with an invasive species.
The unexpected result of our study could be due to interactions between root nutrient acquisition and mutualistic mycorrhizal interactions, which are generally reported to be complementary(Nasto et al., 2017; Png et al., 2017; Soper et al., 2018; Qiu et al.,2021). For example, plants that rely more on roots for nutrient acquisition will reduce their dependence on mycorrhizal fungi(Liu et al.,2015a;Chen et al.,2016;Bialic-Murphy et al.,2021).Although no such tradeoff was found in our study in the native plant(Fig.4a),we did observe a tradeoff between root nutrient acquisition and mycorrhizal interactions when we compared changes in root N concentration and mycorrhizal colonization rate of the native plant in monocultures with those of native plants grown with invasive species (Fig. 4b). Specifically, a larger invasive plant can cause a greater reduction in root N concentration but only a small reduction in mycorrhizal colonization rate in the native plant. We speculate that the smaller reduction in mycorrhizal colonization rate by larger invasive plants may be beneficial to the native plant. For example, despite a relatively weak dependence of the native plant on mycorrhizal interactions, the smaller reduction in mycorrhizal colonization may partially meet nutrient demand of the native plant when nutrient acquisition by the root is greatly suppressed by larger invasive plants.In contrast,when the native plant was grown with a smaller invasive plant, invasive plants suppressed mycorrhizal colonization (Fig. 2a) more than they suppressed root nutrient acquisition(Fig.2b).It is likely that these smaller invasive plants interfere with mycorrhizal colonization through allelopathy(Kato-Noguchi,2020;Kalisz et al.,2021;Zhu et al.,2021).If so,the allelopathic effects of smaller invasive plants warrant further investigation.
5. Conclusions
In this study, we examined how nutrient acquisition strategies of native plants responded to size differences in invasive plants.We found that invasive plants suppressed root nutrient acquisition rather than mycorrhizal colonization of native plants. To our knowledge,we are the first to show a tradeoff between the changes in root nutrient acquisition and mycorrhizal colonization rate in a native plant in response to exotic plant invasion. This tradeoff suggests that larger invasive plants can outcompete native plants through their greater influence on the root nutrient acquisition in native plants, whereas smaller invasive plants likely lean toward allelopathic effects on mutualistic mycorrhizal interactions.Finally,it should be noted that this study was conducted in the temperate zone where plants are usually not limited by soil phosphorus (P).Future studies could test generalizability of this study by selecting more invasive and native species across sites differing in soil P content and plant dependence on mycorrhizal fungi.
Author contributions
H.Z. and D.K. designed the study, D.K. and H.Z. conducted the study,C.J.wrote the manuscript.C.J.,D.K.,M.L.and M.H.contributed to the results,analyses,writing,and revisions.All authors approved the submission.
Declaration of competing interest
We declare that we have no conflict interest.
Acknowledgments
We are grateful for Miss.Xiaocheng Guo,Xinyu Lu and Jinqi Tang for their assistance in field work and lab analysis.We also thank the editor office for assistance in polishing this manuscript patiently.This study was supported by the National Natural Science Foundation of China (31870522, 32171746 and 32171662) and the Scientific Research Foundation of Henan Agricultural University(30500854), Research Funds for overseas returnee in Henan Province, China.
- 植物多样性的其它文章
- GUIDE FOR AUTHORS
- Taxonomic synopsis of Berberis (Berberidaceae) from the northern Hengduan mountains region in China,with descriptions of seven new species
- Evolutionary importance of the relationship between cytogeography and climate: New insights on creosote bushes from North and South America
- SSR markers development and their application in genetic diversity evaluation of garlic (Allium sativum) germplasm
- Mapping the habitat suitability of Ottelia species in Africa
- Adaptive responses drive the success of polyploid yellowcresses(Rorippa, Brassicaceae) in the Hengduan Mountains, a temperate biodiversity hotspot

